Non-cardiac chest pain is defined as a clinical syndrome characterized by retrosternal pain similar to that of angina pectoris, but of non-cardiac origin and produced by esophageal, musculoskeletal, pulmonary, or psychiatric diseases.
AimTo present a consensus review based on evidence regarding the definition, epidemiology, pathophysiology, and diagnosis of non-cardiac chest pain, as well as the therapeutic options for those patients.
MethodsThree general coordinators carried out a literature review of all articles published in English and Spanish on the theme and formulated 38 initial statements, dividing them into 3 main categories: (i)definitions, epidemiology, and pathophysiology; (ii)diagnosis, and (iii)treatment. The statements underwent 3rounds of voting, utilizing the Delphi system. The final statements were those that reached >75% agreement, and they were rated utilizing the GRADE system.
Results and conclusionsThe final consensus included 29 statements. All patients presenting with chest pain should initially be evaluated by a cardiologist. The most common cause of non-cardiac chest pain is gastroesophageal reflux disease. If there are no alarm symptoms, the initial approach should be a therapeutic trial with a proton pump inhibitor for 2-4weeks. If dysphagia or alarm symptoms are present, endoscopy is recommended. High-resolution manometry is the best method for ruling out spastic motor disorders and achalasia and pH monitoring aids in demonstrating abnormal esophageal acid exposure. Treatment should be directed at the pathophysiologic mechanism. It can include proton pump inhibitors, neuromodulators and/or smooth muscle relaxants, psychologic intervention and/or cognitive therapy, and occasionally surgery or endoscopic therapy.
Dolor torácico no cardíaco (DTNC) se define como un síndrome clínico caracterizado por dolor retroesternal semejante a la angina de pecho, pero de origen no cardiaco y generado por enfermedades esofágicas, osteomusculares, pulmonares o psiquiátricas.
ObjetivoPresentar una revisión consensuada basada en evidencias sobre definición, epidemiología, fisiopatología, diagnóstico y opciones terapéuticas para pacientes con DTNC.
MétodosTres coordinadores generales realizaron una revisión bibliográfica de todas las publicaciones en inglés y español sobre el tema y elaboraron 38 enunciados iniciales divididos en tres categorías principales: 1)definiciones, epidemiología y fisiopatología; 2)diagnóstico, y 3)tratamiento. Los enunciados fueron votados (3rondas) utilizando el sistema Delphi, y los que alcanzaron un acuerdo >75% fueron considerados y calificados de acuerdo con el sistema GRADE.
Resultados y conclusionesEl consenso final incluyó 29 enunciados Todo paciente que debuta con dolor torácico debe ser inicialmente evaluado por un cardiólogo. La causa más común de DTNC es la enfermedad por reflujo gastroesofágico (ERGE). Como abordaje inicial, si no existen síntomas de alarma, se puede dar una prueba terapéutica con inhibidor de bomba de protones (IBP) por 2-4semanas. Si hay disfagia o síntomas de alarma, se recomienda hacer una endoscopia. La manometría de alta resolución es el mejor método para descartar trastornos motores espásticos y acalasia. La pHmetría ayuda a demostrar exposición esofágica anormal al ácido. El tratamiento debe ser dirigido al mecanismo fisiopatológico, y puede incluir IBP, neuromoduladores y/o relajantes de músculo liso, intervención psicológica y/o terapia cognitiva, y ocasionalmente cirugía o terapia endoscópica.
Non-cardiac chest pain (NCCP) is a condition whose clinical picture is indistinguishable from that of ischemic heart disease. Even though the condition does not produce an increase in mortality, it is associated with greater use of medical services and reduced quality of life.1 There is much evidence of and numerous guidelines and consensuses on chest pain of cardiovascular origin,2–4 but there are no previous consensuses on NCCP. Several clinical guidelines mention the theme as part of the evaluation of other topics, such as esophageal motility disorders (EMDs), gastroesophageal reflux disease (GERD), and esophageal manometry. However, most of the general information comes from review articles, and the evidence on the usefulness of diagnostic methods and treatments is indirect or has been extrapolated to the causes of NCCP (e.g., GERD, EMD). Evidence on the usefulness of each diagnostic study and treatment for NCCP has gradually begun to emerge. The Asociación Mexicana de Gastroenterología (AMG) summoned a group of experts on the theme to establish recommendations based on an extensive review of the medical literature and to produce a document on the definitions, epidemiology, pathophysiology, diagnosis, and treatment of NCCP that are useful for the medical community.
MethodsThe present consensus was developed utilizing the Delphi process,5 whose main steps were: a) selection of the consensus group, b) identification of the areas of clinical importance, c) systematic review of the literature to identify the evidence supporting the statements, d) formulation of the statements, e) anonymous, electronic voting rounds, discussion and analysis of the results, and correction and modification of the statements.
Three general coordinators of the consensus were designated (MAB, ECA, and OGE) and 17 gastroenterologists that are specialists in gastrointestinal motility and/or neurogastroenterology were invited, along with specialists in the areas related to the theme in question (cardiologists) that agreed to participate in the consensus and the formulation of the present document. The general coordinators carried out a thorough search utilizing the following databases: CENTRAL (the Cochrane Central Register of Controlled Trials), MEDLINE (PubMed), EMBASE (Ovid), LILACS, CINAHL, Bioma Central, and the World Health Organization International Clinical Trials Registry Platform (ICTRP). The time frame of the search was from January 1, 2000 to March 31, 2018, and in PubMed, it went back 20 years to 1980. The search criteria included the following terms: “dolor torácico” (chest pain, thoracic pain), “dolor torácico no cardíaco” (non-cardiac chest pain, noncardiac chest pain), “dolor torácico de origen esofágico” (chest pain of esophageal origin or presumed esophageal origin), combined with the following terms: “epidemiology”, “incidence”, “prevalence”, “pathophysiology”, “pathogenesis”, “evaluation”, “diagnostic tests”, “endoscopy”, “biopsies”, “pH monitoring”, “impedance”, “esophageal manometry”, “high-resolution esophageal manometry”, “differential diagnosis”, “treatment”, “therapy”, “management”, “surgery”, “review”, “guidelines”, “consensus”, “systematic”, “meta-analysis” and their equivalent terms in Spanish. The search included articles in English and Spanish. The complete bibliography was available online to the members of the consensus through Google Drive, so they could consult it at any time during the entire process.
The general coordinators then formulated 38 statements that underwent a first anonymous electronic voting round (May 15 to 22, 2018) to evaluate their composition and content. The consensus participants voted according to the following responses and criteria: a) in complete agreement (signifying complete acceptance of the composition, content, and concept of the statement), b) in partial agreement (signifying acceptance of the statement and agreement with the general concept, but proposing changes in the composition and/or content), c) uncertain (signifying that the content of the statement was insufficient for acceptance), d) in partial disagreement (signifying that the statement could not be accepted, mainly due to discrepancies related to the composition and/or content, but could be accepted after certain modifications), and e) in complete disagreement (signifying that the concept, content, and composition of the statement could not be accepted).
After the first round of voting, the coordinators made the corresponding modifications to each statement, according to the results and comments of the participants. The statements that reached complete agreement > 75% were kept, and those in which complete disagreement was > 75% were eliminated. The statements with < 75% complete agreement and < 75% complete disagreement were revised and restructured, considering the comments of the participants. In addition, each of the new statements was given a strength of recommendation grade and the quality of evidence for sustaining said recommendation was evaluated through the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.6–8 In the GRADE system, the quality of evidence is graded not only on the design or methodology of the study, but is also judged by whether it has a clearly posed question related to a clearly formulated outcome variable. Thus, the quality of evidence can be high, moderate, low, or very low. According to the GRADE scale, “high” means it is very unlikely that further research would change the estimate of effect, “moderate” means further research would probably change the estimate of effect, “low” means further research would very likely change the estimate of effect, and “very low” means any estimate of effect is very uncertain. In addition, the GRADE system establishes the strength of recommendations as “strong” or “weak” and “in favor of” or “against” the intervention or statement. A recommendation is “strong” when it applies to the majority of patients, the majority of the time, and “weak” when it applies only to a subgroup of patients. The characterization “in favor of / against the statement” was applied to definitions, pathophysiology, and descriptions of techniques and “in favor of / against the intervention” was applied to diagnostic tests and treatment. The GRADE system employs a code that utilizes an uppercase letter for the quality of evidence, followed by a number indicating the strength of the recommendation in favor of or against a statement or intervention, as shown in Table 1.
Classification of the evidence and strength of the recommendation according to the GRADE system.
| Quality of evidence: |
| High: unlikely that further research will change the estimate of effect (code A) |
| Moderate: further research will probably change the estimate of effect (code B) |
| Low: very likely that further research will change the estimate of effect (code C) |
| Very low: the estimate of effect is uncertain (code D) |
| Strength of recommendation: |
| Strong: applies to the majority of patients the majority of the time (code 1) |
| Weak: applies only to some patients (code 2) |
| In favor of/against the statement: |
| Definitions, epidemiology, pathophysiology, technical description |
| In favor of/against the intervention: |
| Diagnostic tests, treatment |
The statements that were revised and categorized by the GRADE system underwent a second round of anonymous, electronic voting (June 15 to 22, 2018) and the results were presented on August 29, 2018 at a face-to-face meeting held at the offices of the AMG in Mexico City. At that meeting, the statements with > 75% agreement were ratified. The sentences that did not reach > 75% agreement in the previous rounds of voting were discussed in an effort to either reach an agreement or eliminate them, and the third round of voting was conducted.
Once all the consensus statements were agreed upon, the coordinators formulated the final manuscript, which was reviewed and approved by the members of the consensus group. An internationally-known expert on the theme (SRA) accepted our request to be the technical reviewer of the document for the final manuscript revision.
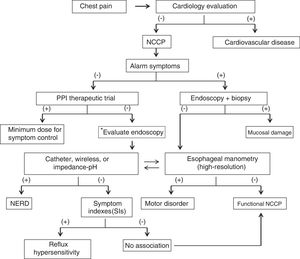
ResultsThe coordinators initially formulated 38 statements. In the first round of electronic voting, 4 of the statements were eliminated because a consensus was not reached and because two statements were fused into one. Thirty-four statements were included in the second round of electronic voting and were ratified for the face-to-face vote. Nineteen of the 20 members of the consensus group (95%) were present for the face-to-face vote. Of the 34 final statements included in the round of voting, 2 were eliminated and 3 were fused together, leaving 29 final statements in the consensus. The final statements and the voting results follow below. Table 2 summarizes the general results of the consensus, together with the recommendations derived from each statement. At the face-to-face meeting, a diagnostic algorithm was designed and agreed upon and is presented in Fig. 1.
Summary of the recommendations in the statements on diagnosis and treatment.
| Theme | Statement | Total agreement percentage | Recommendation |
|---|---|---|---|
| Diagnosis | The clinical characteristics of cardiac chest pain and non-cardiac chest pain are very similar, and so their etiologies are difficult to differentiate. Patients with chest pain should first been evaluated by a cardiologist.Once a cardiovascular cause is ruled out, other causes should be studied, such as musculoskeletal,pulmonary, and gastrointestinal alterations, including esophageal disorders, as well as psychiatric disorders.The esophagram has a poor diagnostic yield for NCCP. It can be used in cases associated with dysphagia.Tests that cause esophageal pain, dysmotility, or hypersensitivity lack availability and are limited to research studies.The double-dose proton pump inhibitor (PPI) therapeutic trial is useful for identifying patients with NCCP secondary to GERD.Endoscopy has a low diagnostic yield for NCCP, and so should be performed in patients with alarm signs and symptoms.Esophageal biopsies in NCCP are useful for making the differential diagnosis with other entities, such aseosinophilic esophagitis (EoE), infections, and when Barrett's esophagus is suspected.24-h pH monitoring with a catheter or wireless capsule and impedance-pH monitoring are the most useful tests for detecting GERD as the cause of NCCP.The symptom association indexes evaluated during ambulatory pH monitoring increase the diagnostic yield of the test for NCCP secondary to GERD.Esophageal manometry (EM) is the most useful test for detecting motor disorders as a cause of NCCP.Psychiatric evaluation is recommended in patients with NCCP that have negative tests and do not respond to a therapeutic intervention. | 90.91%90.91%81.82%90.91%90.91%86.36%90.91%86.36%81.82%77.27%90.91% | Initial evaluation by a cardiologist is recommended for all patients presenting with chest pain (B1 strong, in favor of the intervention)Once a cardiovascular problem is ruled out, the differential diagnosis should be made, beginning with the most common causes (esophageal) (C1 strong, in favor of the intervention)It can be considered for a structural evaluation if there is dysphagia; it should not be used for diagnosing GERD or motor disorders (C1 strong, in favor of the intervention)Useful in selected clinical settings, not recommended for general use (C2 weak, against the intervention)Recommended as the initial test in all patients with no alarm symptoms (A1 strong, in favor of the intervention)Recommended in cases of treatment failure or when there are alarm symptoms (A1 strong, in favor of the intervention)Biopsies should only be taken to histologically confirm the differential diagnosis, not to confirm GERD (B1strong, in favor of the intervention)Recommended for confirming esophageal acid exposure or in the case of impedance, non-acid or refractory reflux (A1 strong, in favor of the intervention)They require at least 3 episodes of each symptom, only GERD-related symptoms, and are the only parameter for differentiating functional pain reflux hypersensitivity (C1 strong, in favor of the intervention)It is recommended for ruling out spastic esophageal motor disorders associated with pain and GERD (B1 strong, in favor of the intervention)Referral to a psychologist or psychiatrist is recommended in cases of suspected psychiatric disorder or when the evaluation is normal (B1 strong, in favor of the intervention) |
| Treatment | Ideally, treatment of NCCP should be directed at the underlying pathophysiologic mechanism (e.g., gastroesophageal reflux, esophageal dysmotility, hypersensitivity, psychiatric comorbidity).Treatment with a PPI is indicated when gastroesophageal reflux disease has been documented as a cause of NCCP.Treatment with a double dose of PPI for at least 2 months is recommended for GERD-related NCCP.Smooth muscle relaxants, such as nitric oxide donors, calcium antagonists, and anticholinergics are used in NCCP associated with spastic motor disorders, but their efficacy is limited, and they are associated withnumerous adverse effects.Transendoscopic injection of botulinum toxin is an alternative for treating NCCP associated with esophageal spastic disorders in patients that are not candidates for myotomy or pneumatic dilation.Peroral endoscopic myotomy (POEM) is a therapeutic option in NCCP for selected patients with spastic disorders of the esophagus.Visceral pain neuromodulators are useful in functional NCCP when there is no satisfactory response to other treatments.Cognitive behavioral therapy, hypnotherapy, biofeedback, and Johrei healing are alternatives in refractory cases or complements to other treatment modalities for NCCP.Surgery as treatment for NCCP is based on myotomy of the affected esophageal segment and limited to spastic disorders of the esophagus. It should be performed by an expert surgeon and in highly selected cases. | 94.45%90.91%95.45%81.82%95.45%90.91%95.45%95.45%90% | Establish a management plan based on the mechanism involved (B1 strong, in favor of the intervention) Regardless of the therapeutic trial, PPI use is recommended as treatment of NCCP when GERD is documented through endoscopy or pH monitoring (A1 strong, in favor of the intervention)A PPI dose of 40mg b.i.d. or its equivalent for at least 8 weeks is recommended, with the same schedule as in typical GERD cases (B1 strong, in favor of the intervention)Useful in the short term for NCCP associated with spastic motor disorders in non-candidates for other treatments, or as bridging therapy before definitive treatment; adjust dose based on response and adverse effects (C2 weak, in favor of the intervention)The same indications as with smooth muscle relaxants: as a temporary measure before definitive therapy or in cases in which more invasive therapy is contraindicated. Avoid multiple administrations if later definitive therapy is considered (B2 strong, in favor of the intervention)Recommended as a definitive measure in spastic motor disorders and only in centers with experience and qualified personnel (B1 strong, in favor of the intervention)Low doses of imipramine, amitriptyline, paroxetine, or sertraline are recommended as a neuromodulator in functional pain (B1 strong, in favor of the intervention)Cognitive behavioral therapy (B1 strong), hypnotherapy (B2 weak), and biofeedback (C2 weak) should be administered by qualified personnel. Alternative medicine (Johrei healing: C2 weak)Extended longitudinal myotomy is recommended after documentation of the extension of the area of spasticity through high-resolution manometry, in nonresponsive cases, and by a qualified surgeon (C2 weak, in favor of the intervention) |
Definitions, epidemiology, and pathophysiology
- 1.
Non-cardiac chest pain is defined as the presence of recurrent retrosternal pain, in which a cardiovascular cause has objectively been ruled out by a cardiologist.
Quality of evidence and strength of the recommendation: C1 strong, in favor of the statement.
Level of agreement: in complete agreement 82%, in partial agreement 14%, uncertain 4%.
Non-cardiac chest pain (NCCP) is characterized by the presence of pain located in the retrosternal area. Its clinical presentation is indistinguishable from pain of cardiac origin, which can lead to numerous studies, partially due to the fact that some patients tend to augment their symptoms, utilizing a more sensorial and affective vocabulary when describing them, than patients with heart disease. Such manners of expression can alert the clinician in the initial evaluation.1,8 All patients that first present with retrosternal pain, albeit not necessarily precordial pain, require a cardiology evaluation due to the need for ruling out heart disease, whose morbidity and mortality is considerable, compared with that conditioned by the esophageal pathology.9 NCCP symptoms can be similar to those of angina, with oppressive chest pain that radiates to the back, neck, arms, and jaw. Said radiating pain does not aid in distinguishing the true origin of the pain.10 The typical symptoms of cardiac chest pain are characterized by retrosternal pain or discomfort, perceived as oppression or heaviness, lasting 5-15min, that is usually induced by physical activity, stress, overeating, or exposure to the cold, and that improves with rest or nitroglycerine use. Acute heart failure must first be ruled out, then chronic heart failure, which includes carrying out an electrocardiogram and stress test. Coronary angiography, CT angiography, or complementary studies may also be required, and their use should be decided on by the treating cardiologist.4 It should be understood that heart disease and esophageal pathology can coexist, which is why some experts in the past suggested using the term “unexplained chest pain” to refer to NCCP.11 In some cases, the origin of the pain is unable to be identified with current technology or because the necessary studies for diagnosing the underlying pathology are not available at all levels of care. However, patients with chest pain must always first be evaluated by a cardiologist, carrying out the studies that are considered pertinent for ruling out heart disease.
Key point: Retrosternal pain can only be considered of non-cardiac origin once a cardiovascular cause has been objectively ruled out by a cardiologist.
- 2.
NCCP of probable esophageal origin can be divided into three groups: associated with GERD, associated with motor disorders, and related to esophageal hypersensitivity.
Quality of evidence and strength of the recommendation: C1 strong, in favor of the statement.
Level of agreement: in complete agreement 91%, in partial agreement 9%.
The primary mechanisms of NCCP include GERD, esophageal motility disorders (EMDs), and esophageal hypersensitivity. GERD is the most common cause of NCCP, motility alterations affect a minority of patients, and esophageal hypersensitivity can be present in patients with or without GERD or EMDs.12–14 Close to 50% of patients with NCCP have abnormal esophageal acid exposure (EAE) measured by 24-h pH monitoring,15 and between 15 and 30% have alterations in esophageal manometry.16,17
Visceral hypersensitivity is a phenomenon in which there is an increased perception produced by a stimulus, regardless of its intensity.18 Various studies have demonstrated the presence of esophageal hypersensitivity in patients with NCCP, whether or not there is GERD or an EMD. Nasr et al.19 evaluated 332 patients with NCCP with no evidence of structural esophageal pathology, carrying out an esophageal balloon distension test during which 37% of the patients presented with hypersensitivity and 75% reproduced their chest pain. Thus, the authors concluded that one out of every three subjects with NCCP have visceral hypersensitivity. That NCCP mechanism is important because there are neuromodulators that can increase pain perception thresholds and improve hypersensitivity.
Key point: Three main causes of retrosternal pain of esophageal origin, the main cause of NCCP, are GERD, motor disorders, and visceral hypersensitivity, all of which may coexist.
- 3.
GERD is the most common cause of NCCP of esophageal origin.
Quality of evidence and strength of the recommendation: A1 strong, in favor of the statement.
Level of agreement: in complete agreement 100%.
The term NCCP is designated when cardiac etiology has been ruled out. Esophageal causes hold first place among the non-cardiac causes of retrosternal pain, at 80.5%, and GERD is the most common.1,10,11 Some reviews mention the term “chest pain of probable esophageal origin” to denote the strong association and the majority of articles in the literature on the subject suggest that GERD is the primary cause to be looked for, once cardiac pathology has been ruled out.14,15 Locke et al.20 showed that 37% of the patients that had heartburn ≥ 1 time per week complained of retrosternal pain as a secondary symptom, as did 30% of the patients with < 1 episode of heartburn per week, compared with only 8% of the patients with no heartburn. Other studies have reported an association from 60 to 90% of typical GERD symptoms in patients with NCCP.21,22 Not only has an association been established between GERD symptoms and NCCP, objective studies measuring acid, such as 24-h pH monitoring, have also documented a greater prevalence of GERD in patients with NCCP that varies from 48 to 70%.23,24 Currently, GERD is the primary pathophysiologic mechanism contributing to NCCP and retrosternal pain is considered an atypical manifestation of the disease.25
Key point: At least half of the cases of NCCP are associated with GERD. NCCP can present with or without classic GERD symptoms, such as heartburn and regurgitation.
- 4.
Functional chest pain is defined as recurrent retrosternal pain of probable esophageal origin that is not associated with GERD, esophageal motor disorders, or mucosal involvement.
Quality of evidence and strength of the recommendation: C1 strong, in favor of the statement.
Level of agreement: in complete agreement 91%, in partial agreement 9%.
Functional chest pain falls within the context of functional esophageal disorders catalogued by the Rome IV consensus. Said consensus defines functional chest pain as the presence of recurrent retrosternal pain, unexplained by GERD, motor disorders, or esophageal mucosal diseases, and with no organic cause conditioning it.26 Those patients must have a negative cardiac evaluation, as well as normal endoscopy, reflux tests (pH monitoring or impedance-pH monitoring), and esophageal manometry. Functional heartburn, reflux hypersensitivity, globus sensation, and functional dysphagia belong to the same group,26 as they are conditions with negative structural and physiologic tests.27 The prevalence of functional chest pain is not fully known. In some studies, it is estimated as a cause of NCCP in 19 to 33% of cases, but some of those analyses included GERD, EMDs, and eosinophilic esophagitis as other causes of chest pain, thus real prevalence appears to be lower.28
Key point: Structural, mucosal, reflux, and motor disorders must be ruled out as causes of functional chest pain of esophageal origin.
- 5.
The worldwide prevalence of NCCP is 13 to 30%. In Mexico, it varies between 1.9% and 8% and incidence is unknown.
Quality of evidence and strength of the recommendation: B1 strong, in favor of the statement.
Level of agreement: in complete agreement 90%, in partial agreement 5%, in complete disagreement 5%.
- 6.
NCCP is more common in young persons. In Mexico, it is slightly more frequent in women.
Quality of evidence and strength of the recommendation: B1 strong, in favor of the statement.
Level of agreement: in complete agreement 76%, in partial agreement 14%, uncertain 10%.
NCCP etiology has not been fully studied. Analyses from the United States estimate that 23% of persons will present with symptoms at some point in their lives29 and Australian studies report prevalence of up to 39%,30 with equal distribution between the sexes in both countries. In epidemiologic studies in Mexico, utilizing the Rome II criteria, prevalence of 8.3% (95% CI: 5.7-11.9) was reported in a healthy population in Mexico City 31 and of 3% (95% CI: 1.7-4.9) in the State of Tlaxcala.32 A prevalence of 1.83% in an open population (95% CI: 1.5 to 2.42) was recently found, utilizing the Rome III criteria, with a mean age at presentation of 41.1 ± 11.9 and a predominance in women (61%). As mentioned above, no statistically significant differences in relation to sex have been found worldwide, and it should be kept in mind that women seek medical attention more frequently, which could explain those differences. A decrease in presentation as age increases has been reported in epidemiologic studies, with higher prevalence rates in women < 25 years of age and between 45 and 55 years of age.30 Rao et al.33 evaluated the effects of age and sex on biomechanical properties and esophageal sensitivity and found no changes associated with sex in diameter, muscle distensibility, and sensory thresholds. In contrast, older subjects presented with changes in diameter, greater wall stiffness, and higher pain thresholds (p < 0.05), suggesting that aging, not sex, influences esophageal function. Finally, a poorer quality of life has been described in patients with NCCP.30
Key point: The prevalence of NCCP varies and there is no difference according to sex, albeit it appears to be more common in women in Mexico.
- 7.
Patients with NCCP present with higher levels of anxiety and depression, producing a greater decline in quality of life.
Quality of evidence and strength of the recommendation: A1 strong, in favor of the statement.
Level of agreement: in complete agreement 95%, in partial agreement 5%.
Between 17 and 75% of the patients with NCCP present with a psychiatric disorder, and anxiety and depression are the most common.34 That group of patients utilizes a disproportionately high level of health resources, seeks medical attention in emergency services more frequently, requires numerous medical consultations in different specialties, and takes a greater number of medications, including those for heart disease, even when there is no evidence of that pathology and/or it has not been diagnosed.35 The majority of patients with NCCP complain of a lack of satisfaction with medical treatment, causing frequent seeking of medical attention and alternative treatment options because they do not feel confident about their diagnosis.36 Several studies have shown that the causes of death in patients with NCCP are not related to their symptoms. Wielgosz et al.37 followed 821 patients with NCCP for one year. A total of 0.3% died, but none of the deaths were cardiac in origin, despite the fact that 67% of those patients complained of persistent chest pain during the period up to their deaths. In a similar study, Potts and Bass38 followed 46 patients for 11 years, and of those patients, only 4.3% died from a cardiovascular cause, even though 74% stated they continued to have chest pain throughout the follow-up. The psychiatric comorbidities of stress, anxiety, and depression are more prevalent in patients with GERD and approximately 60% complain of symptom worsening during episodes of stress, which is related to an increase in symptom perception.39 Psychologic comorbidities have been documented to lead the patient to a state of hypervigilance of sensations, which can result in an increased response to a stimulus or an increase in or worsening of pain intensity.40,41 Psychiatric disorders, as well as stress and the fear of pain, have been independently associated with a decline in quality of life. Of those disorders, depression and anxiety are the most common, with a prevalence of 30 to 34% in patients with GERD.42 If physical symptoms have a negative influence on the mental state, the presence of an alarm symptom, such as chest pain, which can be associated with a possibly fatal condition, contributes to higher levels of stress and that condition has been coined “cardiophobia”.43 It has been reported in up to 50% of patients with NCCP and is also associated with a poorer quality of life. Zhang et al.44 evaluated patients with GERD and NCCP, and GERD and cardiac chest pain, and found that levels of anxiety and depression were most related to poor quality of life in the two groups of patients, but particularly in the group with GERD and NCCP. The Gastrointestinal Symptoms in Mexico survey (SIGAME, for its Spanish acronym) conducted on populations in different Mexican States, showed a significant decrease in the scores of the Patient Assessment of Gastrointestinal Disorders Symptom Severity Index (PAGI-SYM) in subjects with retrosternal pain. The overall score was 76 ± 27, with a greater impact on dress (63 ± 23), diet (77 ± 21), and psychologic compromise (68.13 ± 20) (p < 0.05), appearing to confirm that association in the Mexican population.45
Key point: Regardless of the cause of NCCP, patients with said condition frequently present with a psychiatric comorbidity, which considerably affects quality of life, increasing both the use of health resources and treatment dissatisfaction.
- 8.
The pathophysiology of NCCP is complex and can include a series of factors, such as abnormal esophageal exposure to acid and non-acid substances, delayed esophageal emptying, hypersensitivity, motility disorders, and esophageal circulation abnormalities.
Quality of evidence and strength of the recommendation: A1 strong, in favor of the statement.
Level of agreement: in complete agreement 100%.
NCCP can be conditioned by gastrointestinal causes, including esophageal ones, as well as those unrelated to the digestive tract, such as rheumatologic, musculoskeletal, and pulmonary causes.46–48 The most common cause of NCCP of esophageal origin has previously been stated to be GERD.1,10,14,15,21–24 In a review by Fass and Dickman, they estimated that in NCCP cohorts, 50-60% presented with GERD, 15-18% had esophageal dysmotility, and 32-35% presented with functional chest pain.49 Even though multiple studies have shown an association between esophageal exposure to acid and non-acid substances, the mechanism of pain is not clear. It appears to involve chemoreceptor and mechanoreceptor stimulation. That precipitates secondary esophageal sensitization, sensory afferent sensitization that produces increased responses to physiologic and pathologic stimuli, secondary allodynia, and modulation of the afferent neural function at the level of the neural dorsal root of the central nervous system.50–52 In studies with pH monitoring and impedance-pH monitoring, the presence of large-volume reflux episodes, and for longer periods of time, has been reported to be more frequently perceived as pain, rather than as heartburn.53 The relation between NCCP and motility disorders is also complex, and even though some disorders, such as aperistalsis and ineffective motility, can be associated with delayed esophageal emptying and altered acid clearance, spastic disorders can involve mechanoreceptor or esophageal microcirculation alterations.54 Similar to acid, spasm and repeated mechanical stimuli can sensitize peripheral afferent nerves and reduce the pain threshold.55
There is a complex relation between the esophagus and the heart, given that the two organs share sensory innervation.56 Esophageal acidification produces a decrease in the coronary flow in patients with the so-called “X syndrome”. Said syndrome is characterized by typical symptoms of angina, with a positive stress test (typical descent of the ST segment), but with coronary arteries that are angiographically normal and no extracardiac causes.57 The reduction in the coronary blood flow precipitates pain that is typical of angina, suggesting the presence of an esophageal cardiovascular reflex mediated by vagal fibers. Likewise, esophageal ischemia induced by the esophageal spasm or abnormal contraction of the esophagus has been proposed as one of the causes of pain.58 The pathophysiologic mechanisms in functional chest pain are even more complex and involve a combination of esophageal hypersensitivity, central and peripheral sensitization, altered central processing of esophageal stimuli, alterations in the physical and mechanical properties of the esophagus, autonomous deregulation, and psychologic comorbidities.59
Key point: Different pathophysiologic mechanisms can coexist in the patient with NCCP, causing esophageal sensitization arising from central and peripheral stimuli.
- 9.
Esophageal reflux hypersensitivity is characterized by retrosternal pain or heartburn, with normal endoscopy, no eosinophilic esophagitis, no motor disorders, and with evidence of symptoms associated with reflux events but with normal pH monitoring (total acid exposure) and/or normal impedance-pH monitoring.
Quality of evidence and strength of the recommendation: C1 strong, in favor of the statement.
Level of agreement: in complete agreement 82%, in partial agreement 13%, uncertain 5%.
Reflux hypersensitivity identifies patients with the esophageal symptoms of heartburn or retrosternal pain, with no endoscopic or pH monitoring evidence of abnormal reflux, but with symptoms triggered by physiologic reflux. As additional proof, the patient should have no other inflammatory diseases of the esophagus, including eosinophilic esophagitis (EoE) or an EMD. Although the main pathophysiologic mechanism is visceral hypersensitivity, it is sometimes difficult to distinguish that group of patients from those with true non-erosive GERD (NERD), due to the daily variability of acid exposure and symptoms and to the fact that there can be overlap of NERD and functional heartburn.27,28 More than 10% of pH monitoring studies have been reported to be consistent in their diagnosis of reflux hypersensitivity. That percentage increases to 36% if impedance-pH monitoring is performed.60,61 Up to 80% of patients with esophageal functional disorders have another functional pathology associated with visceral hypersensitivity, especially irritable bowel syndrome (27%) and functional abdominal bloating (22%).62
Key point: Reflux hypersensitivity, previously known as hypersensitive esophagus, forms part of the spectrum of non-erosive reflux disease, in which esophageal exposure to acid is normal but the symptomatic association with physiologic reflux is positive.
Diagnosis- 10.
The clinical characteristics of cardiac or non-cardiac chest pain are very similar. Therefore, their etiologies are clinically difficult to differentiate and so patients with chest pain should first be evaluated by a cardiologist.
Quality of evidence and strength of the recommendation: B1 strong, in favor of the intervention.
Level of agreement: in complete agreement 91%, in partial agreement 9%.
Every patient that presents with chest pain for the first time should be evaluated by a cardiologist to rule out cardiac causes.10,63–65 The description of chest pain obtained during the clinical history can be categorized as cardiac or non-cardiac (NCCP) in origin.13 Cardiologists subclassify it into three subgroups, according to the possibility of the presence of coronary artery disease: typical angina (80-90% probability of obstructive coronary artery disease), atypical angina (40-80% probability of coronary artery disease), and non-cardiac pain (20-70% probability of coronary artery disease).65 Typical angina symptoms are characterized by a sensation of pressure or heaviness, lasting 5 to 15min, induced by stress or effort, overeating, or exposure to the cold, that improves with rest or after the administration of nitrates. Atypical angina has at least two of the criteria for typical angina, and NCCP has one or none of the criteria for typical angina.65 From the physiologic perspective, there is a unique relation between the heart and the esophagus, given that they have the same embryonic origin, share the same sensory innervation, and the acidification of the distal esophagus can alter coronary flow and cause pain.66 In addition, coronary artery disease can coexist with other esophageal disorders, such as GERD and/or spastic motor disorders, which in turn, can be associated with coronary spasm.67,68 The role of the cardiologist is to determine whether the pain is due to coronary artery disease, and only after the cardiologist has confirmed that the symptoms are NOT associated with ischemic heart disease or another cardiovascular pathology, can we proceed to the evaluation of ruling out esophageal pathology.65
Key point and recommendation: Based on the semiology of pain, it is not possible to distinguish its cause. Therefore, the recommendation is that the initial evaluation be made by the cardiologist in all patients that present with chest pain.
- 11.
Once a cardiovascular cause is ruled out, other causes, such as musculoskeletal, pulmonary, and gastrointestinal alterations, including esophageal conditions, as well as psychiatric disorders, should be studied.
Quality of evidence and strength of the recommendation: C1 strong, in favor of the intervention.
Level of agreement: in complete agreement 91%, in partial agreement 9%.
The differential diagnosis of NCCP is extensive and includes thoracic, vascular, gastrointestinal, musculoskeletal, and psychiatric conditions.69–72 Of the gastrointestinal diseases, the esophageal ailments of GERD and motor disorders of the esophagus, especially spastic disorders, are certainly the most common. Likewise, other digestive conditions, such as biliary pain, cholecystitis, colonic flexure syndrome, peptic acid disease, and pancreatitis must be considered. The thoracic causes include pneumonia, pleurisy, pulmonary embolism, pneumoperitoneum, mediastinitis, and pericarditis. The non-cardiac vascular conditions, such as thoracic aortic dissection and superior vena cava syndrome, are rare. Different musculoskeletal pathologies, such as costochondritis, fibrositis, cervical or thoracic disease, Mondor's disease, pectoral muscle syndromes, sternoclavicular diseases, thoracic outlet syndrome, and fibromyalgia can cause NCCP, as can soft tissue diseases (herpes zoster, mammary disease) and psychiatric disorders (depression, anxiety, hypochondriasis).46,69–72 A review and meta-analysis that included 11 studies and 6,500 patients, found that the most common causes of chest pain in patients that consulted a primary care physician were: chest wall syndrome (24.5-49.8%), cardiovascular diseases (13.8-16.1%), stable coronary disease (6.6-11.2%), acute coronary syndrome (1.5-3.6%), respiratory diseases (10.3-18.2%), psychogenic disorders (9.5-18.2%), gastrointestinal diseases (5.6-9.7%), and esophageal diseases (6.0-7.1%).69 Once cardiovascular disease is ruled out, the proportions change: the authors of a study that evaluated 123 patients with recurrent NCCP concluded that 80.5% had a probable or possible diagnosis of pain of esophageal origin, the most common of which was GERD (44.7%), followed by GERD with a secondary motor disorder (26.8%).48 NCCP secondary to musculoskeletal causes varies between 11 and 28%, depending on the case series, and the finding of those causes does not exclude the coexistence with other causes, such as esophageal or pulmonary etiologies. Thus, a multidisciplinary evaluation including a gastroenterologist, pneumologist, rheumatologist, orthopedist, or even a neurologist, may be required.35,71 Finally, when the evaluation is negative, there is no response to treatment, or there is a psychologic background, psychiatric comorbidity must also be ruled out.72
Key point and recommendation: Because the differential diagnosis is extensive, once a cardiovascular problem has been ruled out, the diagnostic approach must be advanced, beginning with the most common causes (esophageal), of which GERD is the first option. Some cases may require a multidisciplinary approach.
- 12.
The barium swallow test has a poor diagnostic yield in relation to NCCP evaluation but may be used in cases associated with dysphagia.
Quality of evidence and strength of the recommendation: C1 strong, in favor of the intervention.
Level of agreement: in complete agreement 82%, in partial agreement 13%, in partial disagreement 5%.
Radiologic studies with contrast media are useful in the morphologic evaluation of the digestive tract.73 The barium swallow test enables the visualization of the esophagus and the detection of macroscopic abnormalities or extrinsic compressions.74 Nevertheless, it has low sensitivity for detecting mucosal inflammation in NCCP associated with GERD, and “abnormal reflux” can be detected during the test in up to 20% of healthy subjects75,76. The diagnostic yield of the barium swallow was recently compared with impedance-pH monitoring, considered the gold standard, and sensitivity, specificity, positive predictive value, and negative predictive value of the esophagogram were 46, 44, 50, and 40%, respectively. Therefore, GERD cannot be diagnosed by barium swallow, regardless of whether the patient presents with typical symptoms or NCCP.77 The guidelines of both the American College of Gastroenterology78 and the AMG79 do not recommend its use as a diagnostic test for GERD. The test is more useful when, in addition to pain, the patient presents with dysphagia, because it can detect narrowing of the barium column at the distal level, epiphrenic diverticula, or other structural abnormalities, such as membranes, hernias, or rings. Even so, endoscopy has a greater diagnostic yield. When achalasia or a major motor disorder is suspected, diagnosis should be confirmed through esophageal manometry.76
Key point and recommendation: The barium swallow test should be considered for structural evaluation in the presence of dysphagia. It is not recommended for the diagnosis of GERD or motor disorders, except when achalasia is suspected.
- 13.
Provocation tests, dysmotility tests, or esophageal hypersensitivity tests are not widely available and are limited to research studies.
Quality of evidence and strength of the recommendation: C2 weak, against the intervention.
Level of agreement: in complete agreement 91%, in partial agreement 9%.
There are several tests of pharmacologic stimulation of retrosternal pain, such as the Bernstein test (esophageal perfusion of HCl) and edrophonium (cholinergic stimulation), which historically have reported extremely variable sensitivities of 6-60% and 0-55%, respectively.80 More recently, the esophageal distension test through a specially designed balloon was evaluated in NCCP in 128 patients with NCCP that did not have erosive esophagitis, EMDs, or GERD, and it showed esophageal hypersensitivity in 37% of the patients and reproducible pain in 75%.19 In recent years, impedance planimetry, a test that evaluates the sensory and biomechanical (distensibility) properties of the esophagus, has been assessed in different clinical settings, including motor disorders of the esophagus, but its real usefulness is still under investigation.81 In short, even though they are tests that could be useful in very selected clinical settings, they are available in very few centers and are presently used only for research purposes.
Key point and recommendation: Those tests cannot be used in a general manner, given their low sensitivity or availability, albeit they could be used in selected clinical settings.
- 14.
A double-dose proton pump inhibitor therapeutic trial is useful for identifying patients with NCCP secondary to GERD.
Quality of evidence and strength of the recommendation: A1 strong, in favor of the intervention.
Level of agreement: in complete agreement 91%, in partial agreement 9%.
The so-called “proton pump inhibitor (PPI) therapeutic trial” consists of the short-term administration (7-28 days) of a “high dose” of a PPI (double-dose, twice a day) to identify patients with NCCP secondary to GERD, before a formal diagnostic evaluation.64,76,82 That test was originally described to be given for 7 days,83,84 but later studies evaluated a response at 2 weeks, or up to 28 days, because its usefulness depended on symptom frequency, and in the case of chest pain, symptoms may not be as frequent as in cases of heartburn or dyspepsia, which are the other indications for a PPI trial.85–89 There are two approaches: the “short trial” of 1-28 days and the empiric therapy of 2-3 months, which is used as the formal treatment of GERD. The short trial is considered positive when there is at least a 50% improvement in the intensity and frequency of retrosternal pain, and it has been evaluated with almost all the commercially available PPIs.49,63,64,76,82–92 Depending on the duration of the test, sensitivity (S) varies from 69 to 95% and specificity (Sp) from 67 to 86%.64 For example, a 7-day trial with 40mg of omeprazole in the morning and 20mg at night had S of 78.3%, Sp of 85.7%, and a positive predictive value (PPV) of 90%.85 One study described that same dose of omeprazole as having high S for predicting esophageal acid exposure (S 80, p < 0.03).86 The test with 20mg of rabeprazole twice a day for 7 days produced a 75% improvement rate in patients with NCCP secondary to GERD, compared with 11% improvement in NCCP with no GERD, versus 19% with placebo, with 75% S and 90% Sp.21 Different PPIs (rabeprazole, esomeprazole, pantoprazole, lansoprazole) have been studied in relation to their diagnostic potential in NCCP. Several studies with diverse designs and samples have evaluated diagnostic S and Sp in different populations, as well as the predictive value of those compounds. The majority of the studies employed a double dose for a period of 2-4 weeks. Diagnostic S varied from 78 to 92% and Sp from 62 to 80%, with a PPV of 58 and a negative predictive value (NPV) of 94.87–89 The same therapeutic trial has also been assessed in patients with demonstrated coronary artery disease and persistent angina, showing modest symptom improvement, but a significant reduction in visits to the emergency room and hospitalizations due to acute pain.90–92 Two subsequent meta-analyses and a systematic review confirmed those findings: Cremonini et al.93 included the results of 8 parallel and cross-over studies and reported a lower risk for persistent pain with a PPI (0.54, 95% CI: 0.41-0.71) and a diagnostic OR of 13.83 (95% CI: 5.48-34.91), when compared with pH monitoring, as well as a number needed to treat (NNT) of 3, and 80% S and 73% Sp. In another meta-analysis, Wang et al.94 evaluated 6 studies and the diagnostic OR was 19.35 (95% CI: 8.54-43.84) vs 0.61 (95% CI: 0.20-1.86) with placebo, with 80% S and 74% Sp. A systematic review with 6 studies compared the response to a PPI, according to the presence or absence of objective evidence of GERD, measured through endoscopy and/or 24-h pH monitoring. Response was defined as a therapeutic gain > 50%, over placebo. The risk for said therapeutic gain was 4.3 (95% CI: 2.8-6.7, p < 0.0001) for patients with GERD and 0.4 (95% CI: 0.3-0.7, p = 0.0004) for patients without GERD.95 The evidence of all those studies supports the use of the therapeutic trial as an initial approach for identifying patients with NCCP secondary to GERD.96 The trial has been validated in older adults, as well as in adults below 40 years of age, with no differences in the results.97 The authors of a cost-effective analysis reported that thanks to its high sensitivity and specificity, the therapeutic trial as the initial test in a patient with NCCP could result in an average effective savings of $573 USD per patient in evaluation and was associated with an 81% reduction in endoscopies and a 79% reduction in pH monitoring studies.84
Key point and recommendation: Because GERD is the most common cause of NCCP and the PPI therapeutic trial is a very sensitive, noninvasive, and readily available therapy, it is recommended as the initial test in all patients with NCCP that do not present with alarm symptoms.
- 15.
Endoscopy has a low diagnostic yield for NCCP and so should be performed in patients with alarm signs and symptoms.
Quality of evidence and strength of the recommendation: A1 strong, in favor of the intervention.
Level of agreement: in complete agreement 86%, in partial agreement 9%, in partial disagreement 5%.
Endoscopy, in any of its modalities (conventional white light endoscopy or magnification endoscopy with conventional or electronic chromoendoscopy), is useful for ruling out organic disease, evaluating the endoscopic phenotypes of GERD, and ruling out the presence of eosinophilic esophagitis (EoE) and other painful mucosal lesions, including those produced by infections or medications, or even proximal gastric mucosal lesions that cause chest pain.63,76,98,99 In 1990, the American Gastroenterological Association published the first guidelines for “chest pain of esophageal origin” and recommended the routine performance of endoscopy.100 However, later evidence showed that its diagnostic yield was variable and its sensitivity in NCCP was low. Hsia et al.101 evaluated 100 patients with NCCP and found that 24% of the patients had studies identifying erosive esophagitis and 38% had studies that were completely normal. In their Mexican study, García-Compeán et al.102 evaluated a group of patients suspected of GERD that were referred to the gastroenterologist by other specialists, including otorhinolaryngologists, pneumologists, and cardiologists, and only 10% of the endoscopic studies showed erosive esophagitis. In a transnational study by Dickman et al.,103 the authors evaluated the results from a database of 3,668 patients that underwent endoscopy due to NCCP and found a 19% prevalence of erosive esophagitis. Other findings were hiatal hernia (29%), esophageal stricture (4%), and Barrett's esophagus (4.4%). Forty-four percent of the endoscopies were normal. Similar studies conducted in Denmark and China have shown very variable rates of esophagitis (31 and 11%, respectively).104,105 In the last 15-20 years, new image-magnifying technologies have emerged that utilize a greater number of pixels, filters for selectively blocking color wavelengths (narrow band imaging [NBI], i-SCAN, and Fujinon Intelligent Chromo Endoscopy [FICE]), light excitation, or that have the potential for real-time histologic evaluation.106–109 Several of those technologies have demonstrated greater sensitivity than conventional endoscopy for detecting micro-erosions (magnification endoscopy: 62% S, 74% Sp; FICE: 76.9% S, 51.6% Sp; confocal endoscopy: 68-86% S, 72-91% Sp) and intestinal metaplasia (chromoendoscopy with methylene blue and acetic acid: 100% S, 66% Sp; NBI: 100% S, 66% Sp; confocal endoscopy: 98% S, 94% Sp, 98% NPV).106,108,110,111 Nevertheless, the Porto and Lyon consensuses, which are the most recent on GERD, conclude that up to 15% of the general population may present with grade A esophagitis and that interobserver variability with grade B esophagitis is high. Therefore, they state that only the presence of grade C and/or D esophagitis should be considered diagnostic of GERD.112,113 Thus the gain in diagnostic yield with high-definition endoscopes can include patients with micro-erosions that are not necessarily the cause of the patient's symptoms.114 In addition, the fact that erosions or peptic acid lesions are found at endoscopy does not change the initial therapeutic management because those patients can be treated empirically with a course of PPIs. In brief, the prevalence of erosive esophagitis in NCCP varies greatly, between 10 and 70%, according to the type of population studied and reference biases. Therefore, endoscopy should be performed in patients with NCCP that also present with alarm symptoms, such as dysphagia, persistent odynophagia, anemia, or weight loss, or in those in whom a double-dose PPI therapeutic trial has failed for a period not greater than 6-8 weeks.
Key point and recommendation: Endoscopy is an invasive study that has a low diagnostic yield in NCCP with no other symptoms. It is recommended when there are alarm symptoms or failure to respond to a PPI therapeutic trial.
- 16.
Esophageal biopsies are useful in NCCP for making the differential diagnosis with other entities, such as eosinophilic esophagitis, infections, and suspected Barrett's esophagus.
Quality of evidence and strength of the recommendation: B1 strong, in favor of the intervention.
Level of agreement: in complete agreement 91%, in partial agreement 9%.
Esophageal biopsies should be taken at endoscopy when there are alterations in the mucosa suggestive of infectious pathology (e.g., Candida albicans, herpes simplex virus), inflammatory causes (Crohn's disease, radiotherapy), precancerous lesions (intestinal metaplasia, dysplasia), or neoplasia, and when EoE is suspected.110,115–118 Up to 7% of the endoscopies in patients with EoE appear to be normal, with no characteristic lesions, such as longitudinal grooves, felinization, trachealization, or food impaction.118 Therefore, if suspicion is high, biopsies should be taken to evaluate the number of eosinophils per high power field. In a group of consecutive, non-selected patients with NCCP referred for endoscopic evaluation, abnormal eosinophilic infiltration (6-15 eosinophils/high power field) was identified in 14% and EoE (> 15 eosinophils/high power field) was diagnosed in 6%.119 Biopsy should not be taken to confirm the diagnosis of GERD, given that the characteristic histopathologic findings described (e.g., spongiosis and basal cell layer hyperplasia) can be observed in the healthy population.110,117 Several research groups have described mast cell infiltration in the esophageal biopsies of patients with NCCP secondary to GERD, motor disorders, and functional NCCP, and have proposed that said infiltration can belong to the pathophysiologic mechanisms associated with distal esophageal hypercontractility in NCCP. However, that is still considered a line of research.120,121
Key point and recommendation: The histologic alterations associated with GERD may be seen in the healthy population. Biopsies are recommended only to histologically confirm the differential diagnosis, but not to confirm GERD.
- 17.
24-h pH monitoring with a catheter or wireless capsule and impedance-pH monitoring are useful tests for detecting GERD as the cause of NCCP.
Quality of evidence and strength of the recommendation: A1 strong, in favor of the intervention.
Level of agreement: in complete agreement 86%, in partial agreement 14%.
GERD has been demonstrated to be the most common cause of NCCP, regardless of the presence of the classic symptoms of heartburn and/or regurgitation. Between 50 and 60% of the patients with NCCP have abnormal esophageal acid exposure (EAE), when measured through ambulatory pH monitoring.9,15,49,63,64 It is not clear whether there is an association or causality between GERD (erosive or non-erosive) and the presence of pain.122 The sensitivity of pH monitoring with a catheter varies between 79 and 96%, with 85-100% specificity, albeit some studies have reported a lower specificity (60-78%) and a variable symptomatic correlation (12-50%).48,123,124 However, upon performing pH monitoring as a confirmatory test for NERD, a 75% response to a PPI has been shown after fundoplication, when abnormal EAE has been documented.125 Some studies have compared the short therapeutic trial with pH monitoring, finding similar sensitivities. Thus, pH monitoring appears to have more value when objective evidence of EAE is required or when the PPI trial fails.49,64 Because it is a study that can modify the patient's diet during the test, due to effects related to the presence of the transnasal catheter, wireless pH monitoring has been proposed as an alternative. It entails the endoscopic placement of a capsule that measures pH in the lower third of the esophagus, 6cm proximal to the squamocolumnar junction. The two main advantages of that method are the absence of a transnasal catheter during the study and the fact that measuring can be extended up to 96h, enabling an initial measurement of 48h with no medical treatment to demonstrate EAE and a subsequent measurement during the next 48h to evaluate treatment response.126 In a study that evaluated said strategy in NCCP, Prakash et al.127 reported that the extended measuring only modestly increased the diagnostic yield: 10% for EAE, 7.3% for greater symptom report, and 21% for greater detection of chest pain episodes. The method also has several disadvantages: wireless pH monitoring, itself, has been reported to cause chest pain in 16% of cases, at an intensity that required its removal in 5% of those cases. In a group of patients that required endoscopic capsule dislodgement, the initial indication for wireless pH monitoring was chest pain in 62.5% of them.128 Two additional points to consider are its higher cost, compared with conventional pH monitoring with catheter, and a 12% potential risk for premature dislodging of the capsule during the evaluation period.129 Several authors have proposed that pH monitoring combined with multichannel intraluminal impedance (MII-pH) can be more sensitive than conventional pH monitoring in patients with atypical clinical manifestations of GERD and in patients that are nonresponders to double-dose PPI.112,113,130–137 The design of the impedance catheter enables the detection of reflux according to its chemical (acid, non-acid, less acid) and physical (liquid, gaseous, mixed) characteristics. It can also measure different variables that are not available to conventional pH monitoring with catheter, such as the proximal extension of each refluxate, nocturnal baseline impedance, post-reflux swallow-induced peristaltic wave indexes, and exposure time to bolus. The real value of those new variables is still under study. MII-pH can be performed with or without PPI, according to the indication (to document abnormal EAE in patients with no previous diagnosis of GERD or the evaluation of refractory GERD or treatment failure, respectively.)112,113,130–132 There is less evidence on the role of non-acid reflux as the cause of NCCP.133,134 In a comparative study on 48 patients with NCCP and 50 with typical GERD symptoms, the majority of reflux episodes in the group with NCCP were acid and mixed, and they had a longer period of time of exposure to bolus with altered clearance.133 However, its greatest usefulness appears to be in NCCP that does not respond to PPIs.134 In summary, ambulatory intra-esophageal pH monitoring is the best test for detecting EAE, but in NCCP, its main usefulness is when objective evidence of GERD (EAE measurement without a PPI) is required or when there is treatment failure or refractory symptoms (non-acid and mixed EAE measurement with a PPI).
Key point and recommendation: pH monitoring is considered the gold standard for diagnosing GERD, but it is invasive. Intra-esophageal pH, in any of its variants, is recommended when confirmation of esophageal acid exposure is required, and with impedance, for the evaluation of non-acid, mixed, or refractory reflux.
- 18.
The symptom association indexes evaluated during ambulatory pH monitoring increase the diagnostic yield of the test in detecting NCCP secondary to GERD.
Quality of evidence and strength of the recommendation: C1 strong, in favor of the intervention.
Level of agreement: in complete agreement 82%, in partial agreement 14%, uncertain 4%.
The symptomatic events reported during a pH-monitoring study enable the presence or absence of a temporal relation between a reflux episode and a particular symptom to be established. However, only those symptoms that can be directly related to reflux (heartburn, retrosternal pain, regurgitation, cough) should be considered for the analysis of symptom association.112,113 There are three symptom association indexes: the symptom index (SI), the symptom sensitivity index (SSI), and the symptom association probability (SAP). The SI and SAP have shown predictive value for the effect of medical therapy or surgical treatment. The SI is defined as the percentage of symptomatic events related to reflux events and the SAP is a statistical parameter that utilizes a Fisher's exact test to measure the strength of the relation between symptomatic events and reflux.135–137 However, their correct interpretation involves several limitations: there is symptom variability between days, they require the patient to mark the symptom button at the moment the symptom begins, and their validity requires the presence of at least three symptomatic episodes during the study period, so that when there is a greater number of symptoms there will be a greater probability of establishing an association. Current evidence shows that the majority of patients with NCCP have an inconsistent relation between reflux events and pain, with a correlation that varies from 12 to 50%.59 Prakash et al. evaluated the value of 2 symptom indexes: the SI and the Ghillebert probability estimate (GPE), and found an 8% variability between days with the SI and 21% with the GPE.127 The authors of two studies in the surgical literature reported a good correlation between the SI and clinical outcome in NCCP: DeMeester et al.138 concluded that the SI was highly predictive of postoperative symptom improvement, and Patti et al.139 found 96% improvement if the SI was positive versus 65% if it was negative. One study evaluated the usefulness of pH monitoring and manometry performed during a stress test in 111 patients with typical angina chest pain that had no improvement with PPIs. The patients with a SI > 50% were catalogued as having pain associated with GERD and the authors described an association between esophageal acidification during the test and the presence of pain, especially when the reflux episodes lasted more than 10seconds, with low sensitivity but 83% specificity.140 Even though there is little evidence, the Rome IV group recently introduced the term “reflux hypersensitivity” to refer to patients with esophageal symptoms -including retrosternal pain- and no endoscopic evidence of esophagitis and no pathologic reflux determined through pH monitoring, but with a positive SI. Those patients may also have overlap with other forms of NERD.26,28 Therefore, even though the real value of the association between symptoms and reflux is a subject of debate, at present, in patients with pain and physiologic reflux parameters, the SI is useful for differentiating between reflux hypersensitivity and functional pain.
Key point and recommendation: At least three episodes of each symptom are required for the SI to be valid, and only symptoms associated with GERD should be evaluated. It is the only test for differentiating reflux hypersensitivity from functional pain.
- 19.
Esophageal manometry is the most useful test for detecting motor disorders that cause NCCP.
Quality of evidence and strength of the recommendation: B1 strong, in favor of the intervention.
Level of agreement: in complete agreement 77%, in partial agreement 18%, uncertain 5%.
Esophageal manometry is the best test for detecting esophageal motor disorders (EMDs), which usually manifest as retrosternal pain and/or dysphagia. Several studies and reviews evaluating NCCP with conventional manometry have found various proportions of esophageal motility anomalies (6-70%, average 30%, and only 2% of cases of achalasia).63,64 Dekel et al.141 evaluated a total of 587 consecutive patients that underwent motility studies within the time frame of 1998 to 2001 and chest pain was the primary symptom in 24%. The authors reported that 70% of the studies were normal and nutcracker esophagus was present in only 10%. In a Brazilian study that evaluated 240 patients with NCCP, manometry was normal in 63%, 25% of the patients had nonspecific disorders, 16% presented with lower esophageal sphincter (LES) hypotension, 6% had nutcracker esophagus, 2.5% had achalasia, and 1.6% presented with diffuse esophageal spasm.142 In a study conducted in Chile, 36% of patients had nutcracker esophagus, 28% had nonspecific disorders, 9% had diffuse spasm, 28% had LES hypotension, and only 2% presented with aperistalsis.17 The authors of a Mexican study on 33 patients documented the following causes of NCCP: GERD in 48%, achalasia in 34%, and functional pain in 18%.143 The relation between motor abnormalities found through conventional manometry and NCCP is not very clear in the majority of cases, and like GERD, could be the cause or an epiphenomenon. Given that some motor disorders can be associated with GERD, the majority of experts suggest ruling out reflux first. The performance of manometry extended to 24h has a low additional diagnostic yield, compared with short conventional manometry (6.8% additional diagnoses).123,144 In the last 20 years, there have been advances in the development of manometry catheters, with a higher number of sensors, as well as improvement in image processing software that enables pressure data to be presented in the form of color space-time traces.145 High-resolution manometry (HRM) with esophageal pressure topography applying those concepts has brought about better understanding and evaluation of the motor function of the esophagus,146,147 as well as a simpler interpretation of the studies, with less interobserver variability.148 That technology has introduced new variables, changing the diagnostic criteria of the EMDs. They have been incorporated into the third edition of the Chicago classification, redefining several motor disorders (e.g., “jackhammer” esophagus instead of “nutcracker” esophagus) and reclassifying others (e.g., distal esophageal spasm [DES] and its variants, instead of diffuse esophageal spasm). The term “spastic disorders” has been coined, which includes complex disorders, such as type III achalasia.149 The usefulness of HRM has been well-demonstrated in the evaluation of dysphagia: in a study that compared 245 manometry tracings (122 conventional, 123 HRM) from 247 patients with dysphagia, the initial diagnosis was more frequently confirmed by expert review in the HRM group, including tracings that had been interpreted as normal through conventional manometry (52% vs 28%, p < 0.05).150 In recent years, information has begun to emerge with that new test in NCCP. A European study described the behavior of 34 cases of jackhammer esophagus (JE) and found that 47% of the patients had NCCP.151 The authors of a Mexican study reported an association between NCCP and the joint presence of EoE and JE.152 In a retrospective study utilizing HRM, Gómez-Cifuentes et al.153 evaluated 177 patients with NCCP and found GERD in 35% and EMDs in 31% (ineffective esophageal motility in 14.1%, JE in 6.8%, DES in 5.1%, and achalasia in 2.3%). The risk factors for the development of an EMD were age (OR increased by 1.2 every 5 years, 95% CI: 1.0-1.3) and dysphagia as an accompanying symptom (OR 3.8, 95% CI: 1.9-9.75). Even though a direct association between a variable of HRM and NCCP has not been described,154 an increase in the contraction amplitude in segment 3 has been reported in patients with acid hypersensitivity and NCCP. In addition, a study reported 75% sensitivity and 98% specificity for HRM with esophageal topography for diagnosing esophageal spasm.155. The Italian guidelines on indications for manometry156 suggest that “ideally, HRM should be performed on all patients with NCCP with uniform instruments and standard parameters”. However, from the cost-effectiveness perspective, several aspects should be considered: 1) if GERD is suspected, manometry is obligatory for establishing the position of the pH/MII-pH electrode, 2) if NCCP is accompanied by dysphagia, it is strongly indicated for ruling out spastic disorders and obstruction (major motility disorders), 3) if NCCP is isolated and there is no improvement with PPIs, and 4) to rule out accompanying systemic diseases with potential esophageal compromise. The performance of manometry is recommended in the Rome group's “Algorithms for diagnosing common gastrointestinal symptoms” as part of the evaluation of NCCP.156 A group of international experts recently published the first in a series of consensuses on the indications for motility, function, and gastrointestinal sensitivity studies in different gastrointestinal diseases, including NCCP, and they recommend HRM as the first study, as well as provocation studies (multiple, rapid swallow sequencing and rapid swallow test with 200ml), combined with pH monitoring with or without MII. As a second test, they recommend prolonged wireless pH monitoring in cases in which there is diagnostic doubt.157 The consensus also states that the diagnosis of GERD can be established in cases with grey area EAE (4-6%), in the presence of unstable esophagogastric junction (type III), or cases of ineffective esophageal motility.157
Key point and recommendation: Manometry is an invasive test that requires expert personnel for its performance and interpretation. Although it has multiple indications, for the purpose of the present consensus, it is recommended for ruling out esophageal spastic motor disorders and achalasia associated with pain and GERD.
- 20.
Psychiatric evaluation is recommended in patients with NCCP whose tests are negative and who do not respond to a therapeutic intervention.
Quality of evidence and strength of the recommendation: B1 strong, in favor of the intervention.
Level of agreement: in complete agreement 91%, in partial agreement 9%.
Between 17 and 75% of the patients with NCCP are estimated to present with a psychologic abnormality,158 and psychiatric disorders coexist with up to 60% of the patients with “nonsignificant coronary artery disease”.159 Those comorbidities can modulate pain perception or induce the perception of nonpainful stimuli as painful ones. They can be associated with hypervigilance or hyperventilation, which can cause reversible esophageal manometric abnormalities. The most widely associated psychiatric disorders with NCCP are: panic disorder, anxiety, depression, hypochondriasis, and neuroticism.160 Psychiatric comorbidities can, in turn, coexist with other causes of NCCP: in one study, 80% of the patients with an EMD had an adjacent psychiatric disorder versus only 30% of the patients with normal esophageal motility.161 Due to those associations, patients that do not respond to a therapeutic intervention, whose tests for reflux or esophageal motility are negative, or that are suspected of having a psychologic comorbidity, should be referred to a psychologist or psychiatrist for additional evaluation and/or management.162
Key point and recommendation: Referral to a psychologist or psychiatrist is recommended for patients whose diagnostic evaluation is normal, who do not respond to treatment, or in whom a psychiatric disorder that can coexist with other causes of NCCP is suspected.
Treatment- 21.
Ideally, NCCP treatment should be directed at the underlying pathophysiologic mechanism (e.g., gastroesophageal reflux, esophageal dysmotility, hypersensitivity, psychiatric comorbidity).
Quality of evidence and strength of the recommendation: B1 strong, in favor of the intervention.
Level of agreement: in complete agreement 95%, in partial agreement 5%.
NCCP pathophysiology is complex and heterogeneous and there is no single marker that can explain the pain process in all cases. Therefore, the associated factors of GERD, esophageal dysmotility, and different mechanisms related to visceral hypersensitivity have been proposed.14 The Rome criteria make the generic diagnosis of NCCP but due to the numerous pathophysiologic mechanisms involved, it is very difficult to establish a management plan based only on clinical criteria, given that none of the section in the semiology of pain has diagnostic sensitivity or specificity. In fact, the Rome criteria diagnose functional chest pain, when esophageal and cardiovascular causes have been adequately ruled out.26 Thus, the corresponding treatment must be established once the diagnostic evaluation has been carried out and the mechanism(s) involved in producing NCCP in each particular case has/have been determined.163
Key point and recommendation: Due to the numerous causes of NCCP and its mechanisms, a management plan based on the mechanism(s) involved should preferably be established.
- 22.
Treatment with a PPI is indicated when gastroesophageal reflux disease has been documented as the cause of NCCP.
Quality of evidence and strength of the recommendation: A1 strong, in favor of the intervention.
Level of agreement: in complete agreement 90%, in partial agreement 5%, in partial disagreement 5%.
As mentioned above, GERD can be diagnosed in patients with NCCP through a positive therapeutic PPI trial.82,93–95 In patients that require additional evaluation, GERD has been established to be linked to chest pain in 30 to 60% of the cases, based on pH monitoring, with or without symptom association.15,84,137,164 The use of MII-pH has recently determined that non-acid reflux events are associated with episodes of pain,134 and that chest pain may not improve in all cases with PPI therapy when non-acid reflux is the main promotor.131,132 Categoric endoscopic diagnosis is lower due to the study's poor diagnostic yield (30% sensitivity) and the greater prevalence of non-erosive reflux disease (NERD),103 as well as to the recent change in diagnostic criteria that indicate that only grade C or D esophagitis constitutes a categoric diagnosis of GERD.112,113 Therefore, once GERD is documented as the cause of NCCP, whether through endoscopy or pH monitoring, an initial treatment plan can be established with a double dose of a PPI, followed by its reduction, in accordance with the maintenance of the clinical response.49,96
Key point and recommendation: Regardless of the therapeutic trial, PPI use is recommended as treatment for NCCP when GERD has been documented through endoscopy (grade C or D esophagitis with the Los Angeles classification) or pH monitoring (abnormal esophageal acid exposure > 6%).
- 23.
Double-dose PPI treatment is recommended for at least 2 months for GERD-related NCCP.
Quality of evidence and strength of the recommendation: B1 strong, in favor of the intervention.
Level of agreement: in complete agreement 95%, in partial agreement 5%.
PPIs are the best medical treatment for NCCP when it is associated with GERD. They have been used as both therapeutic trials and as prolonged formal treatment. Results have been satisfactory with the different types of PPIs,49,82–95,165 including 20mg of omeprazole b.i.d. or 40mg in the morning and 20mg at night,83–86 20mg of rabeprazole b.i.d.,21,87 30mg of lansoprazole b.i.d.,22,88 and more recently, 40mg of esomeprazole b.i.d.89 Several meta-analyses have demonstrated the superiority of PPIs over placebo in NCCP and the double dose b.i.d. is the dose evaluated in the majority of the studies.93,94,166 In the most recent meta-analysis on patients with GERD confirmed through upper GI endoscopy or pH monitoring, the odds ratio (OR) for response was 11.7 (95% CI: 7.5-25), whereas in patients with uninvestigated NCCP, the OR was lower (4.2, 95% CI: 2.7-6.7). In the patients in whom NCCP was studied but the results were negative, the possibility of success with PPI-based therapy was just 0.8 (95% CI: 0.2-2.8).166 In the meta-analyses by Cremonini and Wang, the OR for improvement with PPIs was 13.83 and 19.35, respectively.93,94 The majority of studies show improvement no higher than 50% during the first 2 weeks. Thus, as in cases of other atypical GERD symptoms, the double-dose PPI treatment is recommended for at least 2 months to evaluate results, albeit a longer period may sometimes be needed.49,59,167 Long-term treatment appears to be efficacious in maintaining symptom remission in patients with NCCP associated with GERD. The same as in other indications for PPI use as maintenance, leaving the smallest dose that is efficacious for each patient is suggested.167,168 No study has compared one PPI with another to determine which is the best, leaving the choice of the initial type of PPI open. The recommendations regarding the schedule for PPI administration are similar to those in GERD with typical symptoms.78,79 H2 antagonists have also been evaluated in open studies with small samples. They have shown subjective improvement at high doses, but no correlation between a positive SI and response to ranitidine has been established.167
Key point and recommendation: Studies have shown that a conventional dose of a PPI is insufficient for achieving clinical improvement in NCCP. A PPI (20mg of omeprazole or its equivalent b.i.d.) for at least 8 weeks is recommended, with the same administration schedule and maintenance as in GERD with typical symptoms.
- 24.
Smooth muscle relaxants, such as nitric oxide donors, calcium antagonists, and anticholinergics are used in NCCP associated with spastic motor disorders, but their efficacy is limited and associated with adverse effects.
Quality of evidence and strength of the recommendation: C2 weak, in favor of the intervention.
Level of agreement: in complete agreement 82%, in partial agreement 13%, in partial disagreement 5%.
Esophageal smooth muscle spasm is an important mechanism in NCCP, which may be associated with spastic motor disorders.16,17,168,169 Smooth muscle relaxants have been used for the treatment of the subgroup of patients in whom distal esophageal spasm (DES), hypercontractile esophagus, or type III achalasia have been detected. However, most evidence is from old studies with deficient methodologies or with outdated manometric criteria.170,171 The smooth muscle relaxants that have been evaluated in NCCP are calcium antagonists (nifedipine, diltiazem), nitrates, and phosphodiesterase-5 inhibitors, such as sildenafil.172–177 Nifedipine at a dose of 10-30mg 3 times a day has been shown to reduce the contraction amplitude in patients with nutcracker esophagus but with no clinical correlation.172 In another study, its use was associated with improvement in pain intensity scores after 16 months, compared with placebo, but there were no changes in the frequency or duration of pain.173 Adverse events are an important limitation of nifedipine and they include facial flushing, headache, dizziness, and anxiety.167 Three randomized studies with modest-sized samples evaluated diltiazem at doses between 60 and 150mg. No superiority over placebo was demonstrated in the treatment of NCCP in patients with esophageal spasm,174 whereas the other two studies showed a decrease in the pain scores in patients with nutcracker esophagus.175,176 As with nifedipine, the adverse events are the main limitation of its use. The evidence on nitrates in EMDs only comes from open studies with limited samples.167 Finally, sildenafil has been evaluated in small case series, with inconsistent results, and for short periods of time due to side effects.177 Smooth muscle relaxants can be used in some well-selected cases, while definitive therapy for spastic motor disorder is being defined. Before considering the use of those agents, it is imperative to rule out gastroesophageal reflux because those medications induce LES relaxation and therefore can potentially aggravate reflux.
Key point and recommendation: Smooth muscle relaxants are useful medications for short-term administration in patients with NCCP associated with spastic motor disorders that are not candidates for other treatments or as bridging therapy before definitive treatment. Dose should be adjusted based on response and adverse effects. Given their effect on the LES, before prescribing smooth muscle relaxants, we must be certain the patient does not have gastroesophageal reflux.
- 25.
Transendoscopic botulinum toxin injection is an alternative for the treatment of NCCP associated with spastic disorders of the esophagus in patients that are not candidates for myotomy or pneumatic dilation.
Quality of evidence and strength of the recommendation: B2 strong, in favor of the intervention.
Level of agreement: in complete agreement 95%, in partial agreement 5%.
Botulinum toxin has been proposed for treatment of spastic disorders of the esophagus because it induces selective blockade of acetylcholine in the neuromuscular presynaptic plate. In an open study that included 29 patients with miscellaneous motility alterations, the injection of botulinum toxin in 4 quadrants of the esophagogastric junction induced a decrease in chest pain of at least 50% in 72% of the patients and the mean duration of the effect was 7.3 ± 4.1 months.178 In a small case series of 9 patients with esophageal spasm, the patients received 100 IU of botulinum toxin injected every 1 to 1.5cm above the esophagogastric junction. There was improvement in 8/9 patients with a mean duration of 6 months. Some cases required re-injection.179 In a more recent open study, botulinum toxin was applied to 45 patients with EMDs diagnosed according to current criteria at a single center in France: 22 of the patients had type III achalasia, 8 had hypercontractile esophagus, 7 had DES, 5 had esophagogastric junction outflow obstruction, and the rest were miscellaneous. Injection location was heterogeneous. In some cases, it was in the esophagogastric junction, and in others, it was in the body of the esophagus. Seventy-one percent of the patients improved after 2 months of treatment and 57% maintained response at month 6 of follow-up. Among the complications were one death related to mediastinitis 3 months after the injection and chest pain in 13 cases that did not last more than 7 days. The authors found no difference in the response based on the diagnosis, but the patients with achalasia had the worst outcome.180 Botulinum toxin injection is currently an alternative for pain control in patients with spastic disorders that should be used when life expectancy or the surgical or endoscopic risk is high. It should be applied by expert personnel. Due to the low quality of evidence, its routine use is not recommended. Other endoscopic treatment modalities, such as dilation with bougies (non-hydraulic), have not been widely evaluated and said procedure appears to be associated with an important placebo response. However, more randomized studies are needed to determine the efficacy of that treatment modality.167 In addition, there are no comparative studies on other forms of esophageal dilation in patients with NCCP secondary to EMDs.
Key point and recommendation: The endoscopic injection of botulinum toxin in NCCP has the same indications as smooth muscle relaxants. In other words, it should be used as a temporary measure before definitive therapy or in cases in which more invasive treatment is contraindicated. Numerous administrations should be avoided if a later definitive therapy is being considered, such as POEM or surgical myotomy.
- 26.
Peroral endoscopic myotomy is a therapeutic option in NCCP for selected patients with spastic disorders of the esophagus.
Quality of evidence and strength of the recommendation: B1 strong, in favor of the intervention.
Level of agreement: in complete agreement 90%, in partial agreement 5%, in partial disagreement 5%.
The main spastic disorders of the esophagus, according to the Chicago classification (DES, jackhammer esophagus, and type III achalasia), can be associated not only with dysphagia, but also with NCCP.149 For that EMD subgroup, peroral endoscopic myotomy (POEM) is a therapeutic option because of its capacity to section the affected muscle along the esophageal body and LES.181,182 In a meta-analysis that included 8 observational studies with 179 patients, the conclusion was that, overall, POEM induced a response in 87% of the patients (95% CI: 78-93%) and patients with type III achalasia had the best outcome, with a mean response of 92% (95% CI: 84-96). In the DES analysis, the mean response was 88% (95% CI: 61-97) in 4 studies. In the 5 studies analyzed, the response for hypercontractile esophagus (JE) was 72% (95% CI: 55-83).183 In a multicenter study that retrospectively compared the effectiveness of POEM vs laparoscopic Heller myotomy (LHM) in patients with type III achalasia, POEM was more effective (98% vs 80.8%, p = 0.01). Surgery duration was also shorter (102min vs 264min; p < 0.01), a longer myotomy length was achieved (16cm vs 8cm, p < 0.01), and it was associated with a lower incidence of adverse effects (6% vs 27%, p < 0.01).184 It should be mentioned that no well-designed prospective study has been conducted for comparing the efficacy and safety of the two procedures in patients with esophageal spastic disorders. Finally, as with all invasive procedures, POEM requires a learning curve, and several studies have examined the number necessary for developing said curve.185–187 One study from a single center in the United States concluded that a minimum of 13 procedures were needed to reach the plateau for performing POEM.185 Nevertheless, two more recent publications from China, stated that the mean number of POEM procedures for improving its performance was 25,186 and at least 100 were needed to reduce adverse effects and the risk for therapeutic failure.187 POEM is now being implemented in some centers in Mexico, and therefore the recommendation is that it be performed by expert personnel in centers of excellence.
Key point and recommendation: POEM is a procedure that is not widely available in Mexico and is still in the learning curve stage, but worldwide evidence of its effectiveness in spastic disorders of the esophagus is highly promising. It is recommended as a definitive measure in spastic motor disorders (distal spasm and jackhammer esophagus), to be performed only in centers of experience with qualified personnel.
- 27.
Neuromodulators for visceral pain are useful in functional NCCP when there is no satisfactory response with other treatments.
Quality of evidence and strength of the recommendation: B1 strong, in favor of the intervention.
Level of agreement: in complete agreement 95%, in partial agreement 5%.
Esophageal visceral hypersensitivity is an important mechanism in NCCP, especially in the functional subtype. Up to 50% of patients with NCCP have been shown to have lower sensory thresholds, compared with healthy controls,188 as well as greater esophageal reactivity and less compliance.19 Visceral pain neuromodulators are medications from different classes that act on the peripheral or central sensory pathways, or both, to modify the sensory threshold. Tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors (SNRIs) have mainly been used, but some agents that affect the serotonin receptors, such as tegaserod, and adenosine agonists, such as theophylline, have also been evaluated in small studies.189–199 The only TCA analyzed in a double-blind study controlled with placebo in NCCP is imipramine. Fifty milligrams of imipramine every 24h, 0.1mg of clonidine, or placebo, was administered to a group of 60 patients for 4 weeks. Pain frequency was reduced in the three groups, but only the imipramine group had a statistically significant decrease in pain frequency (52%, p < 0.03) and the response was independent of the presence or absence of a psychiatric comorbidity.189 An open study compared the effect of adding a low dose of amitriptyline (10mg at night) to a conventional dose of rabeprazole (20mg daily) versus a double dose in a group of patients with NCCP that had a suboptimum response to the conventional PPI dose and had normal pH monitoring. Seventy-one percent (vs. 26%, p = 0.008) had over 50% overall improvement and a greater sensation of wellbeing.190. Paroxetine and sertraline are two SSRIs that have been evaluated in NCCP. Paroxetine was analyzed in a double-blind randomized study on 43 patients and induced improvement in the clinical perception of chest pain but not in the specific chest pain perception score after 8 weeks of treatment at a mean dose of 30mg a day.191 In another double-blind randomized study that compared paroxetine with cognitive behavioral therapy or placebo in 69 patients with NCCP, there were no significant differences between groups.192 Sertraline was evaluated in a double-blind randomized study with dose titration up to 200mg daily, according to response, and showed a significant decrease (p < 0.02) in pain perception and improvement with respect to the baseline wellbeing subscores in the SF36 questionnaire.193 In another study with the same design that compared psychologic therapy plus sertraline, sertraline alone, or placebo, the authors concluded that the combination of psychologic therapy plus sertraline was more efficacious in the control of NCCP.194 Trazodone is an SSRI from the group of phenylpiperazines that also has an anxiolytic and hypnotic effect. It was evaluated in a controlled study on 29 patients with NCCP that had manometric abnormalities and a negative Bernstein test at a dose between 100 and 150mg for 6 weeks. There was significant overall improvement (48% vs 11%, p = 0.02), but no effect on pain intensity or manometric abnormalities.195 Venlafaxine is both an SSRI and an SNRI. Its usefulness has only been examined in a randomized, cross-over study controlled with placebo utilizing 75mg for 4 weeks in 43 patients. There was a response (decrease in NCCP) in 52% of the patients versus only 4% with placebo in the intention to treat analysis (OR 26.0, 95% CI: 5.7-118.8, p < 0.001).196 Tegaserod is a 5-HT4 agonist that was evaluated in patients with functional heartburn, and showed that it increased the pain threshold in the balloon distension test.197 Those findings have not been reproduced in NCCP. Theophylline, an adenosine agonist, was evaluated in two studies by Rao et al.198,199 In the first, intravenous open administration was associated with an increase in the pain threshold measure through impedance planimetry and oral intake with a decrease in the frequency and intensity of pain.198 In a later controlled study, the effects of theophylline on compliance and esophageal relaxation were confirmed. In addition, there was symptom reduction in 58% (vs 6% with placebo, p < 0.02).199 Even though the results suggest a potential use of theophylline in NCCP, it has a narrow therapeutic margin in relation to undesirable effects, limiting its current use in NCCP. However, the finding that theophylline improved NCCP provides an opportunity to examine new, previously unevaluated visceral analgesics in NCCP.200 Despite the fact that the evidence comes from studies that are small or not controlled, neuromodulators appear to be efficacious in NCCP secondary to dysmotility or of functional origin. Its use should be individualized, explaining to the patient that the objective is to modulate the nociceptive pathways and pain integration centers, rather than being aimed directly at the psychiatric comorbidity. Treatment should be begun with low doses (e.g., the equivalent of 10mg of nortriptyline/imipramine or 12.5mg of amitriptyline before going to sleep and gradually increase on a weekly basis until obtaining a response, trying to use the lowest dose possible that improves pain; rarely is more than 50mg needed) and explain the potential adverse effects to favor treatment adherence and induce the desired effect.
Key point and recommendation: The aim of different neuromodulators is to reduce visceral hypersensitivity in functional pain. The majority are antidepressants at low doses, whose maximum effect is experienced after 8-12 weeks. Most evidence comes from studies on imipramine, amitriptyline, and venlafaxine. Paroxetine and sertraline have been studied to a lesser degree.
- 28.
Cognitive behavioral therapy, hypnotherapy, biofeedback, and Johrei healing are alternatives in refractory cases or complements to other treatments for NCCP.
Quality of evidence and strength of the recommendation: B1 strong, in favor of the intervention.
HYPNOTHERAPYQuality of evidence and strength of the recommendation: B2 weak, in favor of the intervention.
BIOFEEDBACKQuality of evidence and strength of the recommendation: C2 weak, in favor of the intervention.
JOHREI HEALINGQuality of evidence and strength of the recommendation: C2 weak, in favor of the intervention.
Level of agreement: in complete agreement 95%, in partial agreement 5%.
There are different non-pharmacologic treatment modalities that have been studied in patients with NCCP. The most important essential element of cognitive behavioral therapy (CBT) is that it favors the development of better strategies for problem management, which in this case, are health problems. Thus, patients with NCCP, with or without an accompanying psychiatric comorbidity, can reduce their symptomatology. In a small controlled study (17 patients) that compared CBT with conventional therapy, after 12 weeks of treatment, 31% of the subjects were symptom-free and 34% presented with partial response.201 Another study compared CBT in a group of patients with NCCP and a control group. After 3 months of the intervention, the CBT group had a significant reduction in the number of days and severity of pain, compared with the controls. At 6 months, there was improvement in the social and physical roles of the patients undergoing CBT.202 A controlled clinical trial showed that patients that received CBT improved in the quality of life spheres in general and stated they had less fear of perceiving painful bodily sensations.203 Finally, an open study on 60 patients that underwent CBT had a significant reduction in the number of pain events from 6.5 to 2.5 per week, together with a decrease in the scores on anxiety and depression scales.204
Hypnotherapy has also been used in patients with NCCP. In a single blind study that randomized 28 patients to receive hypnotherapy or placebo (an interview with no hypnotherapy), the patients in hypnotherapy presented with a greater decrease in the intensity, but not the frequency of pain.205 Biofeedback consists of a series of respiratory exercises to induce diaphragmatic contraction, and its usefulness was evaluated in a very small study. There was a decrease in chest pain but not in heartburn in a group of 6 patients with functional esophageal symptoms.206 It is important to emphasize that CBT, as well as hypnotherapy and biofeedback, should be administered by expert personnel to obtain the best results. Johrei therapy (healing with spiritual energy), considered alternative medicine whose mechanism of action is unknown, was studied in 39 patients with NCCP for 6 weeks. The patients that underwent the intervention showed a significant reduction in the pain score, compared with the baseline.207 Because that is the only study on patients with NCCP, more evidence is needed to confirm its benefit.
In summary, CBT has been studied extensively in cases of functional pain and with good results. Other less known psychologic therapies that have not been widely studied appear to be effective in limited numbers of patients. Those alternative techniques still require critical evaluation in NCCP management, and unfortunately, there are not enough trained therapists that are familiar with them.
Key point and recommendation: CBT appears to be useful in NCCP, but must be administered by qualified personnel. The current evidence that is published on other forms of psychologic therapy, such as hypnotherapy and biofeedback, or Johrei healing, which is a form of alternative medicine, is insufficient and additional study is required.
Surgery as treatment for NCCP is based on myotomy of the affected esophageal segment and limited to spastic disorders of the esophagus. It should be performed by an expert surgeon and in highly selected cases.
Quality of evidence and strength of the recommendation: C2 weak, in favor of the intervention.
Level of agreement: in complete agreement 90%, in partial agreement 5%, uncertain 5%.
Surgery can be beneficial in NCCP under two premises: as antireflux treatment in cases of NCCP associated with GERD and for myotomy of a spastic esophageal segment. There are no controlled studies that analyze fundoplication in NCCP. Open studies have shown improvement between 81 and 96% in patients in whom GERD has been confirmed through pH monitoring.138 With respect to surgery as treatment for NCCP associated with spastic disorders, most evidence comes from old or retrospective studies that utilize previously employed manometric criteria. The improvement rates reported reach 80% in some case series that combined longitudinal myotomy with a partial antireflux procedure.208,209 Even though laparoscopic Heller myotomy (LHM) has been successfully performed in cases of achalasia for many years, studies on type III achalasia (in which there is a higher incidence of secondary chest pain) have shown that it is less effective, because the myotomy does not cover the body of the esophagus. One study compared the success rate of the LHM according to the manometric subtype of achalasia and found that subtype III was associated with lower success rates (70-85%), compared with subtype II (95-100%).210 The evidence of success with other spastic disorders has not been as widely studied. In a case series of 20 cases treated with extended myotomy (14cm of the esophagus and 2cm below the esophagogastric junction), Leconte et al.211 reported improvement in dysphagia and chest pain in patients with DES. Another study that compared the myotomy approaches through thoracoscopy and laparoscopy in patients with esophageal spasm and with nutcracker esophagus, found no differences between the techniques in relation to pain control. However, both techniques were more effective in cases of esophageal spasm than in those of nutcracker esophagus.212 Taking the new manometric criteria of the Chicago classification, version 3.0, into account, there are no controlled studies on LHM in spastic disorders different from type III achalasia, only reports or case series. Therefore, there is no evidence for recommending its use, or not. When performed, it should be done so on highly selected patients, with manometric documentation of the extension of the area of spasticity and carried out by a surgeon with expertise in said procedures.
Key point and recommendation: The evidence on the effectiveness of fundoplication in NCCP associated with GERD comes from open, non-controlled studies. Extended longitudinal myotomy, after documentation of the area of spasticity measured by high-resolution manometry, can be considered in patients that have not responded to other measures, and performed by a surgeon that is qualified in that type of procedure. However, there are few randomized studies that compare the efficacy of surgery with other therapeutic measures. Likewise, the potential undesirable effects of surgery should be contemplated when deciding on that alternative.
ConclusionsNCCP is a clinical syndrome characterized by retrosternal pain that is similar to angina pectoris, in which cardiovascular causes have been ruled out. It can be caused by esophageal, musculoskeletal, pulmonary, or psychiatric diseases. GERD is the most common esophageal cause. Other diseases include spastic motor disorders and functional pain associated with visceral hypersensitivity. All patients that present with chest pain should first be evaluated by a cardiologist to rule out coronary artery disease. Given that the most common cause is GERD, if there are no alarm symptoms (e.g., dysphagia/weight loss, unexplained anemia), treatment can be started with a PPI therapeutic trial for 2-4 weeks. If there is dysphagia or manifestations of alarm symptoms, endoscopy should be performed, and biopsies should be evaluated when diseases of the mucosa different from reflux are suspected. Manometry, especially the high-resolution procedure, is the best method for ruling out motor disorders and pH monitoring, with or without a catheter, and aids in demonstrating abnormal esophageal acid exposure. Impedance-pH monitoring demonstrates non-acid or refractory acid reflux. If the evaluation is negative, the ruling out of psychiatric comorbidity should be considered. Treatment should preferably be directed at the pathophysiologic mechanism and can include PPIs, neuromodulators and/or smooth muscle relaxants. In selected cases, endoscopic therapy (botulinum toxin or Botox) or psychologic therapy (cognitive behavioral therapy) can be useful, and in some cases, surgery can also be an option.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestDr. Octavio Gómez-Escudero is a member of the advisory board of Sanofi and Takeda, is a speaker for Laboratorios Menarini, Sanofi, and Takeda, and participates in research protocols funded by Laboratorios Asofarma.
Dr. Enrique Coss-Adame is a member of the advisory board of Takeda Pharmaceuticals, Allergan, Carnot Laboratorios, and Menarini, is a speaker for Laboratorios Asofarma, Alfa-Wassermann, Allergan, Carnot, and Takeda, and participates in research protocols funded by Laboratorios Asofarma.
Dr. Mercedes Amieva-Balmori is a speaker for Laboratorios Sanfer and Takeda.
Dr. Ramón Carmona-Sánchez is a member of the advisory board of Mayoly-Spindler, a speaker for Mayoly-Spindler and Grünenthal, and participates in research protocols funded by Laboratorios Senosian and Asofarma.
Dr. José María Remes-Troche is a member of the advisory board of Takeda Pharmaceuticals, Alfa-Wassermann, and Menarini. He has received research funding from Sanfer. He is a speaker for Takeda, Asofarma, Alfa-Wassermann, Carnot, Menarini, Almirall, and Astra-Zeneca, and participates in research protocols funded by Laboratorios Asofarma.
Dr. Ana Teresa Abreu y Abreu is a member of the advisory board of Laboratorios Biocodex, Sanofi, and Takeda. She is a speaker for Laboratorios Biocodex, Carnot, Mayoli-Spindler, Signafarma, and Takeda.
Dr. Eduardo Cerda Contreras is a member of the advisory board and a speaker for Laboratorios Carnot.
Dr. Paulo César Gómez Castaños is a speaker for Laboratorios Sanofi and Takeda.
Dr. Marina Alejandra González Martínez is a speaker for Grünenthal.
Dr. Francisco M. Huerta Iga is a member of the advisory board of and a speaker for Laboratorios Asofarma and Takeda.
Dr. María Eugenia Icaza Chávez is a speaker for Laboratorios Asofarma and Takeda.
Dr. Miguel Morales Arámbula is a speaker for Laboratorios Takeda and Asofarma.
Dr. Genaro Vázquez Elizondo is a speaker and outside advisor for Alfa-Wassermann and Asofarma and participates in research protocols funded by Laboratorios Asofarma.
Dr. Miguel A. Valdovinos is a member of the advisory board of and a speaker for Takeda, Menarini, and Mayoly-Spindler.
Dr. Alicia Sofía Villar Chávez is a speaker for Asofarma and Carnot.
Dr. Jorge Ibarra Palomino, Dr. Manlio F. Márquez Murillo, Dr. Luis Raúl Valdovinos, Dr. Mónica Zavala Solares, and Dr. Sami Rene Achem have no conflict of interest in relation to the present consensus.
Please cite this article as: Gómez-Escudero O, Coss-Adame E, Amieva-Balmori M, Carmona-Sánchez RI, Remes-Troche JM, Abreu-Abreu AT, et al. Consenso mexicano sobre dolor torácico no cardiaco. Revista de Gastroenterología de México. 2019;84:372–397.