More than 30 million persons worldwide take nonsteroidal anti-inflammatory drugs (NSAIDs) on a daily basis, and annual consumption is increasing. In addition to their analgesic and anti-inflammatory properties, NSAIDs also produce well-known gastrointestinal adverse events. There is no consensus in Mexico on the diagnosis, treatment, and prevention of NSAID-induced gastropathy and enteropathy, and so the Asociación Mexicana de Gastroenterología brought together a group of experts to establish useful recommendations for the medical community. Thirty-three recommendations were formulated in the present consensus, highlighting the fact that the risk for NSAID-induced gastrointestinal toxicity varies according to the drug employed and its pharmacokinetics, which should be taken into account at the time of prescription. The risk factors for gastroduodenal complications due to NSAIDs are: a history of peptic ulcer, age above 65 years, high doses of NSAIDs, Helicobacter pylori infection, and the presence of severe comorbidities. The symptoms and gastroduodenal damage induced by NSAIDs vary, ranging from an asymptomatic course to the presentation of iron-deficiency anemia, bleeding, stricture, and perforation. Capsule endoscopy and enteroscopy are direct diagnostic methods in NSAID enteropathy. Regarding prevention, the minimum dose of an NSAID needed to achieve the desired effect, administered for the shortest period of time, is the recommendation. Finally, proton pump inhibitors are the gold standard for the prophylaxis and treatment of gastroduodenal effects, but they are not useful in enteropathy.
Más de 30 millones de personas consumen diariamente antiinflamatorios no esteroideos (AINE) en el mundo, y este consumo se ve incrementado anualmente. Aunque los AINE poseen propiedades analgésicas y antiinflamatorias, sus eventos adversos gastrointestinales son bien reconocidos. En nuestro país no existía un consenso respecto al diagnóstico, tratamiento y prevención de la gastropatía y la enteropatía por AINE, por lo que la Asociación Mexicana de Gastroenterología reunió a un grupo de expertos para establecer recomendaciones de utilidad para la comunidad médica. En este consenso se emitieron 33 recomendaciones. El consenso destaca que el riesgo de toxicidad gastrointestinal de los AINE varía según el fármaco empleado y su farmacocinética, lo cual debe ser considerado al momento de su prescripción. Los factores de riesgo de complicación gastroduodenal por AINE son: antecedente de úlcera péptica, edad mayor a 65 años, dosis altas del AINE, infección por Helicobacter pylori (H.pylori), y presencia de comorbilidades graves. Los síntomas y el daño gastroduodenal inducido por AINE son variables ya que puede cursar asintomático o manifestarse como anemia por deficiencia de hierro, hemorragia, estenosis y perforación. La cápsula endoscópica y la enteroscopia son métodos diagnósticos directos en la enteropatía por AINE. Respecto a la prevención, se recomienda prescribir la dosis mínima necesaria de un AINE para obtener el efecto deseado y durante el menor tiempo. Finalmente, los inhibidores de la bomba de protones (IBP) representan el estándar de oro para la profilaxis y tratamiento de los efectos gastroduodenales, mas no son útiles en la enteropatía.
More than 30 million persons worldwide take nonsteroidal anti-inflammatory drugs (NSAIDs) on a daily basis.1 Said use is increasing yearly due to the greater life expectancy of the population and the self-prescription of aspirin and other NSAIDs. In addition to their excellent analgesic and anti-inflammatory properties, NSAIDs also produce well-known gastrointestinal, cardiovascular, renal, and hepatic adverse events. The most frequent NSAID-related adverse events are gastrointestinal, and they result in greater morbidity and mortality.
In February 2018, the Asociación Mexicana de Gastroenterología brought together a multidisciplinary group of health professionals made up of gastroenterologists, endoscopists, and surgeons to produce The Mexican Consensus on the Diagnosis, Treatment, and Prevention of NSAID-induced Gastropathy and Enteropathy and establish useful recommendations for the medical community. The specific aim of the consensus was to prepare an updated document applicable to medical practice in Mexico. The recommendations included are based on a thorough review of the medical literature and the consensus opinion of the participating specialists.
MethodsThe consensus was developed using the Delphi process, as has been previously described.2 Three coordinators (MVBF, JLL, JLT de la C) were designated and 20 experts in specialties related to NSAID-induced gastropathy and enteropathy were invited to participate. The coordinators carried out a detailed search of the following databases: The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (PubMed), EMBASE (Ovid), LILACS, CINAHL, BioMed Central, and the International Clinical Trials Registry Platform (ICTRP) of the World Health Organization. The search encompassed articles published within the time frame of January 1, 2008 to February 28, 2018. The search criteria included the term: “nonsteroidal anti-inflammatories” combined with the following terms: “gastric”, “small bowel”, “enteropathy”, “risk” “incidence”, “prevalence”, “Mexico”, “pathophysiology”, “diagnosis”, “differential diagnosis”, “treatment”, “endoscopy”, “therapy”, “management”, “review”, “guidelines”, and “meta-analysis” and their Spanish equivalents. The complete bibliography was made available to all the consensus participants.
The coordinators then formulated statements that underwent a first round of anonymous, electronic voting (from February 2 to 11, 2018) to evaluate the drafting and content of the statements. The consensus participants voted utilizing the following responses: a) in complete agreement, b) in partial agreement, c) uncertain, d) in partial disagreement, and e) in complete disagreement.
After the first vote, the coordinators carried out the corresponding modifications. Statements in which complete agreement was >75% were kept, and those in which complete disagreement was > 75% were eliminated. The statements with complete agreement ≤ 75% and complete disagreement ≤75% were reviewed and restructured. The revised statements underwent a second anonymous, electronic round of voting (from February 11 to March 4, 2018) After the second vote, in addition to drafting and content, each statement was evaluated according to the quality of evidence and grade of recommendation that sustained it, which was done through the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.3 In the GRADE system, the quality of evidence is not graded based solely on the design or methodology of the research, but also on a clearly posed question related to an outcome that is also clearly stated.4 Thus, evidence is described as high, moderate, low, or very low, and the strength of recommendation is strong or weak and in favor of or against the intervention or statement. Importantly, the GRADE system was used in relation to diagnostic tests and therapeutic interventions. Table 1 shows the codes utilized in the GRADE system, in which upper case letters refer to the quality of evidence, followed by a number indicating the strength of the recommendation that is in favor of or against the intervention or statement.
GRADE system codes.
| Quality of evidence | Code |
|---|---|
| ● High | A |
| ● Moderate | B C |
| ● Low | D |
| ● Very low |
| Strength of recommendation | |
|---|---|
| ● Strong, in favor of the intervention | 1 |
| ● Weak, in favor of the intervention | 2 |
| ● Weak, against the intervention | 2 |
| ● Strong, against the intervention | 1 |
Source: Adapted from Guyat et al.3.
The results of the third voting round were presented on April 14, 2018, at a face-to-face meeting held in the city of Durango (Durango, Mexico). At that meeting, the statements in which agreement was > 75% were ratified. The statements that did not reach agreement of 75% in the previous voting rounds were discussed, in an effort to reach a consensus. If no consensus was reached, they were eliminated. A final vote was then carried out. Once all the consensus statements were established, the coordinators formulated the present manuscript, which was reviewed and approved by all the consensus members.
ResultsInitially, the coordinators proposed 39 statements. After the online rounds of voting and the final vote at the face-to-face meeting, the resultant consensus was made up of 33 recommendations. The final recommendations and voting results are presented below.
Generalities and risk factors1.- Nonsteroidal anti-inflammatory drugs (NSAIDs) are one of the most commonly used over-the-counter medications worldwide.
Agreement reached: 90% in total agreement, 10% in partial agreement.
NSAIDs are drugs that reduce pain, fever, and inflammation, and are currently classified as1,5:
- a)
Nonselective NSAIDs (nsNSAIDs), such as naproxen, indomethacin, ibuprofen, sulindac, diclofenac, and piroxicam.
- b)
Selective cyclo-oxygenase-2 inhibitors (selective COX-2 inhibitors), which include the coxibs (such as rofecoxib, etoricoxib, and celecoxib) and the so-called “preferential” inhibitors, such as nimesulide and meloxicam.
Due to the increasing life expectancy of the worldwide population and the corresponding increase in rheumatologic disorders, NSAID use has substantially risen in recent decades.1 In addition to the anti-inflammatory, analgesic, and antipyretic effects of acetylsalicylic acid (ASA), its use in low doses is employed to reduce the risk for ischemic cardiac and cerebrovascular events. That indication has been popularized and many persons use it with no medical prescription or exact indication.1,5 At least in the United States, those medications are the most widely used, either as prescription or over-the-counter drugs.6 There has also been an increase in the clinical application of NSAIDs as prophylactic drugs against neoplasias or as part of the treatment of Alzheimer’s disease.7 Thus, it is likely for NSAID-induced enteropathy to be increasingly diagnosed.
2.- NSAID-induced gastroduodenopathy is due to a systemic effect that causes hypoperfusion and a reduction in bicarbonate synthesis and epithelial proliferation, secondary to prostaglandin synthesis inhibition, as well as to direct topical effects that cause disruption of the cytoprotective barrier of the mucosa.
Agreement reached: 80% in total agreement, 20% in partial agreement.
The harmful effect of NSAIDs on the gastroduodenal mucosa is mediated by 2 mechanisms. The main one is the systemic effect, which is due to the inhibition of cyclo-oxygenase (COX) activity, causing less blood flow, reduced bicarbonate and prostaglandin synthesis, and a decrease in epithelial proliferation.8 They can also cause topical mucosal damage, resulting in the disruption of the gastric mucosal barrier, by action of the nonionized weak acids inside the cells, causing intracellular alterations in the mitochondria due to uncoupling of mitochondrial oxidative phosphorylation, as well as damage to the lipid bilayer.9,10 That direct cytotoxicity is independent of COX inhibition and causes an increase in membrane permeability, resulting in epithelial damage with additional necrosis and apoptosis of the gastric cells.11
3.- The pathophysiology of NSAID-induced enteropathy is different from that of gastroduodenopathy.
Agreement reached: 70% in total agreement, 30% in partial agreement.
The pathogenesis of intestinal lesions induced by NSAIDs is less understood than that of gastroduodenopathy.9 Ulcers typically present as necrotic (or apoptotic) damage of enterocytes that can affect the deepest layers of the mucosa, with acute inflammatory infiltrate and loss of the villi.9 Unlike gastroduodenopathy, the symptoms of NSAID-induced enteropathy are nonspecific and its pathophysiology appears to be different. However, the damage induced by nsNSAIDs and selective COX-2 inhibitors is similar in the small bowel. For example, a study showed there was no difference in relation to intestinal lesions induced by long-term use of nsNSAIDs or selective COX-2 inhibitors (62 vs. 50%).12 Several years ago, the 3-hit hypothesis was posited: first, the NSAID affects the phospholipids of the cell membrane, resulting in mitochondrial injury. That causes the second hit, which is the decrease in the synthesis of energy, resulting in the release of calcium and the production of free radicals. Consequently, the intercellular bonds are broken, and the permeability of the mucosa increases. The third hit is then produced, in which the intraluminal content, such as the bile acids, proteolytic enzymes, and gut bacteria and their toxins, enter the cells and inflammation begins.13,14 At present, there is no evidence that the suppression of gastric acid secretion reduces the incidence or severity of NSAID-induced enteropathy. In fact, the few studies on the theme indicate that the benefit is marginal, and studies conducted on animals suggest that the concomitant use of proton pump inhibitors (PPIs) and NSAIDs exacerbate the existing enteropathy and that said damage appears to be related to changes in the number and type of bacteria in the small bowel during therapy with PPIs.15
4.- NSAID-induced damage along the gastrointestinal tract is greater, with the concomitant use of other NSAIDS, anticoagulants, and antiplatelet drugs.
Agreement reached: 85% in total agreement, 15% in partial agreement.
Antiplatelet agents and anticoagulants per se carry a risk for gastrointestinal complications (ulceration or bleeding) and the risk increases with the concomitant use of NSAIDs and other medications (statements 8 through 12). Therefore, communication between cardiologists, gastroenterologists, and primary care physicians is essential for individually evaluating the risk for ischemic or bleeding events in the patient requiring those medications.16
5.- The risk for gastrointestinal toxicity from different NSAIDs varies, depending on the drug employed and its pharmacokinetics, which should be taken into consideration at the time of prescription.
Agreement reached: 95% in total agreement, 5% in partial agreement.
It is well known that all NSAIDs, albeit to greater or lesser degrees, cause gastrointestinal toxicity. Numerous controlled clinical trials with placebo demonstrate that all NSAIDs, including the selective COX-2 inhibitors, are associated with a higher or lower risk for gastrointestinal lesions, and the relative risk varies among the different NSAIDs. The relative risk for aceclofenac, celecoxib, and ibuprofen is low (relative risk [RR] < 2).17 Diclofenac, meloxicam, and ketoprofen have an intermediate risk (RR = 2-4), whereas for naproxen, indomethacin, and diflunisal, the risk is higher (RR = 4-5). Piroxicam (RR = 7.4) and ketorolac (RR = 11.5) are the drugs that have a greater risk for gastrointestinal toxicity.17
The manner in which to prevent NSAID-induced lesions of the gastrointestinal mucosa is to avoid their use or substitute them with an agent that is less toxic for the gastrointestinal mucosa, such as acetaminophen. But if NSAID use is necessary, an effort should be made to minimize damage.18 Lower effective doses should be searched for, they should be used for the least amount of time possible, and preferably only the same NSAID should be used. Selective COX-2 inhibitors should also be given preference, if there are no major cardiovascular risks, or a safer nsNSAID should be used, such as ibuprofen, diclofenac, or aceclofenac, which have been associated with a lower relative risk for gastrointestinal bleeding.18,19 Selective COX-2 inhibitors have lower gastroduodenal toxicity, compared with nsNSAIDs. The main concern with their use is their association with greater risk for cardiovascular events, but many nsNSAIDs, particularly diclofenac, have also been associated with cardiovascular risk.17–19 On the other hand, the risk for gastrointestinal complications increases if high doses of NSAIDs are used in a continuous manner. That risk is constant, regardless of the dose, and for the entire time the treatment is maintained.20 Thus, different drug regulatory agencies recommend that all NSAIDs be used at the minimum dose, for the shortest time period possible, and in accordance with the indication for which it was prescribed.21,22
6.- Nonselective NSAIDs and selective COX-2 inhibitors induce damage throughout the intestine with the same frequency.
Agreement reached: 55% in total agreement, 25% in partial agreement, 20% uncertain.
Compared with nsNSAIDs, selective COX-2 inhibitors are associated with a significantly lower risk for damage at the gastroduodenal level but not at the distal gastrointestinal level (middle intestine and colon), where the risk appears to be similar. There is increasing evidence of a notable rise in small bowel lesions. NSAID-induced lesions and their complications in the stomach and duodenum have been known about for many years. With the increased availability of endoscopic equipment, they have become more recognized and more easily identified, but the actual magnitude of damage in the distal digestive tract is unknown.23 Enteropathy is often underdiagnosed or even undetected in the majority of studies, because the lesions are beyond the reach of conventional endoscopic examination. Nevertheless, an increasing number of hospitalizations are being reported for complications in the lower gastrointestinal tract.24 Some reports in the literature state that the prevalence of enteropathy is greater than that of gastroduodenopathy and that the serious complications are similar.25 Ulcerations in the small bowel were detected in autopsies of 8.4% of the subjects that took NSAIDs, compared with 0.6% of the subjects that never took them.26 In another study, from 55 to 75% of the patients treated continuously with NSAIDs presented with damage in the intestinal mucosa.27
7.- Risk factors for NSAID-induced gastroduodenal complications are: a) a history of peptic ulcer (complicated or uncomplicated), b) age above 65 years, c) high doses of NSAIDs, d) concomitant use of medications, e) Helicobacter pylori (H. pylori) infection, and f) the presence of severe comorbidities.
Agreement reached: 90% in total agreement, 10% in partial agreement.
The most important risk factors are age and a previous history of peptic ulcer (Table 2). NSAIDs are more frequently used in older patients, and the risk in patients above 70 years of age is similar to that of patients with a history of peptic ulcer. As age increases, risk increases about 4% annually, probably due to the presence of other associated risk factors.19 The role of H. pylori infection and the potential benefit of its eradication in patients that take nsNSAIDs has been controversial.9,19 A meta-analysis of case-control studies demonstrated synergism between H. pylori infection and NSAID use for the development of complicated and uncomplicated ulcers (statement 31).28 ASA, steroids, antithrombotics, and more recently, selective serotonin reuptake inhibitors (SSRIs), as mentioned in statement 4, are among the medications associated with an increased risk of gastrointestinal toxicity from NSAID intake.29 Severe comorbidities (e.g. neoplasias, severe heart diseases, kidney failure, etc.) are also considered risk factors.30
Risk factors for gastrointestinal complications associated with NSAID intake.
| Gastroduodenopathy risk factor | Relative risk |
|---|---|
| Age ≥ 65 years | 4.7 |
| NSAID at high doses | 8.0 |
| History of peptic ulcer | 13.5 |
| Use of two or more NSAIDs | 4.1 |
| Concomitant therapy | |
| Anticoagulants | 12.7 |
| Serotonin reuptake inhibitors | 6.33 |
| Antiplatelet drugs | 3.66 |
| Corticosteroids | 4.4 |
| Severe comorbidities | |
| Cardiovascular disease | 1.8 |
| Kidney disease | 1.27 |
| Helicobacter pylori infection | 3.5 |
| Helicobacter pylori infection + NSAID | 20.8 |
| Type of NSAID | |
| Aceclofenac, ibuprofen, celecoxib | <2 |
| Rofecoxib, meloxicam, nimesulide, sulindac, diclofenac, ketoprofen | 2 – 4 |
| Tenoxicam, naproxen, indomethacin, diflunisal | 4 – 5 |
| Piroxicam, azapropazone, ketorolac | > 5 |
| Enteropathy risk factor | |
| Age ≥ 65 years | 4.16 |
| NSAID + H2 receptor antagonist | 3.95 |
| NSAID + PPI | 5.22 |
| COXIB + PPI | 2.7 |
Source: Modified from Lanza et al.103.
8.- Concomitant intake of nsNSAIDs and selective COX-2 inhibitors can predispose to the development of gastrointestinal complications.
Agreement reached: 75% in total agreement, 20% in partial agreement, 5% uncertain.
The simultaneous use of 2 or more different NSAIDs (selective or nonselective) is not recommended because that strategy does not increase their analgesic, anti-inflammatory, or antipyretic efficacy, but does increase the risk for gastrointestinal toxicity and its complications.20 Evidence from animal studies demonstrates a cytoprotective role of prostanoids in the gastrointestinal tract, derived from the two COX isoenzymes. Prostanoids derived from COX-1 (basically prostaglandin-E2) participate as a defense mechanism that acts on physiologic conditions. In contrast, endogenous prostaglandin-E2 derived from COX-2 plays an important role in the healing of ulcers and the repair of the gastroduodenal mucosa. Thus, the inhibition of either of the two COX isoenzymes predisposes to the development of lesions in the gastrointestinal tract.31
9.- The concomitant use of ASA with nsNSAIDs or selective COX-2 inhibitors, even at low doses, increases the risk for gastrointestinal symptoms and complications.
Agreement reached: 90% in total agreement, 10% in partial agreement.
Many patients that take NSAIDs also require antithrombotic prophylaxis with ASA. ASA, even at low doses, increases the risk for gastrointestinal bleeding by 2.5 times, and increases the risk for upper gastrointestinal complications when combined with an nsNSAID or selective COX-2 inhibitor.
Aspirin alone, at low doses, increases the risk for upper gastrointestinal bleeding (UGIB) by approximately 2 times. When combined with an nsNSAID, that risk increases 2 to 4 times, compared with low doses of ASA.32 The combination of low doses of ASA with selective COX-2 inhibitors is associated with a numerical, but not significant, decrease in bleeding, compared with the combination with nsNSAIDs. On the other hand, hospital admissions for UGIB are significantly fewer in ASA users that are also taking selective COX-2 inhibitors, compared with those that are taking nsNSAIDs.33 When a selective COX-2 inhibitor is co-administered with aspirin, the conversion of arachidonic acid to 15 (r) hydroxyepitetraenoic acid (and, in turn, the production of gastroprotective lipoxin, 15 [r]-EPI-lipoxin A4) is blocked, resulting in gastric damage that is more severe than that seen with aspirin alone, or combined with a selective COX-2 inhibitor.14 In clinical practice, selective COX-2 inhibitor use reduces, but does not eliminate, the risk for gastroduodenal lesions. However, that potential benefit is lost, when combined with ASA, even at low doses.34
There is a lower risk for UGIB with the use of selective COX-2 inhibitors than with nsNSAIDs, but when they are combined with low doses of ASA, the differences between nsNSAIDs and selective COX-2 inhibitors tend to disappear.34,35 Treatment with antiplatelet agents (ASA or others) involves a similar risk for causing UGIB. Lanas et al.35 found that NSAID use increased the risk for UGIB (RR = 5.3), as did treatment with rofecoxib (RR = 2.1), but the use of celecoxib, paracetamol, or the concomitant use of a PPI with an NSAID, did not present a greater risk. Antiplatelet treatment with clopidogrel or ticlopidine had a risk for UGIB (RR = 2.8) similar to that of ASA at a cardio-protective dose (100 mg/day) (RR = 2.7) or to that of anticoagulants (RR = 2.8). Nevertheless, an obvious interaction was found between the use of low-dose ASA with NSAIDs, selective COX-2 inhibitors, or thienopyridines, and an even higher risk for UGIB.35
10.- The risk for upper gastrointestinal bleeding (UGIB) increases with the prescription of clopidogrel plus ASA, compared with ASA alone.
Agreement reached: 85% in total agreement, 5% in partial agreement, 10% uncertain.
The risk for gastrointestinal bleeding with the use of ASA increases even more with the concomitant prescription of clopidogrel. Clopidogrel is often perceived to be relatively safe in terms of gastrointestinal adverse events, but data show that, even as monotherapy, clopidogrel is associated with a high risk for rebleeding in patients with a previous history of bleeding due to peptic ulcer. When clopidogrel is combined with ASA, the risk for bleeding increases even more.16
Three large controlled clinical trials have evaluated the combination of clopidogrel and ASA, compared with ASA alone: CURE,36 COMMIT,37 and CHARISMA.38 The first analysis found a significantly higher risk for bleeds over a 12-month period, whereas the CHARISMA study38 reported a higher risk for moderate bleeding and an increase, albeit not significant, in the risk for severe bleeding over a period of more than 28 months with the combination, compared with ASA alone, but the mean treatment duration was only 15 days. Thus, for patients at high risk for gastrointestinal bleeding, the recommendation is to avoid the combination of clopidogrel and ASA.
11.- H. pylori eradication prevents the recurrence of peptic ulcer in NSAID users.
Agreement reached: 75% in total agreement, 20% in partial agreement, 5% in partial disagreement.
The role that H. pylori infection plays in the development of peptic ulcer is clear, and according to the Mexican consensus on the diagnosis and treatment of H. pylori in Mexico and numerous meta-analyses, the eradication of that bacterium is known to significantly reduce the risk for ulcers (statement 31).28,39 Nevertheless, several clinical trials have shown that the concomitant treatment of NSAIDs with PPIs can be superior to preventive eradication of that microorganism, in relation to peptic ulcer prevention (primary and secondary).32 Thus, the decision to prescribe eradication treatment depends on the local prevalence of the infection and other particular risk factors that each individual can present with, as described in the Mexican consensus on the diagnosis and treatment of H. pylori infection in Mexico.39
12.- The risk for upper gastrointestinal bleeding increases with the concomitant use of NSAIDs and selective serotonin reuptake inhibitors.
Agreement reached: 80% in total agreement, 20% in partial agreement.
There is recent concern about the use of SSRIs, given that studies have shown they can be associated with a higher risk for UGIB, and that risk can increase even more if they are used with an NSAID. Published reports, from a relatively small number of studies, have stated there is a substantial risk for gastrointestinal bleeding with SSRI use. However, more recent studies have produced varying results.
A more recent meta-analysis aimed to provide a more accurate estimate of the risk for UGIB with SSRIs, with or without concurrent NSAID use. The authors analyzed 15 case-control studies (including 393,268 participants) and 4 cohort studies.40 There was a greater risk for UGIB with SSRI medications in the 2 types of studies, with odds ratios (ORs) that varied from 1.66 to 1.68. The number necessary to harm (for UGIB) with SSRI use in a low-risk population was 3.177 and in a high-risk population was 8.81. The risk for UGIB increased even more, with the concomitant use of SSRIs and NSAIDs (OR = 4.25, 95% CI = 2.82-6.42). Those authors concluded that SSRIs were associated with a modest increase in the risk for UGIB, which was lower than that previously estimated.
Therefore, the participants in the present consensus consider that caution must be employed when prescribing the combination of those 2 types of medications.
Clinical manifestations and diagnosis13.- Symptoms and gastroduodenal damage induced by NSAIDs are broad and varied.
Agreement reached: 85% in total agreement, 15% in partial agreement.
The damage caused to the digestive tract by NSAIDs, regardless of their administration route (topical or systemic), is broad, varying from the presence of dyspeptic symptoms, heartburn, and nausea, to the presence of complicated or uncomplicated peptic ulcers.24,41 Importantly, the presence and intensity of those symptoms do not predict the presence of mucosal lesions.24 For example, 50% of NSAID users have symptoms and no mucosal lesion, and 50% of patients with complicated peptic ulcer have reported no previous symptoms.30 On the other hand, in the United States, up to 25% of NSAID users develop peptic ulcer and 2-4% present with the complications of perforation or bleeding.42 The development of those symptoms is not exclusive to nsNSAIDs, given that they have also been demonstrated with selective COX-2 inhibitor use, albeit in smaller numbers.43
Importantly, the period of greater risk for developing symptoms and/or complications is apparently in the first month of use (OR = 5.7).44 Other studies have shown the first 2 months of use to be the period of greater risk for complications, but the risk is maintained over the period of time the drugs are taken.35,45 Approximately 47/100,000 NSAID users are estimated to require hospitalization due to adverse upper gastrointestinal tract events, with a mortality rate associated with severe gastrointestinal complications of 5.57%.46,47
14.- The course of NSAID-induced enteropathy is frequently asymptomatic but can also manifest as iron deficiency anemia, bleeding, stricture, and perforation.
Agreement reached: 100% in total agreement.
There are increasingly more reports of harmful effects of NSAIDs on the lower digestive tract that lead to significant morbidity and mortality.48 The adverse events include increased mucosal permeability, inflammation of the mucosa, protein loss, malabsorption, bleeding or occult bleeding, diarrhea, ulcers, stricture, and perforation.49 Recognition of the toxic effects of NSAIDs on the lower gastrointestinal tract has increased with the advent of new technologies for evaluating intestinal integrity, especially with capsule endoscopy and enteroscopy.50 The damage caused by NSAIDs in not transitory, nor does it decrease over time. For example, with continuous use, at 3 months of NSAID consumption, 71% of users had mucosal lesions and up to 80% had them at one year.51,52 Through capsule endoscopy, Endo et al.53 showed that inflammation of the intestinal mucosa could be caused even with low doses of aspirin. Capsule endoscopy and enteroscopy also showed the presence of erosions, ulcers, and strictures in chronic NSAID users.54
15.- NSAID and ASA use can be associated with complications of diverticular disease of the colon.
Agreement reached: 75% in total agreement, 20% in partial agreement, 5% uncertain.
Evidence, albeit scarce, suggests an increased risk for developing complications related to diverticular disease of the colon. In a systematic review by Laine et al.,55 in which 7 studies with different methodologies were analyzed, 5 of them showed a significant increase in the risk for complication of diverticular disease in chronic NSAID users. In an observational case-control study, Taki et al.56 reported that bilateral diverticular disease (OR = 3.00), nsNSAID use (OR = 3.47), low doses of aspirin (OR = 2.23), and anticoagulants (OR = 3.09) were independent risk factors for diverticular bleeding.
16.- The effect of NSAID intake on inflammatory bowel disease is still uncertain.
Agreement reached: 100% in total agreement.
Some studies have pointed out that nsNSAID and selective COX-2 inhibitor use can be a risk factor for the exacerbation of inflammatory bowel disease, specifically in Crohn’s disease and ulcerative colitis,57,58 but other studies have not found such an association.59,60 Recently, in the systematic review and meta-analysis by Moninuola et al.,61 evaluating the risk for inflammatory bowel disease exacerbation with the use of acetaminophen and NSAIDs, no consistent association was found between the use of acetaminophen, nsNSAIDs, or selective COX-2 inhibitors and disease exacerbation.
17.- Capsule endoscopy and enteroscopy are diagnostic methods that directly evaluate NSAID-induced enteropathy.
Agreement reached: 90% in total agreement, 5% uncertain, 5 in partial disagreement.
Quality of evidence and strength of recommendation: B1, strong, in favor of.
Since capsule endoscopy and enteroscopy have become available for the direct evaluation of the small bowel, the detection of lesions in that segment of the digestive system has been on the rise.62,63 It is known that 50-70% of patients that are long-term NSAID users will develop damage in the small bowel.51
Currently, both diagnostic methods are available, and even though the capacity to detect small bowel lesions is not perfect, enteroscopy is superior to capsule endoscopy in detecting large lesions, such as polyps, whereas capsule endoscopy is superior in detecting small lesions, such as erosions and red spots.62 Although there is no validated terminology for reporting the findings of NSAID-induced enteropathy, Hayashi et al.64 propose the following criteria for diagnosing NSAID-induced enteropathy through double balloon enteroscopy:
- -
A history of NSAID use.
- -
Endoscopic findings: erosion, ulcer, and diaphragm-like stricture.
- -
Improvement of clinical findings (signs and symptoms) or endoscopic findings upon NSAID suspension, with the exception of diaphragm-like stricture.
- -
Exclusion of other causes (tumor, inflammatory bowel disease, infectious disease).
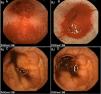
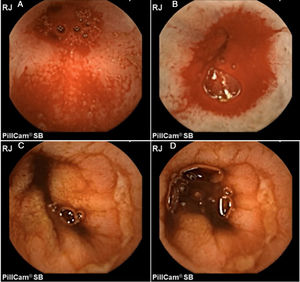
In addition, Maiden et al.65 classified endoscopic findings of NSAID-induced enteropathy into 5 categories (Fig. 1), utilizing capsule endoscopy:
- -
Erythematous folds.
- -
Areas of denuded mucosa.
- -
Red spots.
- -
Disruption of the mucosa.
- -
Bleeding.
Enteroscopy provides direct viewing and better lesion characterization. In addition, biopsies can be taken for additional histopathologic study.42 Unlike capsule endoscopy, it also has therapeutic advantages, given that endoscopic dilations can be performed in cases of diaphragm-like strictures.66
18.- The performance of gastroduodenoscopy, colonoscopy, or both studies, is justified in NSAID users, in the presence of risk factors or alarm symptoms.
Agreement reached: 75% in total agreement, 25% in partial agreement.
Quality of evidence and strength of recommendation: A1, strong, in favor of.
The presence of symptoms is common in NSAID users and their onset appears to vary, depending on the NSAID utilized, as described in statement 13. Even though around 1-2% of NSAID users will develop a serious complication during treatment, it is very important to underline that up to 50% of the patients that present with a severe complication reported no previous symptoms. Therefore, preventive measures should be implemented, based on the presence of risk factors.24
Endoscopy is basically used in clinical evaluations to determine acute or chronic damage caused by NSAID toxicity in the digestive tract. However, none of the present consensuses or guidelines recommends its routine use. The management of patients that are going to receive treatment with NSAIDs must always be preceded by the correct evaluation of the individual gastrointestinal risks of each patient.
For example, the Mexican consensus on dyspepsia suggests performing endoscopy on all patients with uninvestigated dyspepsia that present with alarm symptoms or initial treatment failure directed at the predominant symptom.67 In addition, the American Society for Gastrointestinal Endoscopy (ASGE) states that endoscopic examination is justified when there are alarm symptoms (symptoms of dyspepsia in patients above 50 years of age, weight loss, gastrointestinal bleeding, iron deficiency anemia, obstructive symptoms, a family history of cancer, or imaging studies suggestive of organic disease).68Table 2 shows the main risk factors that justify the performance of endoscopic studies in the context of NSAID-induced enteropathy.
Table 3 shows the stratification of patients into 3 different groups, based on the presence or absence of risk factors.47 For example, in a study conducted by the National Health System in Spain, there was a mortality rate of 15.3 for every 100,000 NSAID-ASA users. Approximately 50% of the patients in that study that died had one or more of the following risk factors: peptic ulcer (22.6%), gastrointestinal bleeding (15.3%), dyspepsia (13.3%), heart disease (65.1%), and high blood pressure (40%).30
Risk categories for the development of NSAID-induced gastroduodenopathy.
| Risk | Events for every 100 patients per year | |
|---|---|---|
| Low | No risk factors and no low-dose ASA intake | < 1.5 |
| Moderate | No history of peptic ulcer, no anticoagulation + 1-2 risk factors | 1.5 a 10 |
| High | A history of peptic ulcer, anticoagulants or > 2 risk factors | > 10 |
Source: Adapted from Lanas et al.47.
Importantly, not all patients with acute or chronic NSAID use require gastroprotection. Therefore, it is necessary to evaluate each patient individually and make the corresponding decision based on that risk.30
19- Capsule endoscopy in NSAID users is used for the evaluation of patients with anemia or gastrointestinal bleeding, when esophagogastroduodenoscopy and colonoscopy have not identified the cause.
Agreement reached: 80% in total agreement, 20% in partial agreement.
Quality of evidence and strength of recommendation: B2, weak, in favor of.
Five to ten percent of all gastrointestinal bleeding originates in the small bowel.69,70 With the advent of capsule endoscopy (2001) and enteroscopy (2004), it is now possible to detect up to 75% of the causes of bleeding that arise in the small bowel.71 The current guidelines of the American College of Gastroenterology suggest performing a second look, utilizing gastroduodenoscopy and colonoscopy in patients with recurrent hematemesis, melena, a previously incomplete endoscopic examination, recurrent hematochezia, or suspicion of distal origin. If the origin of the bleeding is not found, small bowel bleeding should be considered. Therefore, the performance of capsule endoscopy as a first option for evaluating those patients is recommended.72
20.- Enteroscopy in NSAID users is indicated for small bowel evaluation, biopsy, and lesion dilation.
Agreement reached: 100% in total agreement.
Quality of evidence and strength of recommendation: B2, weak, in favor of.
In addition to offering the possibility to directly observe the mucosal surface, characterize findings, and take biopsies, enteroscopy can be therapeutic. For example, it provides the option of hemostatic treatment and the performance of dilations, specifically when there is diaphragm-like stricture (concentric projections of submucosal fibrosis that can cause nonspecific or obstructive symptoms), which is an uncommon but pathognomonic finding of NSAID-induced enteropathy.63,64 The therapeutic success of the endoscopic dilation of those lesions, when indicated, is 80%.73 A systematic review on endoscopic dilation of small bowel stricture showed that said intervention makes surgery unnecessary in 4 out of every 5 patients, with a complication rate of 4.8% per patient and 2.6% per dilation.74
21.- Intestinal damage biomarkers can be useful in evaluating NSAID-induced enteropathy.
Agreement reached: 80% in total agreement, 20% in partial agreement.
Quality of evidence and strength of recommendation: C1, strong, in favor of.
As described in statement 2, both the increase in permeability and inflammation of the mucosa are pathophysiologic mechanisms associated with NSAID use. In that sense, biomarkers that indirectly evaluate intestinal permeability, such as calprotectin, fecal lactoferrin, the urinary excretion of chromium-51 EDTA (51Cr-EDTA), Indium-111 (In-111)-labeled leukocyte scintigraphy, and radiolabeled erythrocyte scintigraphy can potentially be applicable in clinical practice.23 The tests for measuring intestinal permeability are based on the detection of orally administered compounds that are eliminated by urine. Under normal conditions, those compounds should be incapable of being absorbed, but when there is damage to the barrier or intestinal damage, they enter the circulatory system, to then be eliminated.75 The 51Cr-EDTA test is one of the most widely employed, through which 50-70% of long-term NSAID users have been reported to develop an increase in intestinal permeability.76 Many of those tests are promising but more studies are needed to determine the role of each one.77
Prevention and treatment22.- NSAID prescription should always be preceded by an integral evaluation of the gastrointestinal and cardiovascular risks.
Agreement reached: 100% in total agreement.
Quality of evidence and strength of recommendation: A1, strong, in favor of.
Before beginning therapy with an NSAID, the real necessity of it should be carefully evaluated and the cardiovascular and gastrointestinal risks calculated for each individual. Risk factors are those established in statement 18 and Table 1. Likewise, as described in statement 18 (Table 2), with the presence or absence of those factors, 3 different risk groups are established. The gastrointestinal prevention strategy to be adopted will depend on the group in which the patient is included.
23.- NSAID prescription should be avoided, or prescribed together with a PPI, in subjects that have gastrointestinal risk factors.
Agreement reached: 80% in total agreement, 20% in partial agreement.
Quality of evidence and strength of recommendation: B1, strong, in favor of.
If NSAID use cannot be avoided, there is evidence recommending its concomitant prescription with PPIs for prophylactic purposes. For example, the results of 2 identical, randomized placebo-controlled clinical trials by Scheiman et al.,78 the VENUS study (the United States, n = 844) and the PLUTO study (international, n = 585), were published. Both trials included high-risk patients with no active ulcer (≥ 65 years of age or a history of peptic ulcer disease within the last 5 years) that continued under chronic treatment with nsNSAIDs or selective COX-2 inhibitors. Esomeprazole (20 mg or 40 mg administered once a day) was compared with placebo, with respect to the rate of ulcer development at 6 months. The remission rates at the end of the study period were 79.6% for placebo, 94.7% for esomeprazole 20 mg/day, and 95.3% for esomeprazole 40 mg/day (p = 0.001 for both, compared with placebo) in the VENUS study and 87.7% for placebo, 94.8% for esomeprazole 20 mg/day (p = 0.018), and 95.6% for esomeprazole 40 mg/day (p = 0.007) in the PLUTO study. In the grouped analysis that included only COX-2 inhibitors, the remission rates were 83.5% with placebo, 99.1% with esomeprazole 20 mg/day (p = 0.001), and 95.9% with esomeprazole 40 mg/day (p = 0.002). Both trials showed the effectiveness of a PPI in the prevention of gastrointestinal damage in long-term nsNSAID and selective COX-2 inhibitor users in a high-risk population.
24.- Prescribing the minimum dose of an NSAID for the shortest period of time possible to achieve the desired effect is associated with a lower risk for gastrointestinal adverse effects.
Agreement reached: 90% in total agreement, 10% in partial agreement.
Quality of evidence and strength of recommendation: C1, strong, in favor of.
The decision to prescribe an nsNSAID or selective COX-2 inhibitor should be balanced and influenced by possible cardiovascular and gastrointestinal events. Nevertheless, the comparative anti-inflammatory and analgesic efficacy is also important. The comparative effectiveness has been shown in several controlled clinical trials (CCTs). For example, one CCT reported that the efficacy of etoricoxib was similar to that of diclofenac in patients with osteoarthritis.79 Another CCT showed similar efficacy between celecoxib and naproxen.80 In the CONDOR study, analyzing celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis, there was no difference in effectiveness between celecoxib and diclofenac in patients with osteoarthritis.81 A meta-analysis of CCTs in patients with rheumatologic diseases showed no increased cardiovascular risk associated with celecoxib, compared with placebo,82 whereas a safety analysis of 6 CCTs conducted on patients with diseases different from arthritis provided evidence of a cardiovascular risk that was dependent on the dose of celecoxib, the regimen, and the initial cardiovascular risk.83 The risk did not appear to be significant (RR = 1.1) for the 400 mg/day dose, it was intermediate for the 200 mg/day dose twice a day (RR = 1.8), and high for the 400 mg/day dose twice a day (RR = 3.1).84 In a meta-analysis on selective COX-2 inhibitors and nsNSAIDs, there was a trend towards a lower risk with lower doses of celecoxib.84
However, the cost of treatment is also an important factor. After the results of the CONDOR study were published, the economic model from the clinical guidelines of the National Institute for Care Excellence (NICE) of the United Kingdom was updated in 2012 to include the relative risks of adverse events related to the lower digestive tract.85
25.- PPIs are safe and efficacious drugs in the prevention of gastroduodenal complications associated with chronic NSAID use.
Agreement reached: 90% in total agreement, 10% in partial agreement.
Quality of evidence and strength of recommendation: A1, strong, in favor of.
PPIs, such as omeprazole, pantoprazole, lansoprazole, esomeprazole, dexlansoprazole, etc., are effective and well-tolerated drugs that act by inhibiting gastric H/K-adenosine triphosphatase by covalent bonding to the cysteines of the proton pump, thus reducing gastric acid secretion. In a systematic review with a meta-analysis of 31 clinical trials that included 12,532 participants, conducted to evaluate PPI safety and efficacy, PPIs were significantly more effective than placebo in reducing the complications from ulcers (RR = 0.29) and endoscopic peptic ulcers (RR = 0.27).86 When a subgroup analysis was carried out, there were no differences according to the class of NSAID, risk for ulcer, history of ulcer disease, H. pylori infection, or age. The number needed to treat and prevent a complication of ulcer, was 10 high-risk patients and 268 moderate-risk patients.86 A network meta-analysis indicated that the effectiveness of the different PPIs in reducing the complications of ulcers and endoscopic peptic ulcers was similar. PPIs significantly reduced the adverse gastrointestinal events and related treatment withdrawals, compared with placebo. There were no differences in safety among the different PPIs.86
26.- PPIs at standard doses are more recommendable than H2 receptor antagonists (H2RAs) and misoprostol for reducing the risk for peptic ulcer and its complications in patients with long-term NSAID use.
Agreement reached: 75% in total agreement, 25% in partial agreement.
Quality of evidence and strength of recommendation: A1, strong, in favor of.
PPIs have become the cornerstone for gastroprotection, compared with other therapies that inhibit gastric acid secretion. For example, a pivot study compared omeprazole 20 mg/day with ranitidine 150 mg twice a day for 12 weeks, in 425 NSAID-using patients, as prophylaxis for gastric ulcer and duodenal ulcer. Omeprazole was superior to ranitidine in preventing both gastric ulcer (RR = 0.32) and duodenal ulcer (RR = 0.11).87 Four studies that included a total of 1,478 patients compared a PPI with misoprostol 400-800 μg) per day and found that PPIs were superior to misoprostol for the prevention of duodenal ulcer (RR = 0.25), but not of gastric ulcer (RR = 1.61, random effects) or the total of ulcers (RR = 0.90).88–91
Two trials that included 600 patients compared misoprostol (400 to 800 μg) with 150 mg of ranitidine twice a day.92,93 Misoprostol was superior to standard-dose ranitidine for the prevention of gastric ulcers induced by traditional NSAIDs (RR = 0.12) but not for duodenal ulcers (RR = 1.00). In a meta-analysis of patients that received misoprostol plus an NSAID versus placebo, the incidence of gastric ulcers decreased by 74% and duodenal ulcers by 58%.94 The main limiting factor impeding the generalized use of misoprostol as a protective agent is the elevated frequency of side effects, such as diarrhea, abdominal cramps, and nausea in up to 20% of its users, which limits patient compliance.
27.- In patients receiving NSAID therapy with no antiplatelet drugs, PPI prescription is recommended only when there is a risk for gastrointestinal complications.
Agreement reached: 80% in total agreement, 15% in partial agreement, 5% uncertain.
Quality of evidence and strength of recommendation: B1, strong, in favor of.
To evaluate the comparative effectiveness of the different clinical strategies for preventing NSAID-induced gastrointestinal toxicity, Chinese researchers conducted a pairwise and Bayesian network meta-analysis that included 82 CCTs. They compared the risk for gastrointestinal adverse events in nsNSAID or selective COX-2 inhibitor users that received gastroprotection with a PPI, H2RA, or misoprostol.95 For all of the efficacy evaluation criteria, the concomitant prescription of a selective COX-2 inhibitor + PPI was associated with the least absolute probability of an adverse event and the highest safety range, followed by selective COX-2 inhibitors alone, and in third place, nsNSAIDs plus PPIs.95
28.- In patients at high risk for a gastroduodenal event and low risk for a cardiovascular event, the best strategy for preventing NSAID-associated damage is the combination of a selective COX-2 inhibitor and PPI.
Agreement reached: 100% in total agreement.
Quality of evidence and strength of recommendation: C1, strong, in favor of.
As described in statements 8 and 9, the safety profile of selective COX-2 inhibitors is superior to that of nsNSAIDs, in relation to gastrointestinal risk. The CONDOR study,81 and the more recent GI-REASONS study,96 showed that celecoxib exhibited greater safety in the entire gastrointestinal tract, compared with diclofenac plus omeprazole. A recent meta-analysis that included 52 randomized clinical trials demonstrated that celecoxib was associated with a significantly lower risk for all clinically significant gastrointestinal events in the entire digestive tract, compared with nsNSAIDs.97
29.- In patients at high risk for cardiovascular and gastroduodenal events, the combination of naproxen and a proton pump inhibitor is recommended.
Agreement reached: 85% in total agreement, 15% in partial agreement.
Quality of evidence and strength of recommendation: B1, strong, in favor of.
The most recent and extensive meta-analysis of clinical trials with individual patient data points out that both selective COX-2 inhibitors and nsNSAIDs increase cardiovascular risk, compared with placebo, with no significant overall differences between them. Of the nsNSAIDs, diclofenac was the one with greater cardiovascular risk, and it was similar to that of the selective COX-2 inhibitors. Naproxen, at a dose of 500 mg every 12 h, was not associated with an increased cardiovascular risk, unlike ibuprofen and diclofenac, the most widely studied nsNSAIDs.95 A particular case is that of patients that take ASA. It must be kept in mind that NSAIDs, such as naproxen, and especially ibuprofen, interfere with the antiplatelet activity of ASA.98 Given naproxen’s good cardiovascular safety profile, its administration 2 h after ASA intake should minimize that risk, making it a good option in those patients.20
30.- PPIs are not useful for preventing NSAID-induced enteropathy and can even be harmful.
Agreement reached: 85% in total agreement, 10% in partial agreement. 5% in partial disagreement.
Quality of evidence and strength of recommendation: C2, weak, against the intervention.
In contrast to the stomach and duodenum, there is no evidence that gastric acid plays a role in the pathogenesis of NSAID-induced gastrointestinal damage distal to the ligament of Treitz.33 Chronic suppression of gastric acid secretion (with PPIs or H2RAs) causes small intestinal bacterial overgrowth, which can increase the severity of NSAID-induced enteropathy. Studies on rodents suggest that PPIs actually exacerbate NSAID-induced enteropathy, instead of providing a beneficial effect.99 In that study, the rats treated with a PPI (omeprazole or lansoprazole) developed substantially more ulcers and intestinal bleeding when simultaneously treated with an NSAID (naproxen or celecoxib) than the group treated with placebo plus the NSAID. A series of studies with capsule endoscopy showed a high incidence (55-75%) of damage in the small bowel in healthy volunteers that took an NSAID plus a PPI for 2 weeks.51,100–102 Importantly, that high incidence of intestinal damage was observed in a group considered to be at low risk for NSAID-induced gastrointestinal damage that underwent short-term treatment with the co-administration of a gastroprotective medication (PPI). In a cross-sectional study utilizing capsule endoscopy performed on patients with rheumatoid arthritis, treated with an NSAID for more than 3 months, the advanced age patients and acid suppression users (H2RAs and PPIs) had more probability of developing severe enteropathy.50
31.- Before beginning long-term treatment with an NSAID, Helicobacter pylori infection should be evaluated and treated.
Agreement reached: 90% in total agreement, 10% in partial agreement.
Quality of evidence and strength of recommendation: C1, strong, in favor of the intervention.
The detection of H. pylori infection for the prevention of ulcers in asymptomatic patients should be evaluated individually and its routine performance is not recommended. Nevertheless, the study and eradication of H. pylori is recommended in patients with a history of peptic ulcer disease, before beginning treatment with low doses of aspirin or an NSAID.39H. pylori infection is an independent risk factor for developing ulcers and bleeding due to ulcers in NSAID users.103,104 Eradicating H. pylori infection before beginning treatment with NSAIDs reduces the risk for developing peptic ulcers and bleeding due to ulcers.105,106 A meta-analysis of 5 CCTs suggested that the eradication of H. pylori infection in patients that take NSAIDs was associated with a 57% decrease in the incidence of peptic ulcer (OR = 0.43; 95% CI: 0.20-0.93).28 With respect to chronic NSAID users, the evidence suggests that H. pylori eradication per se does not reduce the incidence of peptic ulcers and that therapy with a PPI is a strategy that provides a more efficacious effect on reducing the risk for ulcer than H. pylori eradication.107,108
32.- There are other alternatives for the prophylaxis and treatment of NSAID-induced gastroduodenopathy and enteropathy that have limited usefulness and availability.
Agreement reached: 85% in total agreement, 15% in partial agreement.
Quality of evidence and strength of recommendation: C1, strong, in favor of the intervention.
Misoprostol, metronidazole, and sulfasalazine have been suggested to be beneficial in the treatment or prevention of NSAID-induced enteropathy in humans. Nevertheless, the majority of studies have significant limitations, such as being open, non-controlled studies, with small sample sizes. The observations in animal studies that NSAID-induced enteropathy was accompanied with dramatic changes in the number and type of gut bacteria led to a series of studies on the potential value of probiotics for the treatment or prevention of NSAID-induced enteropathy. The Shirota strain of Lactobacillus casei has been reported to protect against small bowel lesions induced by indomethacin in rats and that its probiotic effects might be mediated by the anti-inflammatory effects of lactic acid, but those findings have not been shown in humans.109 More recently, other authors demonstrated that the treatment with a mixture of probiotics (VSL #3) that contains L. casei significantly reduced fecal calprotectin concentrations in healthy volunteers that received indomethacin.110 Rebamipide is an anti-ulcer cytoprotecting agent that stimulates endogenous prostaglandin production and has been utilized in several Asian countries for the treatment of gastric ulcers and gastric lesions, such as erosions and edema associated with acute gastritis.111–113 There is good evidence that rebamipide increases endogenous levels of prostaglandins, increases blood flow, suppresses the increases in permeability, eliminates free radicals, and suppresses inflammation in the gastric mucosa.114–116 Through those actions, rebamipide has also been shown to be useful in the prevention of aspirin-induced gastrointestinal lesions. It has even been described to have a preventive effect on small bowel erosions of the mucosa in the ileum induced by aspirin, compared with placebo.117 In a randomized controlled trial conducted on patients receiving low doses of aspirin or an NSAID for more than 3 months, rebamipide was efficacious in the cure of erosions and ulcers of the small bowel.118
33.- There is currently no useful treatment for NSAID-induced enteropathy and the recommendation is to suspend the drug, if possible.
Agreement reached: 100% in total agreement.
Quality of evidence and strength of recommendation: C1, strong, in favor of the intervention.
At present there are no therapies that have proven to be efficacious in treating NSAID-induced enteropathy, and detection of the disease continues to be a challenge, especially due to the scant correlation between tissue lesion and symptoms. In addition, recent studies indicate that drugs commonly used to protect the upper gastrointestinal tract (i.e., PPIs) can significantly worsen NSAID-induced damage in the small bowel. The evaluation of risk for enteropathy has been hindered by the lack of knowledge about its corresponding risk factors, unlike those related to complications of the upper gastrointestinal tract. The data analysis of the MEDAL program (i.e., the MEDAL, EDGE-I, and EDGE-II studies) showed that the risk for a clinical event in the lower digestive tract from NSAID use appears to be constant over time and the main risk factors are a previous lower gastrointestinal event and advanced age.119 In a post hoc analysis of the CONDOR trial,81 baseline levels of C-reactive protein, a history of gastritis and gastrointestinal intolerance, H. pylori infection, advanced age, and body mass index were associated with clinically significant blood loss in patients with osteoarthritis that were treated with NSAIDs.
ConclusionsIt is essential for all prescribing physicians to carry out an individualized evaluation of the cardiovascular and gastrointestinal risk profile of each patient receiving treatment with NSAIDs. Once the risk profile is determined, the most adequate therapeutic option for each case must be searched for. Treatment with NSAIDs should be avoided in high-risk patients (those with a history of complicated peptic ulcer or anti-thrombotic therapy). H. pylori should be eradicated in infected patients with a history of ulcer, and selective COX-2 inhibitors plus PPIs prescribed. Naproxen is the ideal NSAID for patients with a high cardiovascular risk, with the addition of PPIs if there is associated gastrointestinal risk. Regarding NSAID-induced enteropathy, it is increasingly more frequent, and although there is greater diagnostic capacity, prophylaxis and treatment are still limited.
Financial disclosureThe present consensus was carried out with the support of the Asociación Mexicana de Gastroenterología, which enabled the participation, transportation, and lodging during the face-to-face voting. No professional fees were received.
Conflicts of interestDr. María Victoria Bielsa-Fernández declares she has no conflict of interest.
Dr. José Luis Tamayo de la Cuesta has been a speaker for and advisory board member of Takeda and Chinoin.
Dr. Jesús Lizárraga López declares he has no conflict of interest.
Dr. José María Remes-Troche is an advisory board member of Takeda and Asofarma. He has received research funds from Sanfer and Asofarma. He is a speaker for Takeda, Asofarma, Alfa-Wassermann, Carnot, Menarini, and Astra-Zeneca.
Dr. Ramón Isaías Carmona-Sánchez is or has been a speaker for Asofarma, Astra-Zeneca, and Chinoin.
Dr. Juan Manuel Aldana Ledesma declares he has no conflict of interest.
Dr. José Manuel Avendaño Reyes declares he has no conflict of interest.
Dr. Mario Arturo Ballesteros Amozorrutia declares he has no conflict of interest.
Dr. Mauricio De Ariño declares he has no conflict of interest.
Dr. Louis de Giau Troulitz declares he has no conflict of interest.
Dr. Ricardo Flores Rendón has been a speaker for Takeda.
Dr. Héctor Huerta Guerrero declares he has no conflict of interest.
Dr. José Alberto González declares he has no conflict of interest.
Dr. Angélica Hernández Guerrero declares she has no conflict of interest.
Dr. Enrique Murcio Pérez declares he has no conflict of interest.
Dr. Joel Jacquez Quintana declares he has no conflict of interest.
Dr. Arturo Meixueiro Daza declares he has no conflict of interest.
Dr. José Ramón Nogueira de Rojas declares he has no conflict of interest.
Dr. Heriberto Rodríguez Hernández declares he has no conflict of interest.
Dr. Ricardo Santoyo Valenzuela declares he has no conflict of interest.
Dr. Sandra Concepción Solorzano Olmos declares she has no conflict of interest.
Dr. Luis F. Uscanga Domínguez is an advisory board member of Asofarma.
Dr. Felipe Zamarripa Dorsey declares he has no conflict of interest.
Please cite this article as: Bielsa-Fernández MV, Tamayo-de la Cuesta JL, Lizárraga-López J, Remes-Troche JM, Carmona-Sánchez R, Aldana-Ledesma JM, et al. Consenso mexicano sobre diagnóstico, prevención y tratamiento de la gastropatía y enteropatía por antiinflamatorios no esteroideos. Revista de Gastroenterología de México. 2020;85:190–206.