The aim of the Mexican Consensus on the Treatment of HepatitisC was to develop clinical practice guidelines applicable to Mexico. The expert opinion of specialists in the following areas was taken into account: gastroenterology, IFNectious diseases, and hepatology. A search of the medical literature was carried out on the MEDLINE, EMBASE, and CENTRAL databases through keywords related to hepatitisC treatment. The quality of evidence was subsequently evaluated using the GRADE system and the consensus statements were formulated. The statements were then voted upon, using the modified Delphi system, and reviewed and corrected by a panel of 34 voting participants. Finally, the level of agreement was classified for each statement. The present guidelines provide recommendations with an emphasis on the new direct-acting antivirals, to facilitate their use in clinical practice. Each case must be individualized according to the comorbidities involved and patient management must always be multidisciplinary.
El objetivo del Consenso Mexicano para el Tratamiento de la HepatitisC fue el de desarrollar un documento como guía en la práctica clínica con aplicabilidad en México. Se tomó en cuenta la opinión de expertos en el tema con especialidad en: gastroenterología, IFNectología y hepatología. Se realizó una revisión de la bibliografía en MEDLINE, EMBASE y CENTRAL mediante palabras claves referentes al tratamiento de la hepatitisC. Posteriormente se evaluó la calidad de la evidencia mediante el sistema GRADE y se redactaron enunciados, los cuales fueron sometidos a voto mediante un sistema modificado Delphi, y posteriormente se realizó revisión y corrección de los enunciados por un panel de 34 votantes. Finalmente se clasificó el nivel de acuerdo para cada oración. Esta guía busca dar recomendaciones con énfasis en los nuevos antivirales de acción directa y de esta manera facilitar su uso en la práctica clínica. Cada caso debe ser individualizado según sus comorbilidades y el manejo de estos pacientes siempre debe ser multidisciplinario.
Specific questions about therapy were identified and addressed by the participants, based on scientific evidence on hepatitis C management collected from a systematic review of the literature. The development process of the present guidelines took nine months and the first meeting of the steering committee was in September 2016. The face-to-face meeting of the working group of the consensus was held in October 2016 and the presentation of the manuscript for its publication took place in May 2017.
Sources and searchesPhysicians at the Department of Gastroenterology of the Universidad Autónoma de Nuevo León performed a systematic search on MEDLINE (starting from 1946), EMBASE (starting from 1980), and CENTRAL (Cochrane Central Register of Controlled Trials) up to August of 2016. The search terms were: hepatitis C, interferon, ribavirin, sofosbuvir, ledipasvir, velpatasvir, daclatasvir, asunaprevir, simeprevir, dasabuvir, ombitasvir, paritaprevir, ritonavir (3 D), elbasvir, and grazoprevir. The search was limited to studies published in English and conducted on humans.
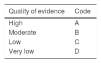
Review and grading of evidenceThe quality of evidence was evaluated according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (Table 1) and carried out by three methodologists (Dr. Roberto Monreal, Dr. Omar David Borjas and Dr. Emmanuel González), who did not vote on the statements. They determined the risk of bias in the individual studies, the risk of bias between studies, and the quality of evidence in general of the studies identified for each statement. The voting members of the working group of the consensus reviewed the GRADE criteria and agreed upon them at the meeting. The quality of evidence for each statement was graded as: high, moderate, low, and very low. The evidence from randomized controlled trials (RCTs) was classified as high-quality but could be downgraded for the following reasons: heterogeneity between the outcomes of individual studies, ambiguous results, indirect study results, reported bias, or a high risk of bias between the studies supporting a statement. The data from cohort studies or case-control studies were initially categorized as having a low quality of evidence but could be downgraded through the same criteria applied to the RCTs or upgraded if a very great treatment effect or a dose-response relation was identified, or if all the plausible biases had a tendency to change the magnitude of the effect into the opposite direction. Product approval and labeling by governmental regulatory agencies vary among countries, and although they were not ignored, the recommendations were based on the evidence in the medical literature and the consensus discussions and may not fully reflect the labeling of products in Mexico.
The consensus processThe working group of the consensus was made up of 34 voting participants that included academic and community-based gastroenterologists, hepatologists, and specialists in infectious diseases with experience in various aspects of hepatitis C management, as well as a non-voting facilitator (Dr. Francisco Bosques). The working subgroups and co-presidents of the meeting (Dr. Aldo Torre Delgadillo, Dr. Graciela Castro Narro, Dr. René Malé Velázquez, and Dr. Rafael Trejo Estrada) developed the initial statements. A web-based platform supported by the Asociación Mexicana de Hepatología (AMH) was used to facilitate the majority of the aspects of the consensus process before the face-to-face meeting. Through the consensus platform, the working groups: 1) reviewed the initial literature search and identified the relevant references that were selected and linked (“tagged”) to each statement; 2) used a modified Delphi process for anonymous voting on the level of agreement of the statements; 3) suggested statement revisions; and 4) provided comments on specific references and background data. The statements were revised through two separate iterations and finally at the consensus meeting. Every participant had access to all the abstracts and electronic copies of the individual “tagged” references. The GRADE criteria in relation to the evidence for each of the statements were given at the meeting. The working group attended a 2-day consensus conference in Mexico City in October 2016, in which the data were presented, the statements were discussed, their final versions were written, and the participants voted on the level of agreement for each statement. A scale from 1 to 5 (in which 1, 2, and 3 indicated “in complete disagreement”, “in disagreement”, and “uncertain”, respectively) was employed and a statement was accepted if > 75% of the participants voted 4 (in agreement) or 5 (in complete agreement). The strength of each recommendation was assigned by the working group of the consensus through the GRADE system as strong (“…is recommended”) or weak (“…is suggested”). The strength of recommendation consisted of four components (risk/benefit balance, patient values and preferences, resource cost and allocation, and quality of evidence). Thus, it was possible for a recommendation to be classified as strong, despite being supported by a low quality of evidence or weak, despite being supported by a high quality of evidence.
Based on the GRADE system, a strong recommendation indicates that the statement can be applied in the majority of cases, whereas a weak recommendation means that clinicians “should recognize that different options are appropriate for different patients and should help each patient decide on the treatment that is consistent with his or her values and preferences”. The characteristics of the GRADE system are shown in Table 1.
The manuscript was integrated and edited by Dr. Francisco Bosques, along with Dr. Roberto Monreal, Dr. Omar Borjas, and Dr. Emmanuel González. It was then reviewed by the steering committee members before being distributed to all the participants for their review and approval. Potential conflict of interest is expressed in writing and presented according to AMH policy and is available to all the consensus working group members and readers. The Consensus document consists of 19 sections that address different clinical scenarios and the quality of evidence and strength of recommendation are shown for each statement, as well as the level of agreement, expressed as a percentage, that was obtained through the voting of all the participants at the face-to-face meeting.
Treatment indications: who should and who should not be treated?Recommendations- •
All patients with chronic HCV infection should be considered for treatment, whether or not they have been previously treated (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
For complete and satisfactory elimination of the hepatitis C virus (HCV), it is necessary to have a solid plan involving the entire country and to have the resource availability and support of the healthcare sector to have effective and easily-accessed treatments. The benefit of curing the infection has important clinical advantages. The goal of treatment is to define a sustained virologic response (SVR) as the persistent absence of HCV for at least 12 weeks after treatment completion. That was demonstrated in a prospective study in which the majority of patients achieved an SVR that lasted for more than 5 years, through a first-line HCV treatment, showing that recurrence was rare after a prolonged follow-up period.1
Patients with SVR have an excellent clinical response, manifested by a significant decrease in fatigue, improved physical activity, a biochemical response with normalized aminotransferase levels, and reduced fibrosis progression.
Over the last 5 years, the treatment of chronic HCV infection has improved considerably with the introduction of new direct-acting antiviral agents (DAAs) that are targeted at different sites of the viral genome, preventing the replication cycle.2
Recommendation- •
Treatment should be a priority for patients with advanced fibrosis or cirrhosis (METAVIR F-3 F-4) (A1).
Level of agreement: in complete agreement 95%.
In agreement with minor reservations: 5%.
Today, patients with advanced disease can receive treatment. That group of patients is at greater risk for complications due to hepatic decompensation (encephalopathy, ascites, gastrointestinal bleeding from esophageal varices, and the appearance of hepatocellular carcinoma). The HALT-C study, supported by the U.S. National Institutes of Health, demonstrated a rise in hepatocellular carcinoma development in patients with chronic hepatitis C under long-term surveillance, with mortality rates increasing 7.5 to 10% per year.3
SVR has been shown to reduce decompensation episodes in patients with advanced disease. There is less need for liver transplantation and a lower mortality rate in patients with advanced liver disease that have SVR, than in untreated patients.3,4 Those data have been reproduced in a European study,5 but there are still no long-term results.
Recommendations- •
Expedited treatment is recommended for patients with cirrhosis of the liver that have Child-Pugh class A or B disease. Patients with decompensated cirrhosis (Child-Pugh class B and C) should also be urgently treated with an IFN-free regimen, even if they are not going to undergo transplantation. Patients with Child-Pugh class C and a MELD score above 20 that are indicated for liver transplantation can undergo the transplant first, and then receive treatment (B1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
IFN use is absolutely contraindicated for patients with decompensated cirrhosis. Post-liver transplantation IFN use is currently not indicated, due to multiple adverse effects and the low SVR rate. With the introduction of the new DAAs, treatment is more effective, with better pretreatment and posttreatment response.
Liver transplantation is the treatment of choice for patients with advanced cirrhosis. It is well-known that post-transplantation hepatitis C recurrence is universal and is associated with reduced graft survival.6 Whether decompensated patients with chronic liver disease should receive pretransplantation or posttransplantation treatment is a subject of debate. At present, there are no controlled studies on which to base an ideal time of treatment.
Prevention of post-transplantation graft infection in patients waiting for liver transplantation is attempted by having them achieve SVR and biochemical response, thus reestablishing liver function. An initial analysis was conducted in France on transplantation waiting list patients that had MELD scores above 14 points. The authors of that study showed that sofosbuvir and ribavirin were well tolerated and SVR was achieved in > 70% of the cases.7 Patients with a history of decompensations or functional Child-Pugh class B or C should not receive treatment with protease inhibitors, due to the toxicity of those antivirals. It is recommended to use only a combination of sofosbuvir plus an NS5A inhibitor.8
Patients with a MELD score > 18-20 should undergo upfront primary liver transplantation, and then be treated. Depending on the transplantation center, patients on the waiting list for more than 6 months can be treated to obtain the benefits of virus elimination.9
Recommendations- •
Treatment should be a priority in patients with any of the following conditions: HIV or HBV coinfection; pre-transplantation or post-transplantation status; clinically significant extrahepatic manifestations (symptomatic vasculitis associated with mixed HCV-related cryoglobulinemia); nephropathy due to immune complexes related to HCV and B-cell non-Hodgkin lymphoma; and debilitating fatigue (A1).
Level of agreement: in complete agreement 95%.
In agreement with minor reservations: 5%.
Patients coinfected with HIV and treated with DAAs have a similar response to those infected only with HCV. Therefore, the former should be treated as if they only had HCV infection, in conjunction with infectious diseases specialists.10,11
There are several management modalities and response rates are similar. The daclatasvir + sofosbuvir regimen was evaluated over a 12-week period in the ALLY-2 study on a group of patients with HIV/HCV coinfection. Some of the patients were treatment-naïve and others were treatment-experienced. The patients were stratified by HCV genotype (1/83%, 2/9%, 3/6%, 4/2%) and doses were adjusted according to the concomitant antiretroviral treatment. Ninety-seven percent of the treatment-naïve patients had SVR, as did 98% of the treatment-experienced patients.12 In the non-randomized open C-EDGE CO-INFECTION study,13 the grazoprevir/elbasvir regimen was evaluated in treatment-naïve patients infected with HCV genotypes 1, 4, or 6 and HIV with or without cirrhosis, resulting in a 96% SVR12 (in 210/218 patients). In the phase II ERADICATE14 study, the ledipasvir/sofosbuvir regimen was assessed in a small group of 50 patients with no cirrhosis. The treatment-naïve group achieved 100% SVR12 (13/13) and the treatment-experienced group had 97% SVR12 (36/37). There is not enough information for treating those patients for 8 weeks, and therefore it is not recommendable to consider short periods. In the TURQUOISE-1 study,11 the combination of paritaprevir/ritonavir/ombitasvir + dasabuvir was evaluated in treatment-naïve and treatment-experienced patients, resulting in SVR12 of 93% and 90-96%, respectively. The COSMOS study 15 produced similar results with the simeprevir/sofosbuvir regimen in patients with genotype 1b infection (92% SVR). Finally, patients with genotypes 1-4 treated with the combination of sofosbuvir/velpatasvir had 100% SVR12.16
Patients coinfected with hepatitis B tend to have a low viral load, making HCV the central focus of treatment. Nevertheless, HBV can reactivate after virus C clearance. Thus, all patients that start treatment with a DAA should have a complete HBV viral panel that includes the DNA of the virus. If the results are positive or there is evidence of occult hepatitis B, treatment should be begun with nucleoside/nucleotide analogs to prevent hepatitis B virus reactivation. Patients coinfected with hepatitis B/C should be treated with a regimen similar to that used in patients with HCV monoinfection.
Those subjects with occult hepatitis B (positive for anti-HB core antigen, but negative for HBsAg and anti-HBs) are at risk for reactivation as soon as they are in treatment for HCV. 17–19 Monitoring of ALT and AST levels is recommended every 4 weeks in patients positive for isolated anti-HB core antigen, during DAA treatment for HCV. If there is an increase > 2-fold the upper limit of normal, HBV DNA levels should be determined, and if positive, treatment for HBV should be considered. Treatment for HBV should begin before (preferably 2 to 4 weeks before) or concomitant with the start of DAA treatment for HCV. The recommended treatment for HBV is entecavir 0.5mg PO once-daily or tenofovir 300mg PO once-daily. Treatment for HBV should be continued for at least 3 weeks after completing DAA treatment for HCV. If the patient has no indication for chronic HBV treatment, treatment for HBV can be discontinued.
DAA and immunosuppressant use, including rituximab, has shown good response in patients with mixed cryoglobulinemia and HCV.20 Non-Hodgkin lymphoma can be associated with HCV and the diffuse B-cell variant is the most common. Lymphoma should be treated with the customary regimen (R-CHOP) plus rituximab. However, rituximab can increase viral replication. Isolated cases of lymphoma remission with antiviral therapy for HCV eradication have been reported.21
There is an association between HCV and immune complex-mediated kidney damage with vasculitis and focal glomerulosclerosis. Those cases should be managed with antivirals adjusted to the grade of kidney damage, in addition to rituximab, plasmapheresis, steroids, and cyclophosphamide. There is no evidence at present of a rapid response to treatment with DAAs, and as stated above, rituximab can be useful. Multidisciplinary management is recommended in such cases.
Recommendation- •
Treatment should be a priority in individuals at risk for transmitting HCV, such as: intravenous drug addicts, homosexuals with high-risk sexual practices (promiscuity, several partners, unprotected sex, sex with persons infected with HBV, herpes virus, or HIV), women trying to get pregnant, patients on hemodialysis, and prisoners (B1).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
HCV transmission is unlikely in patients that have experienced cure. The most common risk for acquiring HCV is injection drug use. De novo HCV infection in subjects that are injection drug users (IDUs) is above 70%.22 In a meta-analysis that evaluated the results of HCV treatment (based on pegylated interferon [pegIFN]) in IDUs, 37% SVR was reported for the patients with genotype 1 or 4, and 67% with genotypes 2 or 3.23 Unfortunately, there is little information on treatment with DAAs and the exact prevalence of reinfection is unknown. Likewise, that group of patients requires a multidisciplinary approach that includes a psychiatrist, a specialized drug and alcohol abuse department, an infectious diseases specialist, and aspects of intense social work.
High-risk homosexuals are defined as those that are promiscuous, have sexual contact with several partners or with partners infected with HBV, HCV, or HIV, that engage in unprotected sex, and men with HIV infection that have sex with men.22,24 Opportune recognition and treatment of HCV infection in that special group of patients is a determining factor in the prevention of later infections, including that of acute HCV. Those patients should receive immediate treatment, as well as information on their disease, so they do not infect other sexual partners.25
Persons in prison are another special group of great epidemiologic importance due to risk for transmission and they have poor access to HCV treatment. Studies conducted in the United States show that seroprevalence varies from 30 to 60%, and of those cases, acute infection occurs in 1%.26 The use of older treatments in that group of patients is not free from side effects and most certainly antiviral therapy could change HCV incidence and reinfection rates, given that many of those patients are IDUs.
In women that wish to become pregnant, there is no possibility of maternal-fetal transmission if the mother does not present with viremia. Those patients can receive treatment before becoming pregnant, but treatment should not be given during pregnancy.
Recommendation- •
Patients with moderate fibrosis (F2) should receive treatment (A2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
It is very useful to treat patients with moderate fibrosis because it reduces liver fibrosis progression, which is one of the basic goals of HCV treatment, and thus treatment should not be delayed. Treatment improves quality of life and increases life expectancy. Noninvasive serologic tests and imaging studies provide better information on early disease-stage patients.27,28
Recommendations- •
Patients infected with HCV, with or without mild fibrosis (METAVIR F0 F1) and with no extrahepatic manifestations, should receive treatment, but the time of treatment can be individualized (B1).
Level of agreement: in complete agreement 88%.
In agreement with minor reservations: 12%.
Beginning treatment in disease stages F0 or F1 increases the SVR rate.
In the past, when patients were treated with interferon (IFN) and ribavirin, the SVR rate was influenced by fibrosis grade, and was more favorable in cases of early-stage fibrosis. Today, with the use of DAAs, that is no longer as relevant.
Patients with early-stage fibrosis documented through biopsy benefit from treatment. In a preliminary study by Jezequel et al.,29 after a 15-year follow-up, they showed greater survival in the treated patients (93%) that had SVR. Survival was lower in the patients that did not respond to treatment (82%) and in untreated patients (88%). Fibrosis progressed 15% in the patients with treatment delay.
Treatment should be individualized in patients with early-stage liver disease (compensated patients with Child A). We believe it is important to evaluate the best therapy or therapies accessible in Mexico and carefully study the group of patients that “can wait” (risk-benefit) in relation to treatment. Unfortunately, there is no solid evidence from Mexican analyses on the theme. Other studies have documented reduced mortality rates and complications, with quality of life improvements upon achieving SVR, in patients receiving treatment at early stages of fibrosis.30–32
Recommendation- •
Treatment should be individualized in patients with a limited life expectancy due to non-hepatic comorbidities (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
There are patients with a very limited life expectancy due to age or severe comorbidities with a short natural history. In those patients, treatment for HCV, as well as liver transplantation or other therapy that does not change the course of their progression, should not be recommended.33,34
Hepatitis C virus treatment goalsThe immediate goal of HCV treatment is SVR, defined as the persistent absence of HCV RNA for at least 12 weeks after the end of treatment. SVR is a marker for HCV infection cure and has been shown to be lasting in more than 99% of patients treated with IFN that have had follow-up of 5 years or more. At present, that benefit has not yet been corroborated in patients receiving treatment with DAAs. Serum anti-HCV antibodies are persistent in patients with SVR, but HCV RNA is no longer detected in serum or in liver tissue, reaching substantial improvement in liver histology.
Both SVR12 and SVR24 have been accepted as HCV treatment goals, given that their concordance is > 99%.35 HCV core antigen that is undetectable 12 or 24 weeks after treatment completion can be used as an alternative to HCV RNA testing to evaluate SVR12 or SVR24.36 Patients that are cured of HCV infection experience benefits in their general state of health that include reduced liver inflammation and a decrease in the progression rate of liver fibrosis and portal hypertension. SVR is associated with a reduction > 70% in the risk for hepatocellular carcinoma and a 90% decrease in the risk for hepatopathy-related death and the need for liver transplantation.3 The cure of HCV infection also reduces symptoms and mortality derived from severe extrahepatic manifestations related to chronic HCV infection. Given the multiple benefits associated with successful HCV treatment, those patients should receive antiviral therapy, with the aim of achieving SVR, preferably at an early stage in the course of chronic infection before the development of severe liver disease or other complications. Numerous studies have shown that HCV treatment that achieves SVR in patients with advanced liver disease (METAVIR F3-F4) results in reduced rates of liver decompensation, hepatocellular carcinoma development, and death. In the HALT-C study, patients with advanced fibrosis that achieved SVR had less need for liver transplantation, a lower hepatopathy-related morbidity and mortality rate, and a lower frequency of hepatocellular carcinoma, when compared with patients that had similar fibrosis grades but did not achieve SVR.37 It should be pointed out that persons with advanced liver disease require long-term follow-up and continuous surveillance with respect to the possible appearance of hepatocellular carcinoma, regardless of treatment result. Real-world multicenter studies in patients with decompensated cirrhosis treated with DAAs have shown that approximately one-third of patients have an improved MELD score, a reduced frequency of decompensation events, and can even be taken off the transplantation list, especially if their MELD score is < 18-20. However, the long-term clinical benefit has not been evaluated in all the studies.38
Recommendations- •
The goals of treatment are to cure the hepatitis C infection, prevent cirrhosis of the liver and its decompensation, and to prevent the development of hepatocellular cancer (HCC), severe extrahepatic manifestations, and death. Treatment goals also include the prevention of disease transmission, as well as recurrence after liver transplantation (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
The goal of treatment is to achieve an undetectable level of HCV RNA through a sensitivity test (≤ 15 IU/ml) at 12 weeks (SVR12) or 24 weeks (SVR24) after treatment completion (A1).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
- •
HCV eradication reduces the decompensation rate and reduces, but does not eliminate, the risk for HCC in patients with advanced fibrosis or compensated cirrhosis. Surveillance for complications and HCC should be continued in those patients (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
HCV eradication reduces the need for transplantation in patients with decompensated cirrhosis that have a MELD score of 18-20. It is not known whether HCV eradication modifies medium-term and long-term survival in that group of patients (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
Pre-therapeutic evaluationAll patients with HCV infection that wish to receive treatment and have no contraindications for it, whether treatment-naïve or treatment-experienced or with compensated or decompensated chronic liver disease, should be treated.
There should be no delay in the treatment of patients with significant fibrosis or cirrhosis, including patients with decompensated cirrhosis, those with clinically important extrahepatic manifestations, patients with recurrence after liver transplantation, those at risk for rapid deterioration of liver disease due to comorbidities, and individuals at risk for disease transmission.
Patients with a limited life expectancy due to hepatopathy should be managed in conjunction with an expert that, if possible, is in contact with a transplantation center. Treatment is not recommended in patients with a limited life expectancy due to comorbidities unrelated to liver disease.
Factors associated with a possibly accelerated progression of liver fibrosis should be evaluated, such as inflammation grade, patient age, HCV progression time, male sex, a history of transplantation, alcohol consumption, fatty liver disease, obesity, insulin resistance, genotype 3, and coinfection with HBV or HIV.
Liver biopsy, imaging studies and/or non-invasive markers to determine the presence of advanced fibrosis are recommended in all patients with HCV infection to facilitate the appropriate decision as to treatment strategy and to determine the need to begin additional measures for the management of cirrhosis.39 Liver fibrosis grade is one of the most important outcome factors for predicting HCV disease progression.40 In some cases, treatment duration is longer in patients with advanced fibrosis or cirrhosis.
Liver biopsy is the diagnostic method probably considered the gold standard, but the possibility of sampling errors, interobserver variability, and the invasive nature of the modality that is not exempt from complications, are all factors that limit its use. Noninvasive tests for establishing liver fibrosis grade in patients with chronic HCV infection include models that incorporate serum biomarkers, direct serum biomarkers, and liver elastography.
Transitory elastography is a noninvasive form of measuring liver stiffness and it correlates well with substantial fibrosis or cirrhosis measurement in patients with chronic HCV infection.41 The most effective approach for evaluating fibrosis grade is probably the combination of direct biomarkers and transitory elastography. Liver biopsy should be considered when conflicting results between the 2 modalities affect clinical decisions or if other causes are suspected. Patients with clinically evident cirrhosis do not require additional stratification studies.
HCV RNA detection is indicated in patients that are candidates for antiviral treatment. Quantification should be carried out through a sensitivity assay (≤ 15 IU/ml) and expressed in IU/ml. HCV genotype, including the genotype 1 (1a or 1b) subtype should also be assessed before starting treatment. However, it is important to know that there are DAA combinations that are pangenotypic (sofosbuvir/velpatasvir and sofosbuvir/daclatasvir), as well as combinations that are non-panogenotyptic (sofosbuvir/ledipasvir, ombitasvir/paritaprevir/ritonavir/dasabuvir, grazoprevir/elbasvir) that are equally effective in genotype 1 disease, with no distinction between subtypes 1a and 1b.
Genotype IL28B has lost predictive value with the advent of treatment regimens based on IFN-free direct-acting antivirals. Thus, that test is only useful when pegylated IFN and ribavirin are the only treatment options.
The systematic determination of HCV resistance prior to treatment commencement is not recommended, because it could limit access to management since treatment regimens can be optimally designed, even without that information.42 However, the presence of certain resistance (NS5A RAVs) significantly reduces SVR12 rates in the 12-week treatment regimen with elbasvir/grazoprevir in patients with genotype 1a infection. Based on the knowledge of an inferior response, resistance testing is recommended in patients with genotype 1a that are being considered for that regimen. If resistance is demonstrated, treatment extension to 16 weeks with the addition of weight-adjusted ribavirin is recommended to reduce the possibility of relapse.42
Recommendations- •
All patients with confirmed HCV infection and viral load should be treated unless there is a contraindication (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Comorbidities that influence liver disease progression should be evaluated and treated (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Liver disease stage should be evaluated before beginning treatment. It is indispensable to identify the patients with cirrhosis because its prognosis is different, and treatment must be modified (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
The grade of fibrosis must be evaluated. It can first be done through noninvasive methods. Liver biopsy should be considered when there is diagnostic doubt or suspicion of other associated causes (A1).
Level of agreement: in complete agreement 95%.
In agreement with minor reservations: 5%.
- •
HCV RNA should be identified and quantified through a sensitivity test capable of detecting ≤ 15 IU/ml (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
It is essential to identify HCV genotype and subtype (1a-1b) before beginning treatment. It is a crucial factor in choosing the treatment regimen (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
The new direct-acting antivirals have made IL28B genotype testing for predicting treatment response unnecessary (A1).
Level of agreement: in complete agreement 92%.
In agreement with minor reservations: 8%.
- •
Determining the baseline resistance-associated polymorphism is unnecessary in patients that have not had previous treatment with DAAs. It should only be considered in patients with treatment regimens that include elbasvir/grazoprevir or daclatasvir/asunaprevir (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
Treatment of hepatitis C virus genotype 1Amazing progress has been made in the treatment for the eradication of chronic hepatitis C virus infection. Without a doubt, improvement in the cure rates with the different treatment regimens has had a favorable impact on outcome and quality of life of infected patients, changing the course of a disease that affects a large number of patients worldwide. This medical breakthrough will surely impact the history of medicine.43,44 Patients with genotype 1 were considered the most difficult to treat, given the low sustained virologic response at 24 weeks from the end of treatment (SVR24) with pegIFN and ribavirin (RBV), with even poorer response in patients with cirrhosis of the liver. Many patients with advanced liver disease were not considered candidates for that therapy due to its adverse effects.45 Today, the new treatments based on direct-acting antivirals (DAAs) can be administered to patients with advanced liver disease, not only improving SVR, but also survival rates and liver function9,46 Some of the DAAs are available in Mexico: asunaprevir (ASV), ombitasvir/paritaprevir/ritonavir and dasabuvir (OBV/PTV/r/DSV), daclatasvir (DCV), simeprevir (SMV), sofosbuvir (SOF), and the combination of sofosbuvir/ledipasvir (SOF/LDV) and grazoprevir/elbasvir (GZV/EBV). Other drugs have been accepted in North America, Europe, and Asia, and although not yet available in Mexico, their process of acceptance has begun. One such case is sofosbuvir/velpatasvir (SOF/VEL). Therefore, the consensus working group decided to include the drugs that are currently available in Mexico, as well as those that are in the process of acceptance, in its recommendations.
Double therapy based on pegIFN + RBV and the triple therapy with pegIFN + RBV + simeprevir are currently considered alternative therapies because their SVR rates are lower than those achieved with interferon-free regimens, and consequently, are recommended only in cases in which there are access limitations to the new DAA drugs.9,46,47 Notwithstanding, the consensus working group believes that the risk for progression in patients with fibrosis stages F3 or F4 must be considered when treatments with suboptimal efficacy are contemplated.9,46,47 Triple therapy with first generation protease inhibitors (boceprevir or telaprevir) is considered unacceptable because of the high percentage of adverse effects.9,46 The Mexican Consensus on Hepatitis C, previously published in 2015, provides a detailed description of IFN-based double therapy and triple therapy with simeprevir.45 It is important to specify that the majority of current treatments achieve SVR12 rates above 90%. Therefore, any treatment with lower SVR rates should be regarded as alternative therapy and recommended only in cases where access to therapeutic regimens with a high SVR rate is difficult.48
Genotype 1 infectionTherapies based on interferonThe consensus working group includes therapy based on pegIFN + RBV and SMV for 12 weeks in its recommendations.45
Recommendations- •
Regimens based on pegIFN/RBV or pegIFN/RBV/SMV are recommended in patients with no previous treatment history, without cirrhosis or with compensated cirrhosis, and only when there is no access to IFN-free regimens with direct-acting antivirals (A1).
Level of agreement: in complete agreement 92%.
In agreement with minor reservations: 8%.
- •
If there is no access to IFN-free regimens with direct-acting antivirals, regimens based on pegIFN/RBV or pegIFN/RBV/SMV are a therapeutic alternative, according to the 2015 Mexican Consensus on the Diagnosis and Management of Hepatitis C (A1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
Therapy with pegylated interferon plus ribavirin plus sofosbuvir- •
Patients with genotype 1 infection that do not have cirrhosis can be treated with a combination of weekly pegIFN, a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or ≥ 75kg, respectively), and sofosbuvir (400mg daily) for 12 weeks (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
The triple therapy regimen based on pegIFN, ribavirin, and sofosbuvir was evaluated in the NEUTRINO study49 that included treatment-naïve patients. The SVR in that study was 89% (259/291): 92% (207/225) in subtype 1a and 82% in subtype 1b (54/66). A lower SVR was observed in the patients with cirrhosis of the liver (80%), compared with patients that did not have cirrhosis (92%). The SVR in patients previously treated with pegIFN and ribavirin and treated with the above regimen was calculated using mathematical modeling, taking the NEUTRINO study results into account. Based on those variables, the mathematical model supposes a 78% SVR in patients with previous double-therapy failure.50 The SVR12 rate with the triple therapy based on pegIFN, RBV, and SOF was 79% in the patients that did not achieve SVR after receiving pegIFN, RBV, and a protease inhibitor under study.51 The TRIO study included real-life patients, 42% of whom were treatment-experienced and 58% treatment-naïve. SVR12 was 81% (112/138) in treatment-naïve patients that did not present with cirrhosis of the liver. In treatment-naïve patients with cirrhosis, SVR was 81% (25/31), and it decreased to 77% (30/39) in treatment-experienced patients with no cirrhosis, and to 62% (53/85) in treatment-experienced patients with cirrhosis.52
The C-EDGE study showed that a DAA regimen (grazoprevir/elbasvir) had a better safety profile and SVR12 rate than the triple therapy based on pegIFN + RBV + SOF.42 The C-EDGE is an important study because it compares an IFN-free therapy with an IFN regimen that contains a DAA that is not a protease inhibitor or an NS5A inhibitor. The SVR in genotype 1a was 100% in the patients treated with GZP/EBV (18/18) and in the group that received the triple therapy with pegIFN + RBV + SOF (17/17). The same as in the NEUTRINO study, SVR in genotype 1b was 90% (94/104) with the triple therapy based on pegIFN + RBV + SOF, which was lower than the SVR12 with the GZV/ EBV regimen (99%) (104/105).7,49 The SVR was even lower with the triple therapy of pegIFN + RBV + SOF. In patients with poor outcome factors, it was 89% (87/98) in those with the non-CC IL-28 B genotype, 76% (16/21) in those with cirrhosis of the liver, 50% (7/14) in nonresponders to pegIFN + RBV, and 88% (7/8) in partial responders to treatment.53
Triple therapy with pegIFN + RBV + SOF for 12 weeks is an alternative treatment when interferon-free DAA therapies are not available. It is important to be aware that SVR is lower in patients with genotype 1b and that said response decreases when there are poor outcome factors, such as patients with cirrhosis, or those with a history of prior pegIFN + RBV treatment failure.49,53
Interferon-free therapies (Tables 2 and 3)In accordance with that suggested by the consensus working group, the medications approved by the National Commission for Protection Against Health Risks (COFEPRIS, Spanish acronym) are listed, along with their level of evidence and strength of recommendation. The medications not yet available in Mexico are listed in the same manner, after the approved available drugs.
Treatment for genotype 1a.
| Regimen | GZR/EBR SVR above 95% | OBV/PTV/r/DSV SVR above 95% | SOF/LDV SVR above 95% | SOF/DCV SVR above 95% | SOF/VEL SVR above 95% |
|---|---|---|---|---|---|
| Tx-naïve, without cirrhosis | 12 weeks without RBV: A1 | 12 weeks with RBV: A1 | 8 weeks without RBV-B1, or 12 weeks without RBV: A1 | 12 weeks without RBV: A1 | 12 weeks without RBV: A1 |
| Tx-naïve, with cirrhosis | 12 weeks without RBV: A1 | 24 weeks with RBV: A1, or 12 weeks with RBV: A2 | 12 weeks without RBV: A1 | 24 weeks ± RBV: B1 | 12 weeks without RBV: A1 |
| Tx-experienced, without cirrhosis | 12 weeks without RBV or 16 weeks with RBVa: A1 | 12 weeks with RBV: A1 | 12 weeks without RBV: A1 | 12 weeks without RBV: A1 | 12 weeks without RBV: A1 |
| Tx-experienced, with cirrhosis | 12 weeks without RBV or 16 weeks with RBV: A1a | 24 weeks with RBV: A1, or 12 weeks with RBV: A2 | 12 weeks with RBV or 24 without RBV: B1 | 24 weeks ± RBV: B1 | 12 weeks without RBV: A1 |
| Regimen | Asunaprevir + daclatasvir SVR insufficient | Sofosbuvir/simeprevir SVR below 95% |
|---|---|---|
| Tx-naïve, without cirrhosis | Not recommended: A1 | 12 weeks without RBV: A1 |
| Tx-naïve, with cirrhosis | Not recommended: A1 | 24 weeks ± RBVb: B2 |
| Tx-experienced, without cirrhosis | Not recommended: A1 | 12 weeks without RBV: A1 |
| Tx-experienced, with cirrhosis | Not recommended: A1 | 24 weeks ± RBV: B2b |
A1, A2, B1, B2: level of evidence; GZR/EBR: grazoprevir/elbasvir; OBV/PTV/r/DSV: ombitasvir/paritaprevir/ritonavir/dasabuvir; RBV: ribavirin; SOF/LDV: sofosbuvir/ledipasvir; SVR: sustained virologic response; Tx: treatment.
12 weeks of treatment without RBV are recommended in patients with previous relapse with pegIFN and ribavirin (PR) and 16 weeks of treatment with ribavirin are recommended in null responders to PR, with or without cirrhosis + AFP (alpha-fetoprotein) under 20 ng/ml, platelets above 90 k, and albumin above 2.5g/dl.
Treatment for genotype 1b.
| Regimen | GZR/EBR SVR above 95% | OBV/PTV/r/DSV SVR above 95% | SOF/LDV SVR above 95% | SOF/DCV SVR above 95% | SOF/VEL SVR above 95% |
|---|---|---|---|---|---|
| Tx-naïve, without cirrhosis | 12 weeks without RBV or 8 weeks without RBVa: A1 | 8b,c or 12 weeks without RBV: A1 | 8 weeks without RBVd: B1, or 12 weeks without RBV: A1 | 12 weeks without RBV: A1 | 12 weeks without RBV: A1 |
| Tx-naïve, with cirrhosis | 12 weeks without RBV: A1 | 12 weeks without RBV: A1 | 12 weeks without RBV: A1 | 24 weeks ± RBV: B1c | 12 weeks without RBV: A1 |
| Tx-experienced, without cirrhosis | 12 weeks without RBV: A1 | 12 weeks without RBV: A1 | 12 weeks without RBV: A1 | 12 weeks without RBV: A1 | 12 weeks without RBV: A1 |
| Tx-experienced, with cirrhosis | 12 weeks without RBV: A1 | 12 weeks without RBV: A1 | 12 weeks with RBV or 24 weeks without RBV: B1 | 24 weeks ± RBV: B1c | 12 weeks without RBV: A1 |
| Regimen | Asunaprevir+ daclatasvir SVR below 95% | Sofosbuvir/simeprevir SVR below 95% |
|---|---|---|
| Tx-naïve, without cirrhosis | 24 weeks without RBV: A1 | 12 weeks without RBV: A1 |
| Tx-naïve, with cirrhosis | 24 weeks without RBV without NS5A RAVs LK31/Y93: B2 | 24 weeks ± RBV: B2 |
| Tx-experienced, without cirrhosis | Not recommended: A2 | 12 weeks without RBV: A1 |
| Tx-experienced, with cirrhosis | Not recommended: A2 | 24 weeks ± RBV: B2 |
A1, A2, B1: level of evidence; GZR/EBR: grazoprevir/elbasvir; OBV/PTV/r/DSV: ombitasvir/paritaprevir/ritonavir/dasabuvir; RBV: ribavirin; SOF/DCV: sofosbuvir/daclatasvir; SOF/LDV: sofosbuvir/ledipasvir; SOF/VEL: sofosbuvir/velpatasvir; SVR: sustained virologic response; Tx: treatment; RAV: resistance-associated variant.
- •
Patients with HCV genotype 1b infection can be treated with a combination of daclatasvir (60mg once-daily) + asunaprevir (100mg twice-daily) for 24 weeks (A1).
Level of agreement: in complete agreement 88%.
In agreement with minor reservations: 12%
- •
Patients with genotype 1b that are treatment-naïve, do not have cirrhosis or have compensated cirrhosis, and do not have the resistance-associated variants (RAVs), NS5A-L31 and NS5A-Y93, should receive a 24-week regimen (B2).
Level of agreement: in complete agreement 92%.
In agreement with minor reservations: 8%.
- •
Patients with genotype 1b that are treatment-experienced, have cirrhosis, or are IFN-intolerant (depression, anemia, neutropenia, and thrombocytopenia) are not considered good candidates for that regimen (A2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Treatment failure with that regimen can lead to a risk for developing variants that are resistant to rescue therapy (C1).
Level of agreement: in complete agreement 90%.
In agreement with minor reservations: 10%.
The daclatasvir (60mg once-daily) plus asunaprevir (100mg twice-daily) regimen for 24 weeks is a treatment option in patients with chronic hepatitis infected with genotype 1b. The HALLMARK-DUAL study included treatment-naïve patients infected with genotype 1b, nonresponders to prior treatment with pegIFN/RBV, as well as patients that were ineligible for or did not tolerate pegIFN, which included patients with depression, anemia < 8.5mg/dl, neutropenia < 1.5 cells/l, or F3/F4 fibrosis with thrombocytopenia < 90,000 platelets. The SVR12 reported in treatment-naïve patients was 91% (182/293), 82% (168/205) in the patients that were nonresponders to prior treatment, and 83% (192/235) in the patients with pegIFN intolerance. SVR12 in the patients with cirrhosis was: 91% (29/32) in the treatment-naïve patients, 87% (55/63) in the nonresponders, and 79% (88/111) in the patients that are ineligible for or intolerant to pegIFN.54
Twenty-seven of the 596 patients (5%) presented with the NS5A-L31 polymorphism, and of those patients, only 41% achieved SVR12. Likewise, 48 patients (8%) (48/596) had the NS5A-Y93 polymorphism, of which only 38% (18 patients) achieved SVR12.
SVR12 was achieved in 39% (29/75) of the patients that had the NS5A L31 and Y93 polymorphisms, compared with the 92% SVR12 in the patients without the RAVs (478/521).54
The AI447-026 study included treatment-naïve patients with genotype 1b infection that were ineligible for or had intolerance to IFN and those that were nonresponders to previous treatment. SVR12 was 88% (119/135) in the treatment-naïve IFN-ineligible or intolerant patients and 80.5% (70/87) in the nonresponders to prior treatment.55
In an analysis of several clinical trials with DCV/ASV for 24 weeks, SVR12 was evaluated in patients with genotype 1b with and without cirrhosis. Treatment-naïve patients, patients that were IFN-ineligible or intolerant, and patients that were nonresponders were included. The general SVR12 in patients with cirrhosis was 84% (192/228). It was 91% (29/32) in the treatment-naïve group with cirrhosis, 80% (98/122) in the IFN-ineligible or intolerant group, and 88% (65/74) in the nonresponders to prior treatment.
The SVR12 results in the patients that did not have cirrhosis were: a general SVR12 of 85% (539/637), 89% (152/171) in the treatment-naïve patients, 86% (213/248) in patients that were ineligible for or intolerant to IFN, and 79% (173/218) in the nonresponders.56
SVR12 in the patients with cirrhosis that were IFN-intolerant due to depression and thrombocytopenia associated with advanced fibrosis was 73% (11/15) and 76% (53/70), respectively. SVR12 in the patients with anemia and neutropenia that were ineligible for IFN was 92% (24/26).56
RecommendationsGrazoprevir/elbasvir- •
Patients with HCV genotype 1 infection can be treated with the fixed-dose combination of grazoprevir (100mg) and elbasvir (50mg) in one tablet administered once-daily (A1).
Level of agreement: in complete agreement 88%.
In agreement with minor reservations: 12%.
- •
Patients with genotype 1b that are treatment-naïve or treatment-experienced and that do not have cirrhosis or have compensated cirrhosis, should be treated with the 12-week regimen (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Patients with genotype 1a that are treatment-naïve or treatment-experienced, that do not have cirrhosis or have compensated cirrhosis, and in whom there is no possibility of NS5A RAV testing, should receive the 12-week treatment regimen, if their HCV RNA < 800,000 IU/ml. If their HCV RNA ≥ 800,000 IU/mL, then treatment should be the 16-week regimen + ribavirin (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Patients with genotype 1a that are treatment-naïve or treatment-experienced, that do not have cirrhosis or have compensated cirrhosis, that have the NS5A RAVs, and their HCV RNA ≥ 800,000 IU/ml should be treated with the 16-week regimen + ribavirin (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
Recommendations for treatment with the fixed-dose combination of grazoprevir (100mg) / elbasvir (50mg) for 12 weeks without ribavirin in patients with genotype 1a or 1b, with or without previous treatment, with no cirrhosis or with compensated cirrhosis, are based on the results of the C-EDGE-TN and C-EDGE-TE phase III studies. In the C-EDGE-TN study, the SVR12 was 92% (144/157) and 99% (129/131) in genotype 1a and 1b, respectively. The presence of cirrhosis did not affect the efficacy of that treatment regimen.42
In the C-EDGE-TE study that included treatment-experienced patients, 34% of whom presented with compensated cirrhosis, the SVR12 in patients with genotype 1a and those with genotype 1b was 92% (55/60) and 100% (34/34), respectively, with the combination treatment of GZV/EBV for 12 weeks without RBV and 93% (56/60) and 97% (28/29), respectively, after 12 weeks with RBV. When treatment was extended to 16 weeks, the SVR12 in the patients with genotype 1a was 100% (55/55) and 94% (45/48), with and without RBV, respectively, and was 100% (37/37) and 98% (46/47), with and without RBV, respectively, in the patients with genotype 1b.57
In an integrated response predictor analysis of phase II and phase III studies on a total of 1,408 patients with genotype 1a that received GZV/EBV for 12 weeks ± RBV, the only variables associated with a lower SVR12 rate in patients with or without previous treatment, and without cirrhosis or with compensated cirrhosis, were the baseline levels of HCV RNA ≥ 800,000 IU/ml and the presence of NS5A RAVs.
Therefore, we recommend that NS5A RAV testing be carried out on all patients with an HCV RNA load > 800,000 IU/ml. In patients with NS5A RAVs or in those in whom the presence of NS5A RAVs cannot be determined, the fixed-dose combination of GZV/EBV for 16 weeks with RBV is recommended.57,58
RecommendationsOmbitasvir/paritaprevir/ritonavir plus dasabuvir- •
Patients with HCV genotype 1 infection can be treated with an IFN-free regimen with the fixed-dose combination of ombitasvir (12.5mg), paritaprevir (75mg), and ritonavir (50mg) in a single tablet (taken once-daily with food) plus dasabuvir (250mg) (one tablet twice-daily) (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Patients with genotype 1b that do not have cirrhosis or have compensated cirrhosis should receive a regimen of ombitasvir, paritaprevir, and ritonavir plus dasabuvir for 12 weeks with no ribavirin (A1).
Level of agreement: in complete agreement 83%.
In agreement with minor reservations: 17%.
- •
Patients with genotype 1a that do not have cirrhosis should receive that combination daily for 12 weeks with a body weight-adjusted dose of ribavirin (1,000 or 1,200mg in patients < 75kg or ≥ 75kg, respectively) (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
In patients with genotype 1a that have compensated cirrhosis, that combination for 24 weeks with a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or ≥ 75kg, respectively) is recommended (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
The regimen can be shortened to 12 weeks in patients with genotype 1a that are treatment-naïve or treatment-experienced, that have compensated cirrhosis, and that present with pre-treatment levels of three baseline response predictors: alpha-fetoprotein (AFP) < 20 ng/ml, platelets ≥ 90 x 109/l, and albumin ≥ 3.5g/dl (A2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
The indication for the ombitasvir/paritaprevir/ritonavir (25/150/100mg once-daily) plus dasabuvir (250mg twice-daily) (OBV/PTV/r/DSV) regimen used in patients with genotype 1 is based on numerous clinical trials. It is contraindicated in patients with decompensated cirrhosis of the liver.
In the SAPPHIRE I study, treatment-naïve patients with no cirrhosis received that regimen plus RBV for 12 weeks. The patients with genotype 1a achieved 95% SVR12 (307/322) and those with genotype 1b reached 98% (148/151).59 The use of that regimen with RBV was evaluated in the PEARL IV study in patients with genotype 1a with no previous treatment and no cirrhosis, resulting in 90% SVR12 (185/205) without RBV and 97% (97/100) with RBV. The PEARL III study showed that patients with genotype 1b with no previous treatment and no cirrhosis that received OBV/PTV/r/DSV with or without RBV, achieved 99% SVR12 (207/209) without RBV and 99% (209/201) with RBV.60
The results of the MALACHITE-I study were 97% SVR12 (67/69) in patients with genotype 1a with no previous treatment and no cirrhosis, treated with that regimen plus RBV for 12 weeks. and 98% SVR12 (81/83) in patients with genotype 1b, treated without RBV for 12 weeks.61
The GARNET study provided recent evidence showing that patients with genotype 1b with no cirrhosis and a METAVIR F0 to F3 fibrosis grade, treated with OBV/PTV/r/DSV without RBV for 8 weeks, achieved 97% SVR12 (161/166). Of the 15 patients with F3 in that study, 13 achieved SVR12. Thus, shortening treatment without RBV to 8 weeks can be considered in treatment-naïve patients with genotype 1b and a F0 to F2 grade of fibrosis.62
In the SAPPHIRE II study, patients that did not present with cirrhosis and were nonresponders to previous treatment with pegIFN + RBV were treated with OBV/PTV/r/DSV plus RBV. The patients with genotype 1a achieved an SVR12 rate of 96% (166/173), and in those with genotype 1b, SVR12 was 97% (119/123). The SVR12 according to the type of response to the previous treatment was 95% (139/146) in the nonresponders, 95% (82/86) in patients with relapse, and 100% (65/65) in the partial responders.63
In the PEARL II study, patients with genotype 1b with no cirrhosis that did not respond to previous treatment with pegIFN and RBV achieved 100% SVR12 (91/91) without RBV and 97% (85/88) with RBV.64
In the MALACHITE II study on patients with no cirrhosis and with genotype 1a or 1b that were nonresponders to previous treatment, there was 99% SVR12 (100/101) with OBV/PTV/r/DSV plus RBV.61
Treatment-naïve patients and patients with compensated cirrhosis that did not respond to pegIFN and RBV were treated in the TURQUIOSE II study with the OBV/PTV/r/DSV plus RBV regimen for 12 or 24 weeks. Patients that received the 12-week regimen had 92% SVR12 (191/208) and those with the 24-week treatment had 96% (165/172). According to genotype, the patients with genotype 1a had 92% SVR12 (239/261) and those with genotype 1b had 99% (118/119).65
In a sub-analysis of the TURQUIOSE II study, the possibility of shortening treatment to 12 weeks was described in patients with genotype 1a and compensated cirrhosis that presented with the following factors: AFP below 20 ng/ml, platelets above 90 x 109/l, and albumin above 3.5g/dl.65
The TURQUOISE III study reported 100% SVR12 (60/60) with a 12-week regimen with no RBV in patients with genotype 1b, with or without previous treatment, and with compensated cirrhosis.66
RecommendationsSofosbuvir/daclatasvir- •
Patients with HCV genotype 1 infection can be treated with an IFN-free combination of sofosbuvir (400mg) and daclatasvir (60mg), daily (A1).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
- •
Patients with genotype 1a or genotype 1b infection, that are treatment-naïve or treatment-experienced, and that do not have cirrhosis should be treated with the 12-week regimen (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Patients with genotype 1a or genotype 1b infection, that are treatment-naïve or treatment-experienced, and that have cirrhosis should be treated with the 24-week regimen ± ribavirin (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
The daily-dose combination treatment of daclatasvir (60mg) and sofosbuvir (400mg) can be recommended, based on the ALLY-2 phase IIb study and the ALLY-1 phase III study. In the phase IIb study, utilizing the DCV/SOF combination in patients with no cirrhosis, the following results were obtained: in treatment-naïve patients with 24 weeks of DCV/SOF ± RBV combination therapy, 100% SVR12 (14/14) was achieved in patients with no RBV and 100% (15/15) in patients with RBV. In patients that did not respond to previous treatment with pegIFN + RBV + telaprevir or boceprevir, SVR was 100% (21/21) with 24 weeks of treatment with daclatasvir/sofosbuvir with no ribavirin and 95% (20/21) with ribavirin. In the third arm of that study, SVR12 was 100% (40/40) in treatment-naïve patients after 12-week treatment with DCV/SOF and no RBV.67
In the ALLY-2 phase III study, the DCV/SOF combination was administered for 12 weeks in patients with genotypes 1-4 coinfected with HIV. Of that cohort, 123 patients had genotype 1 infection, 83 of whom were treatment-naïve. Patients with and without previous treatment achieved 98% SVR12 (43/44) and 96% (80/83), respectively. Of those patients, 104 with genotype 1a had 96% SVR12 (100/104) and 23 with genotype 1b had 100% SVR12 (23/23).12
In the ALLY-1 phase III study, in 45 patients with genotype 1 and advanced cirrhosis, 34 had genotype 1a infection and 11 had genotype 1b. Treatment with DCV/SOF + RBV (initial dose of 600mg, later titrated) was administered for 12 weeks. The patients with genotype 1a achieved 76% SVR12 (26/34), whereas those with genotype 1b achieved 100% SVR12 (11/11).68
Because the patients with compensated cirrhosis were not adequately represented in any of the 3 studies mentioned above, treatment duration was not clearly determined. In an analysis of a patient cohort from a European compassionate use program, it is suggested that treatment should be prolonged to 24 weeks with or without RBV in patients with compensated cirrhosis.69
RecommendationsSofosbuvir/Ledipasvir- •
Patients with HCV genotype 1 infection can be treated with the combination of sofosbuvir (400mg) and ledipasvir (90mg) in a single tablet once-daily (A1).
Level of agreement: in complete agreement 86%.
In agreement with minor reservations: 14%.
- •
In patients with genotype 1a or 1b that are treatment-naïve, do not have cirrhosis, or have compensated cirrhosis, the fixed-dose combination of one tablet of sofosbuvir/ledipasvir daily with no ribavirin for 12 weeks is recommended (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
In patients with genotype 1a or 1b that are treatment-experienced and do not have cirrhosis, the fixed-dose combination of one tablet of sofosbuvir/ ledipasvir daily with no ribavirin for 12 weeks is recommended (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
In patients with genotype 1a or 1b that are treatment-experienced and have compensated cirrhosis, the fixed-dose combination of one tablet of sofosbuvir/ledipasvir daily with ribavirin for 12 weeks (A1) or for 24 weeks with no RBV is recommended (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
In treatment-naïve patients with genotype 1a or 1b that do not have cirrhosis, in whom an F0-F2 grade of fibrosis is determined through a reliable invasive or noninvasive study, and whose HCV RNA level is < 6,000,000 IU/ml (6.8 log), the fixed-dose combination of a single tablet of sofosbuvir/ledipasvir daily can be reduced to 8 weeks, with no ribavirin (B1).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
The phase III ION-1, ION-2, and ION-3 studies showed that the combination of sofosbuvir and ledipasvir is safe and effective for the treatment of patients with genotype 1a or 1b HCV infection, with or without cirrhosis, with compensated or decompensated cirrhosis, and with or without a history of previous treatment.70–72 The ION-1 study included patients with no history of previous treatment and only 16% with compensated cirrhosis. In the ION-1, SVR12 was 99% (211/214) with 12 weeks of treatment without ribavirin and 97% (211/217) with 12 weeks of treatment with ribavirin. Response at 24 weeks with the same treatment regimen was 99% (215/217) with RBV and 98% (212/217) without RBV.70
In the ION-3 study, patients with no history of previous treatment, with no cirrhosis, and with an F0-F2 grade of fibrosis achieved 94% SVR12 (201/215) with 8 weeks of the combination treatment with no RBV, 93% (201/216) with 8 weeks with RBV, and 95% (205/216) with 12 weeks with no RBV. Even though the differences in SVR in the three groups were not statistically significant, the absolute number of relapses after treatment completion was higher in the patients treated for 8 weeks.72 A secondary analysis indicated that only patients with an HCV RNA level < 6 million U/l (6.8 log) could be treated for 8 weeks. The relapse rate in patients treated for 8 weeks was lower in women than in men. The relapse rate was 1% (1/84) and 1% (1/96) in women treated with SOF/LDV with and without RBV, respectively, and 8% (10/129) and 7% (8/114) in men treated with the same regimen with and without ribavirin.72 The results of the ION-3 study were confirmed in real-world studies in Europe and the United States that included 4 different groups of patients with 95 to 99% SVR12 rates.73
Patients previously treated with pegIFN + RBV or pegIFN + RBV plus telaprevir or boceprevir, and 20% of whom had cirrhosis, were included in the ION-2 study. SVR12 was 94% (102/109) with 12 weeks with no RBV and 96% (107/111) with 12 weeks with RBV. However, SVR improved to 99% in the two groups with 24 weeks of treatment with or without RBV.74
An analysis of 513 patients with genotype 1 infection with compensated cirrhosis treated with the combination of SOF/LDV, with or without RBV, that were included in different phase II and phase III studies, showed 95% SVR12 (305/322) with 12 weeks of treatment and 98% (188/191) with 24 weeks. In that study, neither duration (12 or 24 weeks) nor RBV administration impacted SVR in treatment-naïve patients. However, in treatment-experienced patients, SVR12 was 90% with 12 weeks of treatment with no RBV, 96% with 12 weeks with RBV, 98% with 24 weeks with no RBV, and 100% with 24 weeks with RBV.75 In the SIRIUS study that included patients with compensated cirrhosis and previous treatment failure with the triple-regimen of pegIFN + RBV + telaprevir or boceprevir, SVR12 was 96% (74/77) with the combination for 24 weeks with RBV and 97% (75/77) with the combination with no RBV.76
RecommendationsSofosbuvir / simeprevir- •
Patients with HCV genotype 1 infection that do not have cirrhosis can be treated with an IFN-free combination of sofosbuvir (400mg/24h) and simeprevir (150mg/24h), one tablet of each every 24h for 12 weeks (A1).
Level of agreement: in complete agreement 95%.
In agreement with minor reservations: 5%.
- •
Patients with genotype 1a and 1b that are treatment-naïve or treatment-experienced, do not have cirrhosis, and have or do not have the Q80K polymorphism should receive that regimen for 12 weeks (A1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
- •
Patients with genotype 1a that are treatment-naïve or treatment-experienced, have compensated cirrhosis, and do not have the Q80K polymorphism should receive that regimen for 24 weeks ± ribavirin (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Treatment-experienced patients with genotype 1b that have compensated cirrhosis should receive that regimen for 24 weeks ± ribavirin (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
The combination of simeprevir (150mg) and sofosbuvir (400mg) for the treatment of patients with genotype 1 HCV infection was evaluated in the OPTIMIST-1 and OPTIMIST-2 studies. A total of 310 patients were included in the OPTIMIST-1 study. They did not present with cirrhosis and were either treatment-naïve or treatment-experienced. They randomly received treatment with sofosbuvir and simeprevir for 12 weeks or 8 weeks. SVR was 97% (150/155) with 12 weeks of treatment and 83% (128/155) with 8 weeks of treatment. SVR was similar in the patients with or without a history of previous treatment that received 12 weeks of simeprevir + sofosbuvir. There was also no difference in SVR in the patients with genotype 1a with the presence of the Q80K mutation.77
The combination of simeprevir and sofosbuvir in patients with cirrhosis, with or without previous treatment, was assessed in the OPTIMIST-2 study. A total of 103 patients were treated for 12 weeks with a daily dose of 150mg of simeprevir and 400mg of sofosbuvir. Treatment-naïve patients achieved 88% SVR (44/50) and treatment-experienced patients achieved 79% (42/53). SVR was similar in patients with genotype 1a and those with genotype 1b, with no presence of Q80K mutation, at 84% (26/31) and 92% (35/38), respectively. Due to the low response rates in the patients with previous treatment failure, extending that combination treatment to 24 weeks, with or without ribavirin, is recommended. Patients with genotype 1a with cirrhosis and the presence of the Q80K mutation are not considered candidates for receiving the simeprevir/sofosbuvir combination.15,78
RecommendationsSofosbuvir / velpatasvir- •
Patients with HCV genotype 1 infection can be treated with a fixed-dose combination of sofosbuvir (400mg) and velpatasvir (100mg) in one tablet administered once-daily (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
That treatment without ribavirin is recommended for 12 weeks in patients with genotype 1a infection or genotype 1b infection, with or without previous treatment, and with compensated cirrhosis (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
Those recommendations are based on the results of the phase III ASTRAL-1 study, in which patients with genotype 1 HCV infection (22% with cirrhosis, 66% treatment-naïve, 34% treatment-experienced, 44% that had been exposed to direct-acting antivirals) were studied. Those patients were treated with a fixed-dose combination of SOF/VEL for 12 weeks with no RBV.79 SVR12 was 98% (323/328) in the total patient group, 98% (206/210) in the patients with genotype 1a infection, and 99% (117/118) in those with genotype 1b infection.
In the ASTRAL-5 study, patients with HIV infection treated with the same regimen and infected with genotype 1a achieved 95% SVR (62/65) and those with genotype 1b had 92% SVR (11/12). SVRs were 100% (19/19) and 94% (80/85) in patients with or without cirrhosis, and 93% (71/75) and 97% (28/29) in treatment-naïve and treatment-experienced patients, respectively.80
Genotype 2 infectionThere are different treatment options for patients with genotype 2 infection, two of which are first-line: the combination of sofosbuvir and velpatasvir (a combination not yet available in Mexico) and the combination of sofosbuvir and daclatasvir.9,46 Other treatment options can be evaluated with the following evidence:
RecommendationInterferon therapies- •
Patients with compensated cirrhosis and/or treatment-experienced patients can be treated with the combination of weekly pegIFN-α plus a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or ≥ 75kg, respectively) plus sofosbuvir (400mg) for 12 weeks (B1).
Level of agreement: in complete agreement 87%.
In agreement with minor reservations: 13%.
In patients with previous treatment failure, particularly those with cirrhosis, the inclusion of treatment with pegIFN has been evaluated. The LONESTAR-2 study assessed the combination of pegIFN 180μg weekly, sofosbuvir 400mg/daily, and ribavirin by kilogram of weight divided into 2 doses for 12 weeks. Cirrhosis was present in 61% of the patients and SVR at 12 weeks was 96%.81
Recommendations (see Table 4)Sofosbuvir and daclatasvir- •
Treatment-naïve patients and with no cirrhosis can be treated with the combination of sofosbuvir (400mg) plus daclatasvir (60mg) daily for 12 weeks (B1).
Level of agreement: in complete agreement 100%.
Treatment for genotype 2.
| Regimen | SOF + DCV, SVR above 95% | SOF + RBV, SVR below 95% | SOF/ VEL, SVR above 95% |
|---|---|---|---|
| Tx-naïve, without cirrhosis | 12 weeks without RBV: B1 | 12 weeks: A1 | 12 weeks without RBV: A1 |
| Tx-naïve, with cirrhosis | 12 weeks without RBV: B1 | 16 to 20 weeks: B1 | 12 weeks without RBV: A1 |
| Tx-experienced, without cirrhosis | 12 weeks without RBV: B1 | 12 weeks: A1 | 12 weeks without RBV: A1 |
| Tx-experienced, with cirrhosis | 24 weeks without RBV: B2 | 16 to 20 weeks: B1 | 12 weeks without RBV: A1 |
A1, B1, B2: level of evidence; SOF/DCV: sofosbuvir/daclatasvir; SOF/RBV: sofosbuvir/ribavirin; SOF/VEL: sofosbuvir/velpatasvir; SVR: sustained virologic response; Tx: treatment;
In agreement with minor reservations: 0%.
The data from in vitro action of daclatasvir against HCV has been confirmed in clinical practice. The combination of 60mg of daclatasvir plus 400mg of sofosbuvir for 12 weeks demonstrated its efficacy, achieving eradication of the virus in treatment-naïve patients with genotype 2 infection, with a response of 98% upon treatment completion.67
Recommendation- •
Treatment-naïve patients with no cirrhosis or with compensated cirrhosis should receive the combination of sofosbuvir (400mg) plus daclatasvir (60mg) for 12 weeks (B2).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
Extending the 12-week combination treatment of daclatasvir plus sofosbuvir in treatment-naïve patients with compensated cirrhosis to 24 weeks has been associated with 100% SVR.67
RecommendationsSofosbuvir and ribavirin- •
The combination of sofosbuvir 400mg plus body-weight based ribavirin (1,000 or 1,200mg in patients < 75kg or ≥ 75kg, respectively) can be used for a period of 12 weeks (A1).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
Sofosbuvir 400mg daily combined with body weight-adjusted ribavirin in treatment-naïve patients with genotype 2 infection is supported by the FISSION, POSITRON, and VALANCE studies. In the FISSION study, patients were randomized to receive pegIFN and ribavirin 800mg daily for 24 weeks or sofosbuvir 400mg and weight-adjusted ribavirin for 12 weeks.49 Patients under treatment with sofosbuvir and ribavirin had 97% SVR vs 78% SVR in patients under treatment with pegIFN and ribavirin. Combining the results of the 3 studies, the SVR rate was 94%.82–84
Recommendations- •
Therapy should be extended from 16 to 24 weeks in patients with cirrhosis, especially if they are treatment-experienced (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
There are no data determining the extension of therapy that impacts SVR in patients with compensated cirrhosis, with or without previous treatment. Even though experience comes from a reduced number of patients enrolled in clinical studies on patients with cirrhosis infected with genotype 2, the data and results are in favor of extending therapy from 12 to 16 weeks.83,85
The FUSION study reported SVR above 60 to 78% when therapy was extended from 12 to 16 weeks.83 In contrast, the BALANCE study reported a higher SVR rate in patients treated only for 12 weeks (88%).84
RecommendationSofosbuvir and velpatasvir- •
Patients with HCV genotype 2 infection can be treated with a fixed-dose combination of sofosbuvir (400mg) and velpatasvir (100mg) in one tablet, administered once-daily (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
That therapeutic option is based on the results of the ASTRAL-2 phase III study on patients with genotype 2 infection. Eighty-six percent of the patients evaluated were treatment-naïve and 14% had compensated cirrhosis, 14% of whom were treatment-experienced. SVR of 99% was achieved upon completion of the 12 weeks of treatment (133 of the 134 patients included), thus confirming the effectiveness of said treatment in patients with no cirrhosis, with or without previous treatment, as well as in patients with compensated cirrhosis, with or without previous treatment.86
Recommendation- •
Treatment-naïve or treatment-experienced patients with no cirrhosis or with compensated cirrhosis can be treated with a fixed-dose combination of sofosbuvir and velpatasvir for 12 weeks with no ribavirin (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
In the ASTRAL 2 study, the 12-week regimens of sofosbuvir/velpatasvir vs sofosbuvir and ribavirin were compared, resulting in SVR of 99% vs 94%. The ASTRAL 1 study evaluated 104 treatment-naïve patients, with or without cirrhosis, observing 100% SVR.79,86
Treatment of genotype 3-6 HCVGenotype 3 infectionRecommendationsInterferon therapies- •
Patients with HCV genotype 3 infection can be treated with the combination of weekly pegIFN-α plus a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or 75kg, respectively) plus daily sofosbuvir (400mg) for 12 weeks (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
That combination is a useful alternative in patients that did not previously reach SVR with the combination of sofosbuvir plus ribavirin (B1).
Level of agreement: in complete agreement 92%.
In agreement with minor reservations: 8%.
In a randomized, multicenter, phase II clinical trial by Lawitz et al.87 that included 212 treatment-naïve patients with genotype 1, 2, or 3 HCV, they evaluated the efficacy and tolerability of sofosbuvir 200mg (n = 48), sofosbuvir 400mg (n = 48), or placebo (n = 26) in combination with pegIFN (180μg per week) and weight-adjusted RBV, respectively. There was SVR at 12 weeks in 43/48 patients (90%) from the group that received sofosbuvir 200mg, in 43/47 (91%) from the group that received sofosbuvir 400mg, and in 15/26 (58%) from the placebo group.
The results of a randomized, multicenter, phase III clinical trial that evaluated the efficacy and safety of sofosbuvir and RBV, with or without pegIFN-α in patients with genotype 3 HCV, with or without previous treatment, showed an SVR rate at 12 weeks of 71% and of 84% in the patients that received 16 and 24 weeks of sofosbuvir and RBV, respectively. SVR was significantly higher (93%) in the patients that received the triple therapy with sofosbuvir, pegIFN, and RBV.85
For treatment-naïve patients with no cirrhosis, that combination has shown up to 92% effectiveness, according to the study by Lawitz, but only 10 patients with genotype 3 infection were included in that cohort.87
In an open and nonrandomized clinical trial by Lawitz et al.88 on patients with cirrhosis of the liver, 24 patients with genotype 3 were included. All of them had previous treatment failure and half had cirrhosis. The efficacy and safety of SOF + pegIFN + RBV was measured for 12 weeks. SVR12 in the group with genotype 3 was 83%, with no significant difference between patients with or without cirrhosis of the liver.
In the study by Esteban et al., 91% (20/22) of the patients with genotype 3 infection that did not achieve SVR with sofosbuvir and ribavirin treatment, achieved SVR when re-treated with the triple combination of pegIFN-α, ribavirin, and sofosbuvir for 12 weeks. Therefore, that combination is a useful alternative in patients that did not achieve SVR when previously treated with sofosbuvir plus ribavirin.89
Recommendations (see Table 5)Sofosbuvir and daclatasvir- •
Patients with genotype 3 infection with no cirrhosis can be treated with an IFN-free combination of sofosbuvir (400mg/24h) and daclatasvir (60mg/24h) for 12 weeks (A1).
Level of agreement: in complete agreement 91%.
Treatment for genotype 3.
| Regimen | SOF + DCV, SVR above 95% | SOF + RBV, SVR below 95% | SOF/VEL, SVR above 95% |
|---|---|---|---|
| Tx-naïve, without cirrhosis | 12 weeks without RBV: A1 | 24 weeks: A1 | 12 weeks without RBV: A1 |
| Tx-naïve, with cirrhosis | 24 weeks with RBV: B1 | 24 weeks: B1 | 12 weeks with RBV: A1 |
| Tx-experienced, without cirrhosis | 12 weeks without RBV: A1 | 24 weeks: B1 | 12 weeks with RBV: A1 |
| Tx-experienced, with cirrhosis | 24 weeks with RBV: B1 | 24 weeks: B1 | 12 weeks with RBV: A1 |
A1, B1: level of evidence; SOF + DCV: sofosbuvir and daclatasvir; SOF + RBV: sofosbuvir and ribavirin; SOF/VEL: sofosbuvir and velpatasvir; SVR: sustained virologic response; Tx: treatment
GZR/EBR + SOF: treatment-experienced patients with cirrhosis, not discussed in the consensus.
In agreement with minor reservations: 9%.
- •
Treatment-naïve or treatment-experienced patients with HCV genotype 3 infection with cirrhosis should receive that combination with a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or ≥ 75kg, respectively) for 24 weeks (B1).
Level of agreement: in complete agreement 90%.
In agreement with minor reservations: 10%.
In the phase IIb open study by Sulkowski et al.67 on patients with no cirrhosis and with previous treatment failure, 18 patients with genotype 3 infection achieved 89% SVR (16/18) with the combination of sofosbuvir (400mg/24h) and daclatasvir (60mg/24h) for 24 weeks.
In the ALLY-3 study, the efficacy of daclatasvir + sofosbuvir for 12 weeks was evaluated in patients with genotype 3 HCV, with or without previous treatment, and with or without cirrhosis of the liver. In the treatment-naïve patients with no cirrhosis, SVR was 97%, compared with 58% in the treatment-naïve patients with cirrhosis. In the group of treatment-experienced patients with no cirrhosis, SVR was 94% and it was 69% in the treatment-experienced group with cirrhosis. Therefore, it is recommended to add ribavirin and extend treatment to 24 weeks in patients with genotype 3 infection with cirrhosis, whether treatment-naïve or with previous treatment failure, given that the response in that group was suboptimal.90
RecommendationsSofosbuvir and ribavirin- •
Patients with genotype 3 infection with no cirrhosis can be treated with an IFN-free combination of a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or 75kg, respectively) plus sofosbuvir (400mg daily) for 24 weeks (A1).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
- •
That therapy is suboptimal in treatment-experienced cirrhotic patients and in patients that did not reach SVR after a previous treatment with SOF/RBV. An alternative treatment option should be offered to those patients (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
The combination of SOF/RBV for patients with genotype 3 infection has been evaluated in 4 phase III clinical trials. In the FISSION study, the effectiveness and safety of the combination of sofosbuvir with ribavirin was assessed in patients with HCV and genotype 2 or 3. The patients were randomly assigned to receive either 12 weeks of sofosbuvir plus ribavirin or 24 weeks of pegIFN-α-2a plus ribavirin. The SVR rate in the patients that received sofosbuvir-ribavirin was lower in those with genotype 3 infection than in the patients with genotype 2 infection (56% vs 97%), and lower in the patients with cirrhosis than in those with no cirrhosis (47% vs 72%). SVR in the genotype 3 patients was similar to that with the traditional dual therapy of pegIFN-ribavirin (67%).49
It was concluded from 2 randomized phase III clinical trials (POSITRON and FUSION) that the administration of sofosbuvir and ribavirin (n = 207) or placebo (n = 71) for 12 weeks in patients with genotype 2 or 3 HCV in whom pegIFN was not an option, achieved a 78% SVR rate, compared with 0% with placebo (p < 0.001). In the second study, patients that had not had a response to treatment with pegIFN were included, receiving sofosbuvir and ribavirin for 12 weeks (103 patients) or 16 weeks (98 patients). There was 50% SVR at 12 weeks, compared with 73% in the patients treated for 16 weeks. Efficacy was greater in the patients with genotype 2 infection with no cirrhosis of the liver. That study suggested that a longer period of virologic suppression could be necessary to eliminate the residual viral reservoirs in patients with genotype 3 HCV. That observation was supported by the fact that extending treatment duration (from 12 to 16 weeks) significantly improved the SVR rates of the patients with genotype 3 infection (overall genotype 3 SVR of 30% vs 62% at 12 and 16 weeks, respectively; and in the genotype 3 patients with cirrhosis, 19% vs 61% at 12 and 16 weeks, respectively).83
In the VALENCE clinical trial, the SVR rate was 94% (86/92) after 24 weeks of treatment in the patients with no cirrhosis that were treatment-naïve, 92% (12/13) in treatment-naïve patients with cirrhosis, 87% (87/100) in treatment-experienced patients with no cirrhosis, and 60% (27/45) in the treatment-experienced patients with cirrhosis. Those results indicate that 24 weeks is the adequate duration for that treatment regimen in patients with genotype 3 HCV infection with no cirrhosis, but it is suboptimal in treatment-experienced patients with cirrhosis.84
In the study by Esteban et al.89 conducted on patients infected with genotype 3 that relapsed after treatment with sofosbuvir and ribavirin and were re-treated with sofosbuvir and ribavirin for 24 weeks, SVR was achieved in only 63% (24/38) of the cases, indicating that said regimen is suboptimal in those patients.
RecommendationsSofosbuvir and velpatasvir- •
Patients with HCV genotype 3 infection can be treated with a fixed-dose combination of sofosbuvir (400mg) and velpatasvir (100mg) in one tablet, once-daily, with or without ribavirin (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Treatment-naïve patients with no cirrhosis should be treated with a fixed-dose combination of sofosbuvir and velpatasvir for 12 weeks without ribavirin (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
Based on the results of the phase III ASTRAL-3 study on patients with genotype 3 HCV treated with the fixed-dose combination of 400mg of sofosbuvir and 100mg of velpatasvir for 12 weeks without ribavirin (29% with compensated cirrhosis, 74% with no previous treatment, and 26% with previous treatment failure), the SVR12 rates were: 98% (160/163) in the treatment-naïve patients with no cirrhosis, 93% (40/43) in the treatment-naïve patients with compensated cirrhosis, 91% (31/34) in the patients with previous treatment failure and no cirrhosis, and 89% (33/37) in the patients with previous treatment failure and compensated cirrhosis. Therefore, treatment-naïve patients with no cirrhosis do not require ribavirin.86
Recommendations- •
If resistance-associated variant (RAV) testing for NS5A is not performed, treatment-experienced patients with no cirrhosis, as well as treatment-naïve and treatment-experienced patients with compensated cirrhosis, should be treated with a fixed-dose combination of sofosbuvir and velpatasvir for 12 weeks with ribavirin (1,000 or 1,200mg daily in patients < 75kg or ≥ 75kg, respectively) (A1).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
- •
If RAV testing for NS5A is performed, treatment-experienced patients with no cirrhosis, as well as treatment-naïve and treatment-experienced patients with compensated cirrhosis, with baseline detection of NS5A RAV Y93H should be treated with a fixed-dose combination of sofosbuvir and velpatasvir for 12 weeks with ribavirin (1,000 or 1,200mg daily in patients < 75kg or ≥ 75kg, respectively). Patients with no baseline detection of NS5A RAV Y93H should receive a fixed-dose combination of sofosbuvir and velpatasvir for 12 weeks with no ribavirin (A1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
- •
NS5A RAV testing for HCV genotype 3 can be a technical challenge, and thus a reliable result is not guaranteed in all cases (B2).
Level of agreement: in complete agreement 95%.
In agreement with minor reservations: 5%.
- •
Patients with a contraindication for ribavirin or with poor tolerance to treatment with ribavirin should receive the combination of sofosbuvir and velpatasvir for 24 weeks with no ribavirin (C1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
It was shown in the same ASTRAL-3 study that the SVR rate was higher (97%) in the group of patients that had no baseline NS5A RAVs (Y93H), compared with SVR in 88% of the patients in whom baseline NS5A RAVs were detected (present in 16% of the patients). Thus, treatment-experienced patients with no cirrhosis, as well as treatment-naïve and treatment-experienced patients with compensated cirrhosis, with detected baseline NS5A RAV Y93H, should be treated with the fixed-dose combination of sofosbuvir and velpatasvir for 12 weeks plus ribavirin (1,000 or 1,200mg daily in patients < 75kg or ≥ 75kg, respectively). Patients with no baseline detection of NS5A RAV Y93H should receive the fixed-dose combination of sofosbuvir and velpatasvir for 12 weeks without ribavirin. If the patient has a contraindication for ribavirin use or does not tolerate treatment with ribavirin well, he or she should receive the combination of sofosbuvir and velpatasvir for 24 weeks with no ribavirin.86
Genotype 4 infectionRecommendationsInterferon therapies- •
Patients with HCV genotype 4 infection can be treated with a combination of weekly pegIFN-α, a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or ≥ 75kg, respectively) and sofosbuvir (400mg/24h) for 12 weeks (B1).
Level of agreement: in complete agreement 88%.
In agreement with minor reservations: 12%.
That combination was evaluated by Lawitz, et al. in the NEUTRINO study that included 28 patients with genotype 4 infection, achieving 96% SVR.49 That regimen is currently no longer included in the recommendations published by the EASL nor in the 2016 AASLD/IDSA.9,46 Until 2015, the AASLD guidelines proposed that combination, with a B2 level of evidence, as an alternative regimen for patients with no previous antiviral treatment.46 The 2015 EASL gave it a B1 level of evidence,91 but with the development and success of the interferon-free therapies, that combination is no longer recommended and its use is left up to the clinician's judgement, in accordance with his or her experience, as well as with drug availability.
Recommendation- •
Patients with genotype 4 infection can be treated with a combination of weekly pegIFN-α, a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or 75kg, respectively) and simeprevir (150mg/24h) (B1).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
That therapeutic regimen was evaluated in the RESTORE study by Moreno et al.92 It included 107 patients with genotype 4 infection, with an overall SVR of 65.4%. That combination is no longer among the most recently published recommendations of the EASL nor those in the 2016 AASLD/IDSA.9,46 In the 2015 EASL guidelines, that regimen was proposed with a B1 level of evidence.91 With the development and success of the interferon-free therapies, that combination is no longer recommended, and its use is left to the clinician's judgement, in accordance with his or her experience and with drug availability.
Recommendation- •
Simeprevir should be administered for 12 weeks in combination with pegIFN-α and ribavirin. After that, pegIFN-α and ribavirin should be administered for 12 weeks (total treatment duration of 24 weeks) in treatment-naïve patients and those with previous relapse (including patients with cirrhosis). The patients with a partial or null response, including cirrhotic patients, should have an additional 36 weeks of treatment (total treatment duration of 48 weeks) (B1).
Level of agreement: in complete agreement 77%.
In agreement with minor reservations: 23%.
In the study by Moreno et al.,92 that evaluated 107 patients, a 65.4% overall SVR was identified and in the analysis by groups there was 82.9% SVR in patients with no previous antiviral treatment, 86.4% in patients with relapse, 60% in partial responders, and 40% in previous nonresponders. In the patients with no previous antiviral treatment and those with relapse that had response-guided therapy, their SVR was 93.5% and 95%, respectively.
Recommendations- •
HCV RNA levels should be monitored during treatment. Treatment should be interrupted if the HCV RNA level is > 25 IU/ml at treatment week 4, week 12, or week 24 (A2).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
If that therapeutic regimen is prescribed,91 it is recommended to follow the initial evaluation criteria, follow-up, and suspension rules previously indicated in the 2015 Mexican Consensus on Hepatitis C.45
Grazoprevir and elbasvir- •
By analogy with genotype 1a, treatment-experienced patients with genotype 4, with or without compensated cirrhosis, with a baseline HCV RNA level > 800,000 IU/ml should receive the combination of grazoprevir and elbasvir for 16 weeks, combined with a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or ≥ 75kg, respectively) (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Patients with HCV genotype 4 infection can be treated with the fixed-dose combination of grazoprevir (100mg) and elbasvir (50mg) in one tablet, once-daily (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Treatment-naïve patients with genotype 4 infection, with or without compensated cirrhosis, should receive the combination of grazoprevir and elbasvir with no ribavirin for 12 weeks (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
The phase III C-EDGE-TN study included only 18 treatment-naïve patients with genotype 4, 12% of whom had cirrhosis. The participants received the combination of grazoprevir and elbasvir without ribavirin for 12 weeks. One hundred percent (18/18) of the patients achieved SVR12.42 SVR was a bit lower in coinfected genotype 4 patients (C-EDGE-COINFECTION study), with a SVR12 of 96% (27/28).13
In the phase III C-EDGE-TE study conducted on treatment-experienced patients (46% in the cirrhosis phase), SVR12 rates were 87% (7/8) after 12 weeks of grazoprevir and elbasvir without ribavirin; 93% (14/15) after 12 weeks of grazoprevir and elbasvir with ribavirin; 60% (3/5) after 16 weeks of grazoprevir and elbasvir without ribavirin; and 100% (8/8) after 16 weeks of grazoprevir and elbasvir with ribavirin.57
RecommendationsOmbitasvir, paritaprevir, and ritonavir- •
Patients with HCV genotype 4 infection with no cirrhosis can be treated with an IFN-free regimen that consists of a fixed-dose combination of ombitasvir (12.5mg), paritaprevir (75mg), and ritonavir (50mg) in the same tablet (two tablets once-daily with food) for 12 weeks with a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or 75kg, respectively), with no dasabuvir (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
The multicenter, open, randomized phase IIb PEARL-I study93 evaluated the effectiveness of the combination of paritaprevir (P: NS3/4A inhibitor, 75mg), ritonavir (r: potentiator of paritaprevir levels, 50mg), and ombitasvir (O: NS5A inhibitor, 12.5mg), with or without ribavirin (RBV) for 12 weeks in 135 individuals with genotype 4 chronic hepatitis without cirrhosis. Eighty-six of the patients were treatment-naïve and were randomized into one of 2 groups: 44 to PrO (with no ribavirin), achieving 90.9% SVR, whereas the treatment-naive patients that received the same regimen plus ribavirin (PrO + RBV), achieved 100% SVR. In the group of patients that did not receive ribavirin, there were two (5%) posterior relapses and one (2%) viral breakthrough event. A group of treatment-experienced patients that previously received pegIFN and ribavirin was also evaluated that included 49 individuals with chronic hepatitis and no cirrhosis, with genotype 4, that were given the PrO + RBV regimen, achieving 100% SVR. That is the study with the largest number of patients with that genotype that have received said regimen, and even though genotype 4 is very rare, an excellent response can be seen. However, in Mexico, that combination includes dasabuvir, a nonnucleoside NS5B polymerase inhibitor, that does not act against genotype 4.
Recommendations- •
Patients with HCV genotype 4 infection with compensated cirrhosis should be treated with a fixed-dose combination of ombitasvir (12.5mg), paritaprevir (75mg), and ritonavir (50mg) in the same tablet (two tablets once-daily with meals) for 24 weeks with a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients <75kg or 75kg, respectively), with no dasabuvir (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
Provisional results from the ongoing randomized, open, multicenter, phase III AGATE-I study94 on the treatment of genotype 4 hepatitis C with compensated cirrhosis using the combination of paritaprevir/ritonavir and ombitasvir (75/50/12.5) + ribavirin (RBV) for 12 or 16 weeks were presented this year. SVR in the individuals that received the 12-week regimen was 96% (52/54), whereas it was 100% (49/49) in those that received that regimen for 16 weeks. With respect to safety, no combination suspension due to adverse events was reported. However, there were 13 serious adverse events, none of which resulted in death.
RecommendationsSofosbuvir and daclatasvir- •
Patients with HCV genotype 4 infection can be treated with an IFN-free combination of sofosbuvir (400mg) and daclatasvir (60mg) daily, for 12 weeks. The addition of a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or ≥ 75kg, respectively) to the treatment is recommended in patients with cirrhosis (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
In patients with cirrhosis in whom ribavirin is contraindicated, extending treatment duration to 24 weeks should be considered (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
According to real-life experience in the ANRS CO22 HEPATHER French cohort study95 on 47 patients with genotype 4 hepatitis C that received the combination of sofosbuvir and daclatasvir for 12 weeks, 100% of the treatment-naïve patients with no cirrhosis achieved SVR at post-treatment week 12. In the cirrhotic patients, SVR was 85.7% (12 weeks of treatment) vs 92.3% (24 weeks), whereas the treatment-experienced patients achieved SVR of 87.5% (12 weeks of treatment) vs 94.1% (24 weeks). A small subgroup of treatment-experienced cirrhotic patients received that combination plus weight-adjusted ribavirin and reached 100% SVR.
The preliminary results of another ongoing cohort study on 176 individuals with genotype 4 hepatitis C infection, of whom 76% had cirrhosis, 82% were treatment-experienced, and 35% were coinfected with HIV, analyzed SVR with the combination of sofosbuvir plus daclatasvir versus the combination of sofosbuvir, daclatasvir, and ribavirin.96 Eighty-two percent of the patients received the combination of sofosbuvir and daclatasvir and 18% received the triple combination. Overall SVR, regardless of treatment duration (12 vs 24 weeks) was 90% (159/176). When the overall response was analyzed in the patients that did not receive ribavirin, SVR was 90% (123/137) vs 97% (33/34) with ribavirin. SVR was 88% in the patients with cirrhosis that did not receive ribavirin vs 97% of the patients that received the combination with ribavirin. The results for the patients with previous treatment failure and HIV coinfection have not yet been reported. In accordance with those findings and the inferred results with other regimens, in patients with cirrhosis and/or previous treatment failure, the addition of weight-adjusted ribavirin for 12 weeks is suggested, and if that addition is not possible, then extending treatment to 24 weeks should be considered.97,98
RecommendationsSofosbuvir and ledipasvir- •
Patients with HCV genotype 4 infection can be treated with the IFN-free combination of sofosbuvir (400mg) and ledipasvir (90mg) in a single tablet, administered once-daily. Patients with no cirrhosis, including treatment-naïve and treatment-experienced patients, should receive that combination for 12 weeks with no ribavirin (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
The evidence on efficacy in patients with genotype 4 is based on two open studies. The first (SINERGY)99 includes just 21 patients, 95% (20/21) of whom achieved SVR at week 12 after treatment (SVR12). The patient that did not, withdrew from the study at week 4. The other study100 included 44 subjects, demonstrating 96% SVR12 (21/22) in the treatment-naïve patients and 91% (20/22) in the treatment-experienced patients. Surprisingly, upon segmenting the population, there was 91% SVR12 in the patients with no cirrhosis (31/34) and 100% in the patients with cirrhosis (10/10).
Recommendations- •
Based on the data of patients with HCV genotype 1 infection, patients with compensated cirrhosis, including treatment-naïve and treatment-experienced patients, should receive that fixed-dose combination with a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or 75kg, respectively) for 12 weeks (B1).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
It is important to mention that in the study by Abergel et al.,100 in the genotype 4 cases, the patients with cirrhosis had a better relative response (10/10) than the patients with no cirrhosis (31/34). Additionally, in the studies on patients with genotype 1 with cirrhosis that were treated with the abovementioned combination (ION-2), the response was equivalent to that of the patients with no cirrhosis.70,71
Recommendations- •
Patients with compensated cirrhosis, in whom ribavirin is contraindicated or poorly tolerated, should receive a fixed-dose combination of sofosbuvir and ledipasvir for 24 weeks with no ribavirin (B1).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
That recommendation is based on the current focus of reducing potential toxicity attributable to ribavirin and supposing the availability of unrestricted access to treatment for 24 weeks. It is important to keep in mind that there are articles on the use of the combination of SOF-LDV plus RBV in patients with cirrhosis and genotype 4 in whom strict clinical surveillance is recommended.101
Recommendations- •
Based on data from patients with HCV genotype 1 infection, treatment with the fixed-dose combination of sofosbuvir and ledipasvir with ribavirin can be extended to 24 weeks in treatment-experienced patients with compensated cirrhosis and negative response predictors, such as a platelet count < 75 x 103/μl (B1).
Level of agreement: in complete agreement 86%.
In agreement with minor reservations: 14%.
In fact, those therapeutic regimens have been prescribed and well-tolerated, not only in patients with genotype 1 infection, but also in patients with genotype 4. However, strict clinical surveillance must always be carried out.
RecommendationsSofosbuvir and simeprevir- •
Patients with HCV genotype 4 infection can be treated with an IFN-free combination of sofosbuvir (400mg/24h) and simeprevir (150mg/24h) for 12 weeks. Adding a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or ≥ 75kg, respectively) to the treatment is recommended in patients with cirrhosis (B2).
Level of agreement: in complete agreement 95%.
In agreement with minor reservations: 5%.
- •
In patients with cirrhosis in whom ribavirin is contraindicated, extending treatment duration to 24 weeks should be considered (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
According to the open, randomized, phase III PLUTO study102 that evaluated the efficacy of the combination of simeprevir 150mg plus sofosbuvir (400mg) in the treatment of genotype 4 chronic hepatitis, 100% of the 40 patients achieved SVR with 12 weeks of treatment. Of those patients, 7/40 (18%) had compensated cirrhosis, 13/40 (33%) were treatment-naïve, and 27/40 (68%) had previous treatment failure with peg-IFN and ribavirin. With respect to safety, serious adverse events or medication suspension secondary to adverse events were not reported. Even though the results of that regimen are also very promising, the number of patients that have been studied is limited, especially those with cirrhosis and with previous treatment. Therefore, by inference from results with other regimens in cases of cirrhosis and/or previous treatment failure, the addition of weight-adjusted ribavirin for 12 weeks is suggested. If that is not possible, then extending treatment to 24 weeks should be considered.9
RecommendationsSofosbuvir and velpatasvir- •
Patients with HCV genotype 4 infection can be treated with a fixed-dose combination of sofosbuvir (400mg) and velpatasvir (100mg) in one tablet, administered once-daily (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Treatment-naïve patients and patients with previous treatment failure, with or without compensated cirrhosis, should be treated with the fixed-dose combination of sofosbuvir and velpatasvir, with no ribavirin, for 12 weeks (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
The ASTRAL-1 clinical trial included 116 patients with genotype 4 infection (23% with cirrhosis, 55% with no previous treatment, and 45% with previous treatment failure) that received the fixed-dose combination of sofosbuvir (400mg) and velpatasvir (100mg) in one tablet administered once-daily with no ribavirin for 12 weeks, and 100% SVR (116/116) was achieved.79
Genotype 5-6 infectionAt present, the three treatment options for patients infected with the HCV genotypes 5 or 6 are the dose-fixed combination of sofosbuvir and daclatasvir, the dose-fixed combination of sofosbuvir and ledipasvir, and the combination of sofosbuvir and velpatasvir. In populations in which none of those options is available, the combination of pegIFN and ribavirin or the triple combination of pegIFN-α, ribavirin, and sofosbuvir continue to be acceptable.9
RecommendationsInterferon therapies- •
Patients with HCV genotype 5 or 6 infection can be treated with a combination of weekly pegIFN-α, a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or ≥ 75kg, respectively), and sofosbuvir (400mg/24h) for 12 weeks (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
A phase III study (NEUTRINO) evaluated that combination in treatment-naïve patients with genotype 1, 4, 5, or 6 chronic hepatitis C infection. Of the 456 patients included in the study, only 2% of the patients had genotype 5 or 6 infection. All of the patients with genotype 5 (1 patient) and 6 (6 patients) achieved SVR.49 Due to the low prevalence of those genotypes, there are currently no data for knowing if longer treatment duration is required in patients with predictors of low probability for SVR. That regimen has not been assessed in treatment-experienced patients.
RecommendationsSofosbuvir and daclatasvir- •
Patients with genotype 5 or 6 HCV infection can be treated with sofosbuvir (400mg) and daclatasvir (60mg) daily, for 12 weeks. Based on data with other combinations, the addition of a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or ≥ 75kg, respectively) to the treatment is recommended in treatment-experienced patients without cirrhosis or with compensated cirrhosis (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
In patients with cirrhosis in whom ribavirin is contraindicated, extending treatment duration to 24 weeks should be considered (B1).
Level of agreement: in complete agreement 95%.
In agreement with minor reservations: 5%.
The 2016 EASL guidelines state that daclatasvir is active in vitro in genotypes 5 and 6. There are no clinical trials with data on that combination for those genotypes due to their low prevalence.9
RecommendationsSofosbuvir and ledipasvir- •
Patients with genotype 5 or 6 HCV infection can be treated with a fixed-dose combination of sofosbuvir (400mg) and ledipasvir (90mg) in a single tablet, administered once-daily (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Patients without cirrhosis or with compensated cirrhosis, including treatment-naïve and treatment-experienced patients, should receive that fixed-dose combination for 12 weeks, with no ribavirin (B1).
Level of agreement: in complete agreement 95%.
In agreement with minor reservations: 5%.
- •
Based on data from patients with HCV genotype 1 infection, patients with compensated cirrhosis, including treatment-naïve and treatment-experienced patients, should receive that fixed-dose combination with a daily weight-based dose of ribavirin (1,000 or 1,200mg in patients < 75kg or ≥ 75kg, respectively) for 12 weeks (B1).
Level of agreement: in complete agreement 95%.
In agreement with minor reservations: 5%.
- •
The patients with compensated cirrhosis, in whom ribavirin is contraindicated or not tolerated, should receive the fixed-dose combination of sofosbuvir and ledipasvir for 24 weeks, with no ribavirin (B1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
In a phase II multicenter study that recruited 41 patients, 21 of them were treatment-naïve and 20 were treatment-experienced. All the patients completed the 12-week regimen with sofosbuvir and ledipasvir without ribavirin, achieving 95% SVR12 (39/41). SVR12 was achieved in 20 (95%, 76/100) of the 21 treatment-naïve patients and in 19 (95%, 75/100) of the 20 treatment-experienced patients.
That study was the first to evaluate a treatment regimen with DAAs in patients with genotype 5. Eighty-nine percent of the patients with cirrhosis achieved SVR12 (8/9), whereas 97% of the patients with no cirrhosis (31/32) achieved SVR12.103
In a study on 25 patients with genotype 6, the efficacy of the 12-week sofosbuvir and ledipasvir combination with no ribavirin was evaluated in treatment-naïve and treatment-experienced patients. The SVR rate was 96% (24/25).104
Recommendation- •
Patients with HCV genotype 5 or 6 infection that are treatment-naïve or that had previous treatment failure, without cirrhosis or with compensated cirrhosis, can be treated with a fixed-dose combination of sofosbuvir (400mg) and velpatasvir (100mg) in a single tablet, once-daily, with no ribavirin, for 12 weeks (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
That combination has been approved by the EASL9 and the AASLD/IDSA as an initial treatment option, with an A1 level of evidence.46 That therapeutic regimen demonstrated its efficacy in the ASTRAL-1 study that included 35 antiviral treatment-naïve patients with genotype 5 infection with or without cirrhosis of the liver. The patients received 12 weeks of the SOF/VEL combination, reaching 97% SVR, and 41 patients with genotype 6 infection had 100% SVR.79
Recurrence after liver transplantationRecommendation- •
All patients with recurrence of HCV infection after transplantation should be considered for antiviral treatment, ideally begun 3 months after liver transplantation (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
Several facts justify that recommendation: the natural history of post-transplantation HCV relapse is universal, given that graft reinfection is a dynamic process that begins with viral replication immediately after the post-transplantation anhepatic phase.105 In addition, faster progression time to fibrosis with the development of cirrhosis at 5 years has been demonstrated in 3 to 33% of post-transplantation patients.106–108 Those observations are explained through the study of the pathogenic mechanisms of accelerated damage in the graft with post-transplantation hepatitis C recurrence. It is known that the stellar liver cells are the greatest collagen producers and that the number of activated cells and TGFβ-1 expression are correlated with fibrosis stage and disease progression, with no influence from the type of immunosuppressant used.109,110 That explains the accelerated fibrosis in those patients and therefore they should be treated when there is virus C recurrence.
Recommendation- •
The clinical form of fibrosing cholestatic hepatitis, moderate-to-severe fibrosis, or portal hypertension that emerge during the course of the first post-transplantation year, predict rapid disease progression and graft loss and are indications for immediate antiviral treatment (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
Approximately 10% of the patients with post-transplantation HCV relapse develop fibrosing cholestatic hepatitis (FCH), which is characterized by severe jaundice with cholestatic liver dysfunction and a high level of viremia. From the histopathologic perspective, it is manifested by hydropic changes of hepatocytes, vacuolar degeneration, cholestasis, and periportal peritrabecular fibrosis, and a moderate inflammatory process that lead to acute liver failure and consequent loss of the graft. Thus, DAAs should be used as soon as possible.111 After treatment commencement, the laboratory values of INR, bilirubin, and albumin return to normal within a few weeks and are correlated with viral clearance at week 12. SVR in those patients has been reported at 70%, justifying the benefit of early treatment in severe manifestations of hepatitis C recurrence.112 The efficacy of the sofosbuvir and daclatasvir regimen has recently been shown in patients with post-transplantation FCH in a prospective cohort from 25 French and Belgian transplantation centers, in which 96% SVR was achieved at week 12 in 22 of the 23 study patients and the median time between transplantation and treatment commencement was 5 months.113 Regarding portal pressure, a study was conducted on 166 post-liver transplantation patients. Of the patients that had liver biopsy, the hepatic venous pressure gradient was measured to identify those cases at greater risk for post-transplantation HCV recurrence, and it was concluded that a hepatic venous pressure gradient equal to or greater than 6mmHg was a very accurate marker for identifying patients at greater risk for disease progression.114 That occurs because the established portal hypertension determines fibrosis irreversibility, with an endothelial inflammatory state and stellar cell activation, as well as the installation of fibrogenesis,115 making antiviral treatment a priority in those patients.
Recommendation- •
Patients with post-transplantation HCV recurrence should be treated with an IFN-free regimen of ribavirin for 12 or 24 weeks (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
The negative impact of hepatitis C infection on liver transplantation recipients is well known. The effect of HCV infection on patient survival and on the graft was studied in a cohort of 11,036 patients that underwent 11,791 liver transplants within the time frame of 1992 and 1998, according to the United Network for Organ Sharing (UNOS). Transplantation in HCV-positive recipients was found to increase the risk for death with an OR of 1.23 (95% CI: 1.12-1.35) and for graft failure with an OR of 1.30 (95% CI: 1.21-1.39).116 With the recently observed increase in donor age, the number of patients with severe forms of recurrence has increased, as well as the number of patients that require post-transplantation antiviral treatment.117,118 Hepatitis C recurrence causes graft loss and death, and achieving SVR with antiviral therapy improves post-transplantation survival.119 The difference between the reported SVR rates of 20 to 30% in treatment regimens with IFN and of 93 to 100% in IFN-free treatments makes the use of DAAs the best treatment option in those patients.
Recommendation- •
Post-transplantation patients with no cirrhosis or with compensated cirrhosis (Child-Pugh A) can be treated with any of the following combinations, depending on genotype: genotype 2 with sofosbuvir and ribavirin for 12 weeks (B1); genotypes 1, 4, 5, or 6 with sofosbuvir and ledipasvir or with sofosbuvir and daclatasvir for 12 weeks with ribavirin, or for 24 weeks with no ribavirin. Any genotype can be treated with sofosbuvir and daclatasvir with ribavirin for 24 weeks or sofosbuvir and velpatasvir for 12 weeks (A1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
The evidence supporting that recommendation comes from several studies. The sofosbuvir and ribavirin regimen was studied in a compassionate use program that included 104 patients with post-liver transplantation HCV relapse, in whom 57% SVR was achieved.112 The SOLAR-1 study was conducted on 233 patients with genotypes 1 to 4 and compensated cirrhosis of the liver. They were randomized to receive sofosbuvir 400mg and ledipasvir 90mg with 1,000 or 1,200mg of ribavirin, based on weight < 75kg or 75kg, respectively, for 12 or 24 weeks. SVR was achieved in 96% of the patients with compensated cirrhosis.120 In the ALLY-1 study, the combination of daily daclatasvir 60mg and sofosbuvir 400mg and ribavirin for 12 weeks was used in 53 patients with post-transplantation hepatitis C recurrence, resulting in 94% SVR.68 The daclatasvir and sofosbuvir combination in a cohort of 77 patients and daclatasvir with simeprevir in another 18 patients, both cohorts with virus C recurrence and treated for 24 weeks, were also reported on. The general SVR was 87%, and SVR was 91% with the daclatasvir and sofosbuvir combination with or without ribavirin, and 72% with the daclatasvir and simeprevir combination with or without ribavirin.121 At present, there are no reports on the safety and efficacy of the sofosbuvir and velpatasvir combination treatment regimen in liver transplantation recipients.
Recommendation- •
Post-transplantation patients with no cirrhosis or with compensated cirrhosis (Child-Pugh A) can be treated with the combination of paritaprevir potentiated by ritonavir, ombitasvir, and dasabuvir with ribavirin for 12 weeks (genotype 1b) or 24 weeks (genotype 1a with cirrhosis). In addition, the combination of paritaprevir potentiated by ritonavir and ombitasvir for 12 or 24 weeks with ribavirin can be used in patients with genotype 4 without or with cirrhosis, respectively, or with the combination of sofosbuvir and simeprevir with ribavirin for 12 weeks in genotypes 1 and 4, with the understanding that adjustments must be made in the doses of immunosuppressant drugs, and that with the combination of sofosbuvir and simeprevir, cyclosporine A must not be used (B1).
Level of agreement: In complete agreement 100%.
In agreement with minor reservations: 0%.
The combination of the so-called 3D regimen was analyzed in 34 patients that were liver transplantation recipients in the CORAL study. Patients were included that presented with no fibrosis, with moderate fibrosis, and with compensated cirrhosis in Child class A.122 Treatment was begun at one year after transplantation for 24 weeks, resulting in SVR in 33 of the 34 patients (97%). The side effects were mild, with fatigue, headache, and cough. Strict monitoring of the immunosuppressant medication was required, and the following had to be adjusted: tacrolimus was used at a dose of 0.5mg every 7 to 10 days and cyclosporine at one-fifth of the daily dose. The immunosuppressant levels were measured weekly at the discretion of the treating physician. Consequently, that regimen should only be used at specialized centers.
The combination of sofosbuvir and simeprevir with or without ribavirin for 12 or 24 weeks was evaluated in the longitudinal, real-life TARGET study. Twenty-one transplantation centers participated with a total of 151 patients, some of whom had undergone transplantation, with 88% SVR (133/151). There was a 4.81-fold increase in the simeprevir level in the patients treated with cyclosporine, and therefore the combination of simeprevir and cyclosporine is not recommended.123
Recommendations- •
Post-transplantation patients with decompensated cirrhosis (Child-Pugh B or C) can be treated with any of the following combinations: sofosbuvir and ribavirin for 12 weeks (genotype 2), with a fixed-dose of sofosbuvir/ledipasvir or sofosbuvir with daclatasvir, with ribavirin for 12 weeks (genotypes 1, 4,5, or 6), or with the combination of sofosbuvir plus daclatasvir or sofosbuvir/velpatasvir with ribavirin for 24 weeks (all genotypes). In those patients, ribavirin can be started at a daily dose of 600mg, adjusting the dose in accordance with tolerance (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
The patients with decompensated cirrhosis in the Child class B or C stages can be treated with the combination of sofosbuvir plus ribavirin, which was analyzed in a cohort of 51 patients with compensated cirrhosis or decompensated cirrhosis, with a minimum of one year since transplantation.112 They received that regimen as compassionate treatment, resulting in cirrhosis improvement in 23 patients (45%). The combination of sofosbuvir and ledipasvir in post-transplantation patients with decompensated cirrhosis showed an 86% SVR rate in the patients that had 12 weeks of treatment and an 88% rate in those with 24 weeks of treatment. The response rate in patients with Child class C was lower, with 60% SVR for the 12-week regimen and 75% for the 24-week treatment.124
The combination of daclatasvir and sofosbuvir has been analyzed in advanced cirrhosis in the so-called ALLY-1 phase III study. Forty-seven percent of the patients were associated with improvement in the Child and MELD scores, which was more evident in those with Child classes B and C. That improvement is attributable to a modest improvement in liver synthesis function, as well as in the Fibrotest, APRI, bilirubin, and ALT tests.124 There is evidence from the ASTRAL-4 study on the use of the sofosbuvir and velpatasvir combination in patients with decompensated cirrhosis. The study included all the genotypes in both naïve-treatment and experienced-treatment patients, with 3 treatment arms: SOF/VEL for 12 weeks, SOF/VEL for 24 weeks, and SOF/VEL+ RBV for 12 weeks, achieving an SVR rate of 83 to 94%.124 In those patients, ribavirin was started at a dose of 600mg per day, with later tolerance-dependent adjustments.
Recommendations- •
It is usually not necessary to adjust the dose of tacrolimus or cyclosporine with the sofosbuvir-ribavirin, sofosbuvir-ledipasvir, or sofosbuvir-daclatasvir regimens, but periodic surveillance is recommended (B2).
Level of agreement: in complete agreement 87%.
In agreement with minor reservations: 13%.
The dose of tacrolimus or cyclosporine does not require dosing adjustment with the use of sofosbuvir-ribavirin, which was demonstrated in the compassionate use program for patients with post-transplantation recurrent hepatitis C.112,124 It was also recently demonstrated in the preliminary results of the multicenter prospective study on ledipasvir/sofosbuvir with ribavirin in the treatment of post-transplantation hepatitis C virus recurrence, presented at the congress of the AASLD.120 Cyclosporine and tacrolimus increased the area under the curve 40 and 5%, respectively. However, those changes were not clinically significant. Daclatasvir did not cause significant changes of clinical importance with the calcineurin inhibitors, mTOR inhibitors, steroids, or mycophenolate mofetil,68 but periodic surveillance is nevertheless recommended.
Recommendation- •
Concomitant use of simeprevir and cyclosporine A is not recommended in liver transplantation recipients, due to the significant increase in plasma concentrations of simeprevir. No simeprevir dose modification is required with tacrolimus and sirolimus, but regular follow-up of its blood concentrations should be carried out (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
In healthy volunteers, the administration of a single dose of cyclosporine combined with simeprevir resulted in an increase in both cyclosporine and simeprevir concentration. Nevertheless, in a study on the simeprevir and daclatasvir plus ribavirin regimen, a 6-fold increase in simeprevir serum concentration was found, compared with simeprevir in the absence of cyclosporine. The interaction is thought to be caused by inhibition of the organic-anion-transporting polypeptide 1B1 (OATP1B1), glycoprotein p (P-gp), and the cytochrome P450 3A (CYP3A) by cyclosporine.123–125 In contrast, the concurrent administration of tacrolimus with simeprevir did not show notable changes in the serum concentrations.
Recommendation- •
When the combination of paritaprevir potentiated by ritonavir, ombitasvir, and dasabuvir is used, the tacrolimus dose should be adjusted to 0.5mg once-weekly, or 0.2mg every 3 days, whereas the cyclosporine A dose should be adjusted to one-fifth of the daily dose prior to HCV treatment, once-daily. Prednisone at a dose of ≤ 5mg/day is allowed, but mTOR inhibitors are not recommended (B1).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
The use of a post-liver transplantation 3D regimen was reviewed in a multicenter study on patients with virus C recurrence that included 34 patients with F1-F2 mild fibrosis, 1 year after transplantation. Immunosuppressant drug adjustment was required in the patients that received 0.5mg of tacrolimus every 7-10 days, or 0.2mg every 3-5 days. Adjustments should be individualized, according to strict monitoring of the patient, determining tacrolimus levels every week until optimizing the dose. Said surveillance should be carried out in tertiary care centers that specialize in that type of management. In the patients that used cyclosporine, the dose was adjusted to one-fifth of the previous cyclosporine dose before virus C treatment. In addition, the use of prednisone was allowed at a dose of 5mg per day, obtaining 97% SVR (33 of 34 patients).122
Measures for improving treatment adherenceThe lack of adherence to antiviral therapy favors virologic relapse during treatment, resistance selection, and relapse after treatment. Therefore, before beginning treatment, as well as during treatment, the importance of therapeutic adherence should be emphasized to the patients at all times.126
The participation of a multidisciplinary team trained in the care of patients with hepatitis C has been shown to be a factor that improves treatment adherence. Ideally, to have an impact on all the potential factors that can endanger treatment adherence, the multidisciplinary team should be made up of specialists and nurses trained in antiviral treatment, as well as psychiatrists, psychologists, and social workers, given that there are psychosocial factors that must be taken into account and managed in those patients. 127–128
Among the factors that the patients themselves have identified as potential barriers at the time of deciding to begin treatment, as well as maintaining adherence to it, are the fear of death, the shame from the stigmatization of their disease condition, fear of losing their job, treatment side effects, complicated dosing regimens, and treatment access limitations in the public healthcare systems.127
Among the main factors identified by patients as facilitators of treatment adherence are social, family, and emotional support, viewed as an essential part of treatment, as well as the maintenance of effective communication with healthcare professionals adapted to each patient's needs. In that respect, having nurses trained in education about hepatitis C has been shown to be a useful strategy that favors treatment adherence.128
Patients that consume alcohol should not be excluded from the possibility of receiving antiviral treatment, but they should join a support program for maintaining abstinence from alcoholic beverages. Alcohol consumption in patients with hepatitis C is a factor that accelerates the progression of liver damage and increases the risk for developing cirrhosis and hepatocellular carcinoma.129 Alcohol intake ≥ 10g/day has been related to an increase in viral RNA.130 In addition, patients that are active consumers of alcohol are at greater risk for not maintaining treatment adherence or for suspending it prematurely.131
The sharing of needles/syringes among injection drug users (IDUs) is one of the most relevant risk factors associated with hepatitis C infection. It is also known that there is an increased risk in IDUs for hepatitis C virus reinfection, as well as for hepatitis B virus and HIV infection.132,133 The prevalence of chronic hepatitis C is high among IDUs. A recent study found that up to 47.7% of the patients participating in a rehabilitation program had positive antibodies against hepatitis C, when screened for the disease. Despite the elevated prevalence reported, only 2.21% of the patients that were IDUs in that study had access to antiviral treatment.134 Non-injection drug users (non-IDUs) are also a group with greater risk for becoming infected with hepatitis C and other viruses, compared with the general population. The authors of a study in which 182 subjects treated at a rehabilitation center were evaluated found a 12.6% prevalence of hepatitis C among the non-IDUs. The risk factors identified in those patients were the sharing of cocaine inhalation tubes and having a tattoo. Active injection or non-injection drug use is related to risk behaviors that can jeopardize adherence to antiviral treatment. Patients that are IDUs or non-IDUs should be considered for receiving antiviral treatment, but they must enter a rehabilitation program for treating their drug addiction, as well as ensuring greater success in antiviral treatment adherence.136,137
Recommendations- •
HCV treatment, depending on individual patient characteristics, should be administered by health professionals with experience in HCV evaluation and treatment (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Patients with HCV infection should be made aware of the importance of treatment adherence to achieve SVR (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Programs for reducing injection drug use should be obligatory for persons with active parenteral drug consumption (A1).
Level of agreement: in complete agreement 88%.
In agreement with minor reservations: 12%.
- •
Patients should be advised to abstain from alcohol ingestion during antiviral therapy. Patients with continuous alcohol consumption should receive additional support during antiviral therapy (A1).
Level of agreement: in complete agreement 92%.
In agreement with minor reservations: 8%.
- •
HCV treatment can also be considered for patients with active parenteral drug use, as long as they wish to have the treatment, are capable of doing so, enroll in a rehabilitation program, and are willing to maintain their regular visits (A1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
Treatment monitoring and suspension rulesTreatment monitoringTreatment monitoring includes the areas of follow-up, efficacy, safety, and side effects. The follow-up of treatment efficacy is based on the repeated measuring of HCV RNA levels. The decrease in HCV RNA is associated with SVR for the different treatment regimens and thus has been linked to treatment efficacy. A sensitive and specific test with a wide-ranging quantification dynamic, the same test, and the same laboratory should all be used. Test efficacy should be ensured by measuring HCV RNA levels in each patient at different points in time. At present, repeated follow-up of HCV RNA levels is recommended through real-time PCR at specific periods of time to evaluate patient treatment adherence138 with a sensitive and accurate molecular test.91 Currently, there are different types of tests with a lower limit of quantification ≤ 15 and a lower limit of detection of 10 a 15 IU/ml. A lower limit of detection < 10-15 IU/ml is considered adequate for decision-making.91–138
According to the results obtained, response-guided therapy can be carried out.91 Whether or not SVR has been achieved can be determined when those tests are performed upon treatment completion or during the later follow-up.91 In principle, all patients require HCV RNA determination before treatment commencement.138 When treatment based on pegIFN-α and ribavirin was developed, the strategy known as response-guided therapy was created to minimize drug exposure, which meant determining treatment duration based on viral load at a specific time of treatment.139 Patients with HCV treated with pegIFN + ribavirin are considered to have SVR when HCV RNA is undetectable at 24 weeks of follow-up.140 With the use of treatments that incorporate protease inhibitors in the regimens (telaprevir/boceprevir), conduct (to continue/discontinue) was able to be guided by the rapid virologic response (at 4 weeks of treatment), and therefore that evaluation has been adopted even for new drugs.138
The guidelines for pegIFN + ribavirin management recognize SVR at 24 weeks, but in the studies by Martinot-Peignoux et al.35 and Chen et al.,141 they found that carrying out an HCV RNA test at 12 weeks after treatment was as relevant as monitoring at 24 weeks, for predicting SVR and making patient management decisions. Combining that information with the findings of the ATOMIC study (SOF + pegIFN-α, ribavirin), in which the 12-week regimen was effective in genotypes 1, 4, and 6, and given the uniformity of the therapies, the response-guided regimen is not considered necessary and treatment response can be evaluated at 12 weeks.142
Essentially, all patients require HCV RNA testing before beginning treatment.138 In the QUEST-1 study, with treatment-naïve patients, 85% of the patients were candidates for shortening treatment, according to response-guided therapy, and 91% achieved SVR12. The primary aim was met in 80% of the patients, and so the HCV RNA evaluation at 12 weeks was considered important. It should be remembered that the study design shortened treatment to 24 weeks, and thus a viral load is required at that point, if treatment is response-guided.143 In the QUEST-2 study, 91% of the patients were candidates for suspending treatment at week 24, and 86% of those patients had SVR12.144 In the PROMISE study, for patients with relapse, treatment was shortened (24 weeks) in 93%, thus justifying monitoring at 12 and 24 weeks of treatment.145
The development of IFN-free therapy with high efficacy has changed the follow-up paradigm based on IFN, and so monitoring is adjusted to the consequent reduction, and depending on the response, treatment could be reduced.140 Monitoring associated with early response is maintained at week 4. The use of the 8, 12, or 24-week regimens, according to the population treated, enables HCV RNA monitoring to be adjusted to those times. However, SVR is defined by undetectable HCV RNA at 12 weeks after therapy completion. Those intervals have been accepted as goals in the United States and in Europe.91,146 Consequentially, that monitoring scheme only applies to treatments with INF, because there is no evidence on the effect of treatment with IFN-free DAA-based regimens on viral load for defining treatment duration or its interruption.
The findings of the QUEST-1 and QUEST-2 studies were commented on above, but it is important to remember the designs of those studies and the virologic rules utilized for discontinuing treatment. In those cases, simeprevir or placebo were discontinued if HCV RNA was > 1,000 IU/ml at week 4 and treatment was continued with pegIFN and RBV. In that scenario, in the QUEST-1 study,143 9% of the patients had lack of response and was associated with a mutation in NS3 (R155K / D168V) at the time of the evaluation in 92% of the patients. In the QUEST-2 study, 7% had treatment failure, and if the rule for failure is applied, it occurred in 4% of the patients treated with simeprevir.144
Suspension rulesAt present, the rules for treatment suspension have only been defined in relation to the triple combination of pegIFN-α, ribavirin, and simeprevir, and so the consensus recommends the suspension of that treatment if HCV RNA levels are equal to or greater than 25 IU/ml at week 4, 12, or 24 from treatment commencement. Those are the time intervals at which treatment monitoring of the viral load should be performed.91
Suspension rules have not been defined for the new direct-acting drugs. Therefore, treatment with those new drugs should not be suspended if the viral load is detectable during treatment and the null response definition is considered at treatment completion.
Recommendations- •
To determine HCV RNA levels during and after therapy, a real-time PCR test should be used with a lower limit of detection ≤ 15 IU/ml (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
HCV RNA should be measured at treatment commencement and at weeks 4 and 12 after therapy completion, in patients treated with the triple combination of pegIFN-α, ribavirin, and sofosbuvir for 12 weeks (A2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
RNA should be measured at the baseline and at treatment weeks 4, 12, and 24, in patients treated with the triple combination of pegIFN-α, ribavirin, and simeprevir that were treatment-naïve or had relapse (A2).
Level of agreement: in complete agreement 87%.
In agreement with minor reservations: 13%.
- •
HCV RNA should be measured at treatment commencement, at week 4, at treatment completion, and at 12 weeks after treatment completion, in patients treated with an IFN-free regimen (A2).
Level of agreement: in complete agreement 95%.
In agreement with minor reservations: 5%.
- •
If the HCV RNA level is ≥ 25 IU/ml at treatment week 4 and detectable at treatment week 12 or 24 in patients receiving the triple combination of pegIFN-α, ribavirin, and simeprevir, treatment should be interrupted (A2).
Level of agreement: in complete agreement 95%.
In agreement with minor reservations: 5%.
Retreatment in patients with direct-acting antiviral therapy failure (Table 6)Current treatment regimens with DAAs offer SVR rates above 90% in the majority of patients, regardless of genotype and fibrosis grade. However, despite the success of those treatments, some patients do not respond. That small group of patients should be carefully evaluated, given that at present, studies and retreatment options are limited and it is important to know how to act (Table 6).
Retreatment recommendations for patients with chronic hepatitis C that did not reach SVR with previous antiviral therapy containing one or several DAAs.
| Treatment failure | Genotype | Sofosbuvir/ledipasvir | Sofosbuvir/velpatasvir | Ombitasvir/paritaprevir/ritonavir y dasabuvir | Ombitasvir/paritaprevir/ritonavir | Grazoprevir/elbasvir | Sofosbuvir and daclatasvir | Sofosbuvir and simeprevir | Sofosbuvir + ombitasvir/paritaprevir/ritonavir and dasabuvir | Sofosbuvir + ombitasvir/ paritaprevir/ritonavir | Sofosbuvir + grazoprevir /elbasvir | Sofosbuvir + daclatasvir + simeprevir |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PegIFN-α with ribavirin and telaprevir or boceprevir or simeprevir | 1 | 12 weeks with ribavirin | 12 weeks with ribavirin | No | No | No | 12 weeks with ribavirin | No | No | No | No | No |
| Sofosbuvir alone, sofosbuvir plus ribavirin or sofosbuvir plus pegIFN-α and ribavirin | 1 | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | 12 weeks with ribavirin (F0-F2) if HCV RNA < 800,000 IU/ml or 24 weeks with ribavirin if HCV RNA > 800,000 (5.9 log) IU/ml (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | No | No | No |
| 2 | No | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | No | No | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | No | No | No | No | |
| 3 | No | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | 12 weeks with ribavirin (F0-F2) if HCV RNA < 800,000 IU/ml or 24 weeks with ribavirin if HCV RNA > 800,000 (5.9log) IU/ml (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | No | No | No | |
| 4 | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | 12 weeks with ribavirin (F0-F2) if HCV RNA < 800,000 IU/ml or 24 weeks with ribavirin if HCV RNA > 800,000 (5.9 log) IU/ml (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | No | No | No | |
| 5 or 6 | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | No | No | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | No | No | No | No | |
| Sofosbuvir and simeprevir | 1 | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | No | No | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | No | No | No | No |
| 4 | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | No | No | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | No | No | No | No | |
| Regimen containing an N5A inhibitor (ledipasvir, velpatasvir, ombitasvir, elbasvir, daclatasvir) | 1 a | No | No | No | No | No | No | No | 24 weeks with ribavirin | No | 24 weeks with ribavirin | 24 weeks with ribavirin |
| 1b | No | No | No | No | No | No | No | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | No | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | |
| 2 | No | 24 weeks with ribavirin | No | No | No | No | No | No | No | No | No | |
| 3 | No | 24 weeks with ribavirin | No | No | No | No | No | No | No | No | No | |
| 4 | No | No | No | No | No | No | No | No | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | 12 weeks with ribavirin (F0-F2) or 24 weeks with ribavirin (F3-F4) | |
| 5 or 6 | No | 24 weeks with ribavirin | No | No | No | No | No | No | No | No | No |
With regard to patients that are nonresponders to dual therapy with pegIFN plus ribavirin, different studies63,71,91 indicate that SVR is high and similar to that achieved in treatment-naïve patients with the new IFN-free DAA regimens. Thus, those patients should be treated as if they were treatment-naïve, in accordance with the recommendations of the present consensus regarding genotype, subtype, and fibrosis stage.
The patients with HCV genotype 1 infection that received the triple regimen of pegIFN, ribavirin, plus a protease inhibitor (PI) (telaprevir, boceprevir, simeprevir), and did not have SVR, can be retreated with sofosbuvir and an NS5A inhibitor.
The sofosbuvir/ledipasvir combination has been evaluated in the retreatment of patients that are nonresponders to the triple therapy, obtaining a high SVR rate. In the ION-2 study, the patients with no cirrhosis that received treatment with that combination for 12 weeks, with or without ribavirin, achieved 100 and 96% SVR, respectively. Another group received the same regimen for 24 weeks, with or without ribavirin, and also achieved 100 and 96% SVR, respectively.71 In the same study, the patients with cirrhosis received the same regimen for 12 weeks, with or without ribavirin, and SVR was 85 and 86%, respectively. In addition, it increased to 100%, with or without ribavirin, when treatment was extended to 24 weeks. In the SIRIUS study, a large number of patients with cirrhosis that did not respond to triple therapy were evaluated with the 12-week sofosbuvir/ledipasvir combination with ribavirin vs 24 weeks with no ribavirin, achieving 96 and 97% SVR, respectively.147 Based on those studies, the 2016 EASL91 treatment guidelines recommend giving that treatment regimen for 12 weeks with ribavirin in all patients (with and without cirrhosis), unlike the AASLD treatment guidelines46 that recommend various options: 12 weeks with no ribavirin in patients with no cirrhosis; and 12 weeks with ribavirin or 24 weeks with no ribavirin in patients with cirrhosis.
The ASTRAL-1 study evaluated the 12-week sofosbuvir/velpatasvir combination with no ribavirin in patients with and without cirrhosis with genotype 1, 2, 4, 5, or 6 that were treatment-naïve and treatment-experienced with triple therapy with a PI. The overall SVR rate obtained was 99%, and 100% SVR was achieved in the group of treatment-experienced patients with genotype 1.79
In patients with no cirrhosis that were nonresponders to triple therapy, the sofosbuvir/daclatasvir combination for 24 weeks, with or without ribavirin, achieved SVR rates of 100 and 95%, respectively.67
The newest DAA combination, elbasvir/grazoprevir, recently approved by the FDA and the EMA, and now available in Mexico, has been studied in patients that were nonresponders to the triple therapy of pegIFN/ribavirin plus a PI. SVR > 95% was achieved with that DAA combination for 12 weeks plus ribavirin.148
The resistance of HCV to DAAs can play an important role in patients with IFN-free DAA treatment failure. The presence of resistance-associated variants (RAVs) that are resistant to NS5A inhibitors is associated with a lower virologic cure rate in certain groups of patients, such as those with genotype 1a or 3 and with cirrhosis. The viruses that are resistant to the protease inhibitors and the viruses that are resistant to the non-nucleoside polymerase inhibitors progressively decrease until becoming undetectable in a few months or up to 2 years after treatment interruption. In contrast, the viruses that are resistant to NS5A inhibitors can remain detectable for many years.149
Despite the knowledge of the existence of RAVs, ordering resistance tests for making retreatment decisions is not indicated.2
The patients that have treatment failure with the IFN-free DAA regimens, with any genotype, should be treated with another IFN-free combination that includes drugs with a high barrier to resistance, plus one or two medicines that have no crossed-resistance with the drugs used.
Sofosbuvir is a DAA that has a high barrier to resistance. RAVs are exceptionally selected, and if present, they rapidly disappear upon treatment completion. Therefore, the current retreatment strategies should include sofosbuvir.
That group of patients should be retreated with a pegIFN-free regimen for 12 weeks plus weight-based ribavirin in cases of patients with little or no fibrosis (F0-F1). Extending treatment to 24 months can be considered, especially in patients with advanced liver disease (F3-F4) or that do not tolerate ribavirin.
Patients that had treatment failure with sofosbuvir alone, or sofosbuvir plus ribavirin, or sofosbuvir plus pegIFN-α and ribavirin, can be retreated with the combination of sofosbuvir plus simeprevir (genotype 1 or 4), sofosbuvir plus daclatasvir (all genotypes), or sofosbuvir plus ledipasvir (genotypes 1, 4, 5, or 6), with paritaprevir potentiated by ritonavir, ombitasvir, and dasabuvir (genotype 1), or with paritaprevir potentiated by ritonavir and ombitasvir (genotype 4).
Patients with genotype 1 and 4 that had treatment failure with a regimen that combined pegIFN-α, ribavirin, and simeprevir should be retreated with a combination of sofosbuvir with daclatasvir or ledipasvir. Patients with treatment failure that received a regimen combining pegIFN-α, ribavirin, and daclatasvir should be retreated with a sofosbuvir and simeprevir combination (genotype 1 and 4). Patients infected with genotype 1 or 4, that had treatment failure with a regimen that contained sofosbuvir and simeprevir, should be retreated with a combination of sofosbuvir with daclatasvir or ledipasvir, whereas patients that had treatment failure with a regimen that contained sofosbuvir and daclatasvir or ledipasvir should be retreated with a sofosbuvir and simeprevir combination (genotype 1 and 4).
A group of patients with genotype 1 that had treatment failures with sofosbuvir was retreated by Hezode et al.150 with a sofosbuvir/ledipasvir regimen plus ribavirin for 12 weeks, obtaining 98% SVR (50/51). In another study, 15 patients that did not achieve SVR after treatment that contained an NS5A inhibitor were retreated with sofosbuvir and simeprevir with no ribavirin for 12 weeks, achieving SVR12 in 8/10 patients with genotype 1a, 3/3 patients with genotype 1b, and 2/2 patients with genotype 4.151 Ten patients with F3 fibrosis or compensated cirrhosis that did not achieve SVR after an IFN-free regimen, were retreated with the triple combination of sofosbuvir, simeprevir, and daclatasvir with ribavirin for 24 weeks. Six of those patients achieved SVR12.152
In a study that utilized the combination of sofosbuvir and velpatasvir for 4 to 12 weeks, the patients that did not achieve SVR were retreated with the same combination with ribavirin for 24 weeks, achieving 97% SVR12 (33/34) in the patients infected with genotype 1, 91% (13/14) in the patients infected with genotype 2, and 76% (13/17) in the patients infected with genotype 3.153
In the QUARTZ-1 study, 20 patients with genotype 1 were included that had virologic failure to the previous DAA treatment. They were treated with a combination of sofosbuvir, paritaprevir potentiated by ritonavir, ombitasvir, and dasabuvir for 12 or 24 weeks, with or without ribavirin. SVR12 was achieved in 13/14 patients with genotype 1a with no cirrhosis treated for 12 weeks with ribavirin, in 6/6 patients infected with genotype 1a with cirrhosis treated for 24 weeks with ribavirin, and in 2/2 patients infected with genotype 1b treated for 12 weeks without ribavirin.154
In the C-SWIFT retreatment study, patients infected with genotype 1 were treated for 4, 6, or 8 weeks with the combination of sofosbuvir, grazoprevir, and elbasvir with no ribavirin. Another group was retreated with the same drug combination plus ribavirin for 12 weeks and all patients (23/23) achieved SVR.46
At present, there are few studies that support the recommendations for retreatment of therapeutic failures with DAA regimens without pegIFN, and they include a small number of patients. Those recommendations are based on scant evidence and are subject to changes when data become available. Specific algorithms to guide retreatment decisions cannot be derived from those observations. Therefore, retreatment should be based on knowledge of the drugs that are administered (Table 1).
Patients that have treatment failure with an IFN-free regimen, but do not require urgent retreatment (F0-F2), can wait until more data and/or alternative retreatment therapeutic options become available.
Recommendations- •
Patients that do not respond to the combination treatment of pegIFN and ribavirin should be considered treatment-experienced and treated with new direct-acting antivirals, according to genotype and fibrosis stage (A1).
Level of agreement: in complete agreement 90%.
In agreement with minor reservations: 10%.
- •
Patients with HCV genotype 1 infection that had triple combination treatment failure with pegIFN, ribavirin, and telaprevir or boceprevir or simeprevir should be retreated with an INF-free combination based on sofosbuvir/velpatasvir, sofosbuvir/ledipasvir, grazoprevir/elbasvir, or sofosbuvir/daclatasvir for 12 weeks, with or without ribavirin (A1).
Level of agreement: in complete agreement 92%.
In agreement with minor reservations: 8%.
- •
The retreatment recommendations after a second direct-acting antiviral (DAA) regimen are based on indirect evidence and subject to changes, as more information becomes available (A2).
Level of agreement: in complete agreement 88%.
In agreement with minor reservations: 12%.
- •
Patients that have treatment failure with a DAA regimen, with or without pegIFN and with or without ribavirin, should be retreated with a pegIFN-free regimen for 12 weeks, plus a weight-adjusted dose of ribavirin (F0-F2). Treatment can be extended to 24 weeks, especially in patients with advanced liver disease (F3-F4) (B1).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
- •
Patients that have treatment failure with sofosbuvir alone, or combined with ribavirin or pegIFN and ribavirin, can be retreated with a combination of sofosbuvir and simeprevir (genotype 1 or 4), sofosbuvir and daclatasvir (all genotypes), sofosbuvir and ledipasvir (genotypes 1, 4, 5, or 6), or ritonavir + paritaprevir, ombitasvir, and dasabuvir (genotype 1), or with ritonavir + paritaprevir and ombitasvir (genotype 4), or grazoprevir/elbasvir (genotypes 1 and 4) (B2).
Level of agreement: in complete agreement 92%.
In agreement with minor reservations: 8%.
- •
Patients with HCV genotype 1 or 4 infection that had treatment failure with the combination of pegIFN, ribavirin, and simeprevir should be retreated with a combination of sofosbuvir with daclatasvir or ledipasvir or velpatasvir (B1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
- •
Patients with genotype 1 infection that have failure with a regimen containing an NS5A inhibitor (ledipasvir, velpatasvir, ombitasvir, elbasvir, or daclatasvir) can receive sofosbuvir plus ritonavir plus paritaprevir, ombitasvir, and dasabuvir; sofosbuvir plus grazoprevir plus elbasvir; or sofosbuvir plus daclatasvir plus simeprevir, all with ribavirin, for 12 or 24 weeks, depending on genotype 1a/1b or the grade of fibrosis (B1).
Level of agreement: in complete agreement 92%.
In agreement with minor reservations: 8%.
- •
Patients with genotype 4 infection that have treatment failure with a regimen containing an NS5A inhibitor (ledipasvir, velpatasvir, ombitasvir, elbasvir, or daclatasvir) can receive sofosbuvir plus ritonavir + paritaprevir, ombitasvir, and dasabuvir; sofosbuvir plus grazoprevir plus elbasvir; or sofosbuvir plus daclatasvir plus simeprevir, all with ribavirin, for 12 or 24 weeks, depending on the grade of fibrosis (B1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
- •
All patients with no urgent need for retreatment (F0-F2), regardless of genotype, can wait until there are better treatment options (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
The safety and efficacy of a triple regimen of sofosbuvir, an NS3 inhibitor, and an NS5A protease inhibitor in patients that had failed treatment with a DAA regimen are not known (B2).
Level of agreement: in complete agreement 92%.
In agreement with minor reservations: 8%.
- •
The usefulness of HCV resistance testing prior to retreatment in patients that had treatment failure with any regimen containing DAAs is not known (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
Post-treatment follow-up in patients with SVRIn the era of the direct-acting antivirals, SVR is > 90% for all the genotypes, and so now greater survival is expected, as well as improvement in the manifestations associated with the virus, making knowledge of the follow-up of those patients an important requisite.155
The primary endpoint for evaluating the efficacy of the INF-free treatments is undetectable HCV RNA at 12 weeks after treatment completion.155 Nevertheless, it is not yet known if the correlation between SVR12 and long-term undetectable viremia is similar with regimens of short duration.155 Therefore, the EASL guidelines consider an infection cured if patients with no cirrhosis present with undetectable HCV RNA at week 48.91
A retrospective study on 779 patients treated with sofosbuvir showed that only two patients had relapse at week 24.156 However, in the report by Sarrazin et al., it was estimated through a phylogenetic analysis that 58% of patients with late recurrent viremia were successfully treated with a regimen based on sofosbuvir, and they had reinfection from a different strain. Of the patients that presented with late recurrent viremia, HCV RNA became detectable at post-treatment week 24,157 thus that window of time could be considered for HCV RNA control.
Patients that present with reinfection have been shown to have elevated levels of alanine aminotransferase that are > 1.5-fold the upper limit of normal in up to 71%.158 However, in the patients that have persistently high levels of the enzyme and other risk factors, additional associated pathologies (fatty liver, hemochromatosis, etc.) should be evaluated as possible causes of transaminasemia.155
The exact duration of HCC surveillance in patients with advanced fibrosis or cirrhosis has not been established and at present is considered undefined. What has been reported is that, despite HCV eradication, there is still risk for hepatocellular carcinoma.159
It has been estimated in different studies that the risk for developing HCC at 5 years in patients with SVR was 2.9% in the general cohort, 5.3% in the cohort with cirrhosis, and 0.9% in the coinfected patients.155 In a Taiwanese study, the 5-year risk for HCC in patients with SVR was 22.6% in patients with cirrhosis and 3.2% in patients with F3,159 and the annual incidence was 1.16%. Therefore, the recommendation is to continue follow-up in that population, according to the established recommendations.91
Increase in the hepatic venous pressure gradient (HVPG) is one of the complications associated with hepatitis and fibrosis.160 Eighty-two percent of patients treated with pegIFN/ribavirin that have SVR have been shown to have a decrease in HVPG > 20% or a value < 12mmHg.161 Nevertheless, in a study in which sofosbuvir/ribavirin was used for 48 weeks, only 24% of the patients had a decrease in HVPG > 20% and at follow-up, none of the patients had HVPG < 12mmHg.162
According to the results reported by Mandorfer et al.,162 there was a decrease in HVPG in all the stages. When baseline HVPG was 6-9mmHg, it normalized (< 6mmHg) in 63% of the patients and no patient progressed to a value > 10mmHg. In the patients with HVPG > 10mmHg, a clinically relevant decrease > 10% was found in 63% of the patients and only 24% reached values < 10mmHg. Despite those results, not all patients present with a decrease in the gradient and they continue to be at-risk. Surveillance through endoscopy is recommended in those patients.91
It is believed that if risk behaviors are not modified, they could become potential risks for a new infection in patients with SVR. In a meta-analysis, the reinfection risk was as high as 8.2% for injection drug users and 23.6% in patients with HIV/HCV coinfection.163 Other studies have shown a reinfection rate of 2 to 19%.158,164,165 It must also be remembered that patients with risk behaviors can present with a superinfection (infections combined with different hepatotropic viruses).158
Injection drug users are considered at high risk for reinfection. It has been estimated in 13% of patients in the study by Midgard et al.166 and reinfections have been documented with different viral strains.9 Reinfection incidence was 5.8/100 patient-years in those that relapsed into injection drug use after treatment.
It was reported in a meta-analysis that the 5-year risk for reinfection in patients with SVR was 0.9% in those at low risk vs 8.2% in injection drug users or prisoners.167
Recommendations- •
Serum ALT and HCV RNA measurements 48 weeks after treatment completion are recommended in patients with F0 and F2 with SVR. If ALT is normal and HCV RNA is undetectable then the patient can be discharged as cured (B1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
- •
Surveillance for the appearance of hepatocellular carcinoma should be continued every 6 months through ultrasound imaging in patients that have cirrhosis or grade 3 fibrosis and SVR (B1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
- •
Patients that present with cirrhosis and SVR should have endoscopic follow-up for portal hypertension and varices in accordance with international guidelines (A2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Risk for reinfection should be explained to persons with high-risk behavior to positively modify that situation (B1).
Level of agreement: in complete agreement 95%.
In agreement with minor reservations: 5%.
- •
After achieving SVR, persons at high risk for possible HCV reinfection should have follow-up through annual HCV RNA evaluation (B2).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
Hepatitis B virus (HBV) coinfectionHCV/HBV coinfection is more frequent in endemic HBV zones that include Asia, Sub-Saharan Africa, and South America, where coinfection presents in up to 25% of persons with HCV. It is estimated that 2 to 10% of patients with chronic hepatitis due to HCV have simultaneous HBV infection.168–170
The risk factors for simultaneous infection include more advanced age (> 50 years), male sex, Asian ethnicity, illegal drug use (cocaine), greater number of sexual partners, and HIV infection.168–170
The majority of studies indicate that HCV/HBV coinfection is associated with a greater risk for adverse clinical events that include F3-F4 advanced fibrosis in 84.6% of patients vs 29.9% in patients with HCV monoinfection, cirrhosis, liver decompensation, hepatocellular carcinoma, and the need for liver transplantation. 168,171,172
Therefore, carrying out serology for HBV, including HBV surface antigen (HBsAg), HBV core antigen antibodies (total-anti-HBc), and hepatitis B surface antibody (anti-HBs), is recommended in all patients with HCV infection, to determine the presence of coinfection with HBV or occult HBV hepatitis defined as: negative HBsAg, positive anti-HBc, and detectable HBV DNA. All subjects susceptible to HBV infection should be vaccinated.
When HCV/HBV coinfection is diagnosed, it is necessary to determine HCV RNA and HBV DNA levels to know which of the viruses predominates, due to the phenomenon of reciprocal inhibition. In the majority of cases, the dominant virus is HCV. 168,173
Even though treatment is recommended according to the predominant infection, given that SVR rates are the same as in patients with HCV monoinfection, both the therapeutic regimens and the rules for their use are the same as in HCV monoinfection according to genotype. If active replication of both viruses is detected, it is suggested to treat both infections, adding a nucleoside/nucleotide analog against HBV to the regimen.
During treatment for HCV, there is a possibility of HBV reactivation. It has been reported in 36% of patients, including cases of fulminant hepatitis.17,174 Thus, it is necessary to periodically monitor HBV DNA levels during therapy, in periods no shorter than 4 weeks. If HBV DNA is detected, therapy with nucleoside or nucleotide analogs should be started, according to availability.17,173,174 It is important to state that simeprevir increases tenofovir (TDF) levels, with the risk for greater nephrotoxicity, and so strict follow-up is suggested.
Recommendations- •
Coinfected patients should be treated with the same regimens and following the same rules as patients with HCV monoinfection (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
If significant levels of HBV are replicated before, during, or after HCV clearance, concomitant therapy with nucleoside or nucleotide analogs should be indicated (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
Manifestations of immune complex-mediated chronic hepatitis CChronic infection from HCV is associated with multiple extrahepatic manifestations. Because it has been demonstrated that HCV infects hepatocytes, as well as lymphocytes, systemic manifestations mediated by immune complexes, such as B-cell lymphoproliferative disorders that include non-Hodgkin lymphoma (NHL) and mixed cryoglobulinemia (MC), are frequently associated with HCV infection.175,176
There is evidence that subjects with MC can progress to NHL, as was documented in a retrospective study that included 1,255 positive anti-HCV patients with MC and estimated a rate of 660.8 new cases per 100,000 patients/year, representing a 35-fold higher risk, compared with the population in general.177
Diffuse large B-cell lymphoma is the most common type of NHL. Its standard treatment is the R-CHOP (rituximab + cyclophosphamide, adriamycin, vincristine, and prednisone) chemotherapy regimen. In a retrospective case-control study (76 cases vs 228 controls), HCV infection was found to have a negative impact on the survival of patients with NHL and that antiviral treatment against HCV was associated with better response of NHL to chemotherapy and improved 5-year survival.178
There are case reports in which low-grade lymphoma regressed in patients that had obtained SVR with IFN-free regimens.21 In the recent French ANRS HC-13 Lympho-C study, 61 patients with NHL treated with peg-IFN, ribavirin ± first generation protease inhibitors (pegIFN + RBV ± PI) were compared with a control group of 94 patients with HCV and no NHL. A case series of 10 patients with NHL and HCV treated with DAAs was also described. The SVR rate with pegIFN + RBV ± PI in B-cell NHL was 69%, with a premature discontinuation rate of 19.6%. However, the increase in survival was significantly better in the patients with HCV and no NHL, compared with the group with NHL. In the group treated with DAAs, SVR was 90%, with very good tolerance.179
Vasculitis from MC can be severe and life-threatening, given that in addition to palpable purpura, MC syndrome is characterized by multiple organ damage that includes cutaneous ulcers, neuropathy, and glomerulonephritis.175
Achieving SVR is the main goal in patients with MC associated with HCV. The use of IFN-based regimens can result in vasculitis remission in 88 to 97% of patients.180,181 The efficacy and tolerability of the DAA regimens, in combination with IFN or in IFN-free regimens, has been analyzed.20,182 In a recent prospective study, the efficacy and safety of direct-acting antiviral therapy based on sofosbuvir was evaluated. Forty-four consecutive patients with MC associated with HCV were included, and in 2 of them, MC had progressed to indolent lymphoma. SVR was achieved in all the patients at weeks 12 and 24 and vasculitis was resolved in 100% of the patients with isolated MC. There was improvement in the Birmingham Vasculitis Activity Score starting from week 4, as well as a decrease in mean cryocrit values. Interestingly, the two patients with lymphoma had partial response in relation to vasculitis and a 50% decrease in cryocrit values. Adverse events were reported to be mild in 59% of the cases, and one case of anemia required blood transfusion. It was concluded that IFN-free DAA therapy in patients with MC is safe and efficacious.183
Even though results are promising, there is a lack of information related to the efficacy of the combined regimens with B-cell depletion therapy (rituximab) that is usually employed as rescue therapy in cases refractory to antiviral treatment or as an adjuvant in severe cases using the interferon-based regimens.184 Therefore, at present, management of those patients requires the participation of a multidisciplinary team to satisfactorily evaluate the viral response, hematologic-oncologic response, and kidney response, as well as the pertinence and selection of the complementary studies and appropriate regimens in cases that are refractory, despite having achieved SVR.
Recommendations- •
IFN-free regimens should be used in the treatment of lymphoma associated with HCV, even though the effect of SVR on overall outcome is not yet known. Further study is needed on the effect of the new antiviral therapies, together with B-cell depletion, and a multidisciplinary approach with strict liver function monitoring is required in those patients (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Mixed cryoglobulinemia and kidney disease associated with chronic HCV infection should be treated (requiring multidisciplinary management). The role of rituximab in HCV-related kidney disease must be analyzed. HCV replication inhibition and SVR should be correlated with kidney lesion response and cryoglobulinemia. Strict monitoring of adverse effects is essential (B1).
Level of agreement: in complete agreement 92%.
In agreement with minor reservations: 8%.
Advanced chronic kidney failure and patients on hemodialysisThe prevalence of HCV infection in patients with advanced chronic kidney disease is higher than that of the general population and different studies have shown that mortality is greater in patients on dialysis that have HCV infection, compared with uninfected patients.185
Subjects with mild or moderate kidney damage, with a glomerular filtration rate (GFR) of 30-80ml/min/1.73 m2 do not require dosing adjustment for any of the available DAAs and they can be used at the customary doses corresponding to the disease genotype.
Given that sofosbuvir is eliminated via the kidneys and that both its concentrations and its GS-331007 metabolite increase importantly in patients with severe kidney damage with GFR < 30ml/min/1.73 m2 (171 and 451% AUC, respectively, in comparison with those that do not present with kidney damage), its generalized use in that group of patients cannot be recommended with the currently available information.
In the TARGET 2.0 observational cohort study, the safety and efficacy of different regimens with sofosbuvir in patients with kidney damage was reported. Even though SVR (82-83%) was similar between the different grades of kidney damage, there were higher rates of anemia, kidney function deterioration, and serious adverse events in the patients with GFR ≤ 45ml/min/1.73 m2.186
In another study on 17 patients with end-stage kidney disease on hemodialysis or with a GFR < 30ml/min that were treated with simeprevir 150mg and sofosbuvir 200mg daily, with or without ribavirin, one patient with cirrhosis was treated for 24 weeks and the rest were treated for 12 weeks. The adverse events reported were fatigue (28%), anemia (11%), rash or pruritus (11%), and nausea (5%). Two patients were hospitalized: one for diarrhea and the other for encephalopathy. SVR was high. The authors suggested that the treatment could be considered safe and well-tolerated, but the main limitation of their study was the small number of subjects.187
According to the results reported, sofosbuvir-free regimens are preferred in those patients. However, if treatment is urgent, mainly in patients with genotypes 2 and 3, and there are no options without sofosbuvir, then the risk-benefit of sofosbuvir/daclatasvir or sofosbuvir/velpatasvir must be carefully evaluated and the regimen indicated under strict surveillance, adequately informing the patient and his or her relatives, and ideally, requesting their informed consent.
Ribavirin is eliminated via the kidneys and thus is poorly tolerated in patients with advanced kidney damage. Its main adverse event is anemia, and so it is recommended to reduce the dose of ribavirin to 200mg/day or adjust its frequency to 200mg every other day in patients on hemodialysis or suspend it if the hemoglobin value decreases to 8.5g/dl. If ribavirin is indicated, it should be started with a baseline hemoglobin of at least 10g/dl. Surveillance must be strict in patients with ribavirin indication, and hematologic support with erythropoietin or packed red blood cells, as necessary, should be considered. The therapeutic regimens indicated for patients with severe kidney disease are recommended based on two studies on the safety and efficacy of regimens that are INF-free and do not include sofosbuvir.
The first is the RUBY-1 study, which was conducted on 20 treatment-naïve patients with genotype 1 HCV, with no cirrhosis, and with stage 4 advanced end-stage kidney disease (GFR 15-30ml/min/1.73 m2) or on hemodialysis. All were treated with ombitasvir/paritaprevir/ritonavir/dasabuvir for 12 weeks. Thirteen patients with genotype 1a received ribavirin 200mg once-daily and 7 patients with genotype 1b were treated without ribavirin. SVR12 was 18/20 (90%). One patient presented with relapse after treatment completion response and another patient died from a cause unrelated to treatment. In nine of the 13 patients that received ribavirin (genotype 1a), the drug was suspended due to decreased hemoglobin and treated with erythropoietin. Ribavirin could again be added to the treatment of three of those patients.188
The second is the C-SURFER study, with 122 patients with genotype 1 HCV that included cases of compensated cirrhosis (6%) and stage 4 and stage 5 advanced end-stage kidney disease and/or cases on dialysis (75%). They were treated with grazoprevir (100mg) and elbasvir (50mg) for 12 weeks with no ribavirin and SVR was 94% (115/122). The adverse events that most frequently presented were headache, nausea, and fatigue, albeit they occurred with a frequency similar to that of the group that first received placebo and later received grazoprevir/elbasvir. Patients with genotype 4 were not included in that study, but they could be considered in the population with advanced end-stage kidney disease because of the high level of efficacy shown in patients with normal kidney function.189
Recommendations- •
All patients undergoing renal replacement therapy are candidates for antiviral therapy (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Patients undergoing renal replacement therapy should receive an IFN-free regimen that is also ribavirin-free, if possible, for 12 weeks in patients with no cirrhosis and 24 weeks in patients with cirrhosis. If ribavirin is opted for, low doses are recommended (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
The recommended regimens in patients with genotype 1 infection with a low glomerular filtration rate (GFR < 30ml/min) are the combination of paritaprevir/ritonavir/ombitasvir plus dasabuvir, as well as grazoprevir/elbasvir. In patients with genotype 3, sofosbuvir plus daclatasvir or sofosbuvir/velpatasvir can be indicated, under strict surveillance (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
Non-liver solid organ transplantation recipientsAn increased rate of liver fibrosis progression can be associated with non-liver solid organ transplantation recipients with HCV infection. Kidney transplantation performed on an HCV RNA-positive patient is associated with a higher mortality rate in both the patient and graft. Said mortality may be due to a hepatic cause or to an extrahepatic one, mainly of cardiovascular origin, sepsis and/or a new post-transplantation presentation of diabetes.190 Graft survival decreases due to the increase in glomerulopathy, rejection, and diabetes.191
Cirrhosis is a predictive factor of reduced survival after kidney transplantation. Evaluation of liver fibrosis stage in all HCV-positive patients that are candidates for kidney transplantation is recommended.6 The patients with liver cirrhosis and portal hypertension should be evaluated for a combination liver-kidney transplantation.192
In a randomized clinical study, the sofosbuvir and ledipasvir combination, with no ribavirin, was used in patients with or without cirrhosis and with or without previous treatment, resulting in SVR rates of 100% (57/57) and 100% (57/57) in patients with HCV genotypes 1 or 4 that were randomly treated for 12 or 24 weeks. Mean GFR was 50ml/min for the patients that received the 12-week treatment and 60ml/ min for those receiving therapy for 24 weeks. Treatment was well-tolerated and no significant changes in the GFR were observed during or after treatment.193
In a smaller study, 25 kidney transplantation recipient patients with chronic HCV infection were treated with sofosbuvir-based regimens and SVR was 100%. The analysis included patients with genotype 1 infection (76%), GFR > 30ml/min (100%), and advanced fibrosis (44%). The treatments used were ledipasvir/sofosbuvir (n = 9), daclatasvir plus sofosbuvir (n = 4), sofosbuvir plus ribavirin (n = 3), ledipasvir/sofosbuvir plus ribavirin (n = 1), simeprevir plus sofosbuvir plus ribavirin (n = 1), simeprevir plus sofosbuvir (n = 6), and sofosbuvir plus pegIFN/ribavirin (n = 1). Treatment was well-tolerated, no regimen suspensions or reduced doses were required, and there was no graft rejection or changes in creatinine values. Regimen interaction with calcineurin inhibitors was managed according to established guidelines and was not a significant problem.194
In another study that included 20 patients with HCV and kidney transplantation (88% with genotype 1, half with advanced fibrosis, and 60% treatment-experienced), they received several combinations of sofosbuvir-based regimens: simeprevir plus sofosbuvir (n = 9), ledipasvir/sofosbuvir (n = 7), sofosbuvir plus ribavirin (n = 3), and daclatasvir plus sofosbuvir (n = 1). SVR was 100%. Two patients required reduced dosing due to anemia (in subjects receiving ribavirin). There were no significant changes in creatinine values, proteinuria, or graft rejection before or after treatment. Forty-five percent of the patients required reduced immunosuppression dosing while they were receiving antiviral therapy. Other studies reported a high SVR rate and good safety level 195 in patients treated with different treatment regimens after kidney transplantation.196,197
The available data on the management of patients with HCV infection that underwent heart transplantation are scare. With the existing studies, we cannot conclusively establish the long-term benefits of the new therapies in that group of patients. The same holds true for lung transplantation recipients, but based on reports and case series, IFN-free and sofosbuvir-based regimens can be considered safe and efficacious.198 There are no data available on HCV infection and treatment after transplantation of the pancreas or bowel. In those contexts, and given the experience accumulated through the treatment of liver transplantation recipients, it is suggested that those patients can be treated with a high level of expectation in relation to SVR and safety. Given that the combinations of sofosbuvir with an inhibitor of the NS5A region, such as ledipasvir, velpatasvir, or daclatasvir, do not require immunosuppressant adjustments (with the probable exception of everolimus), those regimens can be considered first-line in post-transplantation subjects. The recipients of organ transplantation, including the kidney, heart, lung, pancreas, and small bowel, should be evaluated for HCV and receive treatment.
Recommendations- •
HCV treatment before kidney transplantation can prevent mortality related to a hepatic cause and prevent kidney graft dysfunction. Those patients should receive IFN-free treatment that is also ribavirin-free, if possible, for 12 weeks in patients with no cirrhosis and for 24 weeks in patients with compensated cirrhosis (Child-Pugh A), following the abovementioned recommendations. The use of those medications must be accompanied by strict surveillance (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
In non-liver solid organ transplantation recipients, patients indicated for antiviral therapy should receive an IFN-free regimen, following the recommendations described above, with adequate surveillance of pharmacologic interactions with immunosuppressants (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
Treatment of patients with severe liver diseaseThe patients with decompensated cirrhosis of the liver (Child-Turcotte-Pugh [CTP] class B or C) should be referred to a specialized center that has a transplantation program and physicians that are experts in the management of patients with advanced liver disease. Given that there are not enough randomized prospective studies on DAA use that allow them to be employed with absolute certainty, much in relation to their use depends on expert opinion, small observational studies, and especially on the risk there is for decompensation induced by or during treatment, especially in CTP class C patients.
Interferon is contraindicated in patients with decompensated cirrhosis. DAA treatment regimens are a new option for that group of patients, regardless of whether or not they will undergo transplantation. In fact, it is in that group of patients that treatment should be intermediate and prioritized, in an effort to prevent further decompensation of their liver function reserve.
Liver transplantation is the treatment of choice for patients with chronic liver disease and although the best time for starting HCV treatment is still a subject of debate, it should always be carried out whenever recurrence occurs in the graft, decreasing its expectation of function in the absence of prevention.105 There is no randomized prospective study that determines whether treatment should be carried out before or after transplantation, and therefore the decision is based on each center's experience, results reported by other centers, and expert opinion. Treatment has two goals: to prevent reinfection of the graft and to maintain integrated liver function, all of which makes the medical treatment of the transplantation patient easier. In some cases, HCV eradication delays the need for transplantation, even to the point of removing the patient from the waiting list.6,120 A possible problem is that some patients could undergo transplantation before completing HCV eradication treatment. Another is that the patient with advanced liver disease could be removed from the waiting list, a scenario in which there is always the risk for developing hepatocellular carcinoma or decompensation characteristic of the grade of liver failure, either of which can be the cause of a higher mortality rate in patients that do not undergo transplantation.
The use of sofosbuvir and ribavirin (an initial 600mg, then increased depending on tolerance and according to weight) for 4 weeks prior to transplantation in patients infected with genotype 1 or 4 has been shown to prevent reinfection of the graft in the majority of cases.7 However, today that combination is insufficient and thus not recommendable.
A very important aspect to consider is the contraindication for the use of the protease inhibitors in patients with liver failure, due to inadequate metabolism that causes higher concentrations in those with CTP class C. In fact, they should also be used very cautiously in patients with CTP class B, given that there have been reports in which cases of borderline liver function become decompensated with protease inhibitor administration. 8,199 Therefore, the combinations to consider in that group of patients are: sofosbuvir and an NS5A inhibitor, such as daclatasvir, ledipasvir, or velpatasvir. The SOLAR-1 study was conducted on patients infected with genotype 1 or 4 and with CTP class B decompensated cirrhosis, treated with the combination of sofosbuvir-ledipasvir with ribavirin for 12 to 24 weeks. SVR at 12 months was 87% with 12 weeks of treatment and 89% with 24 weeks of treatment. In CTP class C patients, SVR figures were 86 and 87% with 12 and 24 weeks of treatment, respectively. In that study there was improvement in both the MELD and CTP scales in half of the patients.120 In the SOLAR-2 study, which was identical in design, SVR rates were 87 and 96% in patients with Child B disease stage and 85 and 78% in patients with Child C. Improvement was also documented in the MELD and CTP scales.101
The ASTRAL- 4 study included patients with CTP class B decompensated cirrhosis and genotypes 1 to 4, and 6. They were randomized to receive sofosbuvir-velpatasvir with no ribavirin for 12 weeks or with weight-based ribavirin, or 24 weeks with no ribavirin. SVR at 12 months was 88, 94, and 93%, respectively, in patients with genotype 1a and 89, 100, and 88% with genotype 1b. In the few patients with genotype 2 (4 in each group), SVR was 100% with no ribavirin and with the weight-based dose, but it was only 75% in the patients that received ribavirin for 24 weeks. SVR in patients with genotype 3 was 50, 85, and 50%, respectively. The number of patients with genotype 4 infection was even smaller. In the patients with a MELD score < 15 points, there was improvement in half of the cases, no change in 22%, but 27% of the patients worsened. In the patients with a MELD score > 15 points, 81% had score improvement and only 7% of the cases worsened. As pointed out previously, a limitation of the ASTRAL-4 study was its small sample of genotypes 2, 4, 5, and 6. Despite the high SVR rates with no ribavirin observed, given the small sample sizes, it is recommended to include ribavirin in the treatment, even though it might not be necessary.124
A preliminary practical experience study conducted in England on patients with genotype 1a infection that received a 12-week regimen of sofosbuvir and ledipasvir, with or without ribavirin, showed 85% SVR without ribavirin and 91% when ribavirin was added. A small group of patients treated with sofosbuvir and daclatasvir, with no ribavirin, barely achieved 50% SVR without ribavirin and 88% when ribavirin was added.
In patients with genotype 3 infection, SVR in 2 small groups of 5 patients was 60% without ribavirin and 71% with the addition of ribavirin.38 Again, approximately one-third of the patients had MELD score improvement, one-third had no difference, and one-third worsened.
The experience with DAAs in the group of patients with advanced liver failure demonstrated by a multicenter European daily practice study showed that liver dysfunction could be reverted in approximately one-third of the cases and 20% of the patients had sufficient improvement to be removed from the transplantation waiting list over the course of one year. That was more apparent in the patients with a low MELD score. Nevertheless, those benefits must be considered along with the risk that not undergoing transplantation implies, as well as the risk for decompensation or the development of HCC. 5,200
A recent report of a mathematical analysis on the results of the SOLAR-1 and 2 studies and the experience of the United Network for Organ Sharing (UNOS) suggest that patients with a MELD score < 27, and particularly those with a MELD score of 23 or lower (range 10 to 40), should be treated with DAAs before transplantation, which was associated with improved survival, even when some of the patients did not undergo transplantation.201
Likewise, in another analysis of a mathematical model utilizing information from the 2003-2015 Scientific Registry of Transplant Recipients database that included 49,500 patients that underwent transplantation and DAA responses, a 32% reduction of patients on the transplantation waiting list due to HCV was described. That contrasted with the patients with liver steatosis, in which the need for transplantation increased due to the decompensation of liver function in 42% of the patients within the same time frame.202
Hepatitis C can be treated in patients with HCC with no cirrhosis or with compensated cirrhosis and indicated for transplantation. In fact, treatment should not delay transplantation because it prevents recurrence, improving post-transplantation outcome. Treatment in that group of patients should be the same as in patients with no HCC, depending on genotype, on having received previous treatment, and on the intensity of liver failure.116
In one study, 61 patients with HCC and genotype 2 or 3 HCV infection, according to the Milan criteria for liver transplantation, were treated with sofosbuvir and ribavirin for 48 weeks.7 The virologic post-transplantation response at week 12 was 70%. Twenty-three percent of the patients had recurrence of the virus and 7% died.
Thus, the recommended treatment for patients with genotypes 1 or 4 and decompensated cirrhosis, whether or not they are transplantation candidates, and including patients with HCC, should be the 12-week combination of ledipasvir 90 mg-sofosbuvir 400mg in a single tablet and ribavirin 600mg daily, increasing the ribavirin according to tolerance and weight. In patients with ribavirin intolerance or those with anemia, ledipasvir-sofosbuvir should be administered for 24 weeks. In the patients that have had previous treatment failure with a regimen with sofosbuvir, the recommended treatment is the combination of ledipasvir 90 mg-sofosbuvir 400mg plus ribavirin 600mg for 24 weeks. In patients with genotype 2, the sofosbuvir, daclatasvir, and ribavirin regimen is another alternative.
Recommendations- •
Patients with decompensated cirrhosis (Child-Pugh B and C, up to 12 points) that are not on the liver transplant waiting list and have no concomitant comorbidities that could affect their survival should receive immediate treatment with any of the following regimens: sofosbuvir and ribavirin for 16 to 20 weeks (genotype 2), a fixed-dose combination of sofosbuvir and ledipasvir (genotypes 1, 4, 5, and 6), or the combination of sofosbuvir and daclatasvir or sofosbuvir/velpatasvir (all genotypes) with a weight-based ribavirin dose, for 12 weeks (B1).
Level of agreement: in complete agreement 86%.
In agreement with minor reservations: 14%.
- •
Patients with decompensated cirrhosis, in whom ribavirin is contraindicated or poorly tolerated, should receive the combination of sofosbuvir and ledipasvir (genotypes 1, 4, 5, or 6), or the combination of sofosbuvir and daclatasvir, or sofosbuvir/velpatasvir (all genotypes) for 24 weeks, with no ribavirin (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Patients that underwent resection or ablation due to HCV-associated HCC should receive adequate antiviral treatment for their liver disease, in accordance with the previously mentioned recommendations (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
HCV in patients on the liver transplant waiting list should be treated if the waiting period exceeds 6 months, to prevent post-transplantation recurrence (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Treatment should be started as soon as possible, so it can be completed before transplantation and the effect of elimination of the virus (SVR) on liver function can be evaluated. Liver function could significantly improve, resulting in the patient's removal from the waiting list (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Patients waiting for a liver transplant should be treated with an IFN-free regimen, and the addition of ribavirin, for 12 or 24 weeks, or alternatively, after the transplantation (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Patients with conserved liver function (Child-Pugh A) that are indicated for transplantation due to HCC can be treated with the combination of sofosbuvir and ribavirin for 16-20 weeks (genotype 2); the fixed-dose combination of sofosbuvir/ledipasvir with ribavirin for 12 weeks (genotypes 1, 4, 5, or 6); the combination of paritaprevir potentiated by ritonavir, ombitasvir, and dasabuvir, with ribavirin, for 12 weeks (genotype 1b) or 24 weeks (genotype 1a); the combination of paritaprevir potentiated by ritonavir and ombitasvir, with ribavirin, for 12 weeks (genotype 4); the combination of sofosbuvir and simeprevir, with ribavirin, for 12 weeks (genotypes 1 and 4); or with the combination of sofosbuvir and daclatasvir or sofosbuvir/velpatasvir, with ribavirin, for 24 weeks (all genotypes) (B1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
- •
Treatment with pegIFN-α, ribavirin, and sofosbuvir for 12 weeks is acceptable in patients with compensated cirrhosis (Child-Pugh A) that are waiting for a liver transplant, if the IFN-free combinations are not available. However, it should be specified that there is less efficacy and a lower safety profile in that patient population (B2).
Level of agreement: in complete agreement 92%.
In agreement with minor reservations: 8%.
- •
Patients with decompensated cirrhosis (Child-Pugh B or C) that are waiting for a liver transplant can be treated with the combination of sofosbuvir and ribavirin for 16-20 weeks (genotype 2); with the fixed-dose combination of sofosbuvir and ledipasvir, with ribavirin, for 12 weeks (genotypes 1, 4, 5, or 6); or with the combination of sofosbuvir and daclatasvir or sofosbuvir/velpatasvir, with ribavirin, for 24 weeks (all genotypes). However, there are limited data on patients with cirrhosis that have Child-Pugh C > 12 points or a MELD score > 20 (A1).
Level of agreement: in complete agreement 78%.
In agreement with minor reservations: 22%.
- •
The ideal time for treating patients before or after transplantation continues to be a subject of debate and requires individual evaluation (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Considering the limited treatment safety data available in relation to patients with decompensated cirrhosis that are on the waiting list for a liver transplant, their evaluation and management should be carried out at a medical center with a transplantation program (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
In patients with decompensated cirrhosis, ribavirin can be started at a dose of 600mg daily, with later tolerance-related dosing adjustments (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Patients in whom ribavirin is contraindicated or poorly tolerated during treatment, should receive the fixed-dose combination of sofosbuvir and ledipasvir (genotypes 1, 4, 5, or 6); the fixed-dose combination of sofosbuvir and velpatasvir (all genotypes); or the combination of sofosbuvir and daclatasvir (all genotypes) for 24 weeks, with no ribavirin (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
Special group treatmentActive drug users and stable patients in replacement therapyThere is a high prevalence of chronic hepatitis C virus infection in the population of subjects with injection drug addiction and it is the main known risk factor for that infection in some countries. An example is the cohort from the United Kingdom in which 90% of HCV infections were due to injection drug use.203 Mexico is no exception. In a study conducted in the cities of Tijuana and Juárez, where the prevalence of injection drug addicts is high, it was found that 85% of the addicts had HCV infection after 2 years of injection drug use and 100% of the study subjects with more than 6 years of addiction were infected with hepatitis C.204
Given the high prevalence of hepatitis C virus infection in that population, it is important to include harm reduction programs to reduce infection transmission.205 Such programs include needle and syringe provision, through which a reduction in new infections has been demonstrated.206 However, it is important for Public Safety policies or programs to be linked to those of the Public Health Sector, otherwise harm reduction programs, such as those of needle and syringe provision, will not work and can become deleterious factors. That was demonstrated in a study conducted in Tijuana and Juárez, in which public safety policies became a deleterious factor in the sterile needle and syringe provision program. Despite the fact that it is not illegal to buy or carry syringes, it was common for drug users carrying syringes to be arrested, favoring consequent needle sharing with other drug users.207
Injection drug users should be counselled to moderate marijuana consumption or abstain from it if there is evidence of liver disease. Its use has been clearly shown to promote the progression of inflammation, fibrosis, and liver steatosis. In addition, it is considered an independent fibrosis progression factor in hepatitis C.208,209 Research studies have shown that inhibition of cannabinoid CB1 receptors in the liver reduces fibrosis progression.210,211
Numerous studies have shown that alcohol consumption in persons with hepatitis C results in greater liver damage and more rapid disease progression.212–214 Thus it is essential to counsel injection drug users, as well as the rest of the population with hepatitis C infection, to moderate or abstain from alcohol consumption if there is evidence of liver damage.
Abstinence from alcohol is extremely important for maintaining or prolonging liver health. Studies on injection drug users with hepatitis C and alcohol consumption have demonstrated liver function benefit in the patients that were able to abstain from alcohol consumption.215
Treatment response in the addict populationInjection drug users face more barriers to having access to treatment, compared with other persons,216,217 and for a long time, patients with active addiction to injection drugs were excluded from treatment. However, there is no information in the literature or evidence of greater risk for virologic failure in the population with a history of injection drug use or with active injection drug use. Studies on populations with chronic hepatitis C virus infection secondary to injection drug use have shown that it is not a risk factor for virologic failure. In fact, studies from the era of interferon and ribavirin showed 95% SVR (100% for genotype 3 and 86% for genotype 1), which was most likely associated with a lower fibrosis grade and younger study populations.218 Studies on addict populations have shown a similar response to that of the non-addict population. A study on a Swiss cohort included patients with active addiction, compared with controls (199 addicts and 301 controls), and results were similar in the two groups.219 Therefore, today, active injection drug addiction is not considered a contraindication for treatment and the decision to treat or not to treat should be an individualized one.
Patients with active addiction behave the same as those with a history of injection drug addiction and those in a replacement program, with similar SVR. However, greater patient loss was demonstrated in the subjects that received replacement therapy and IFN, due to side effects (36%) and abandonment (46%).220 Therefore, injection drug users undergoing opioid replacement therapy should receive an IFN-free regimen.
Considering that hepatitis C virus infection prevalence and incidence are high in injection drug addicts, counselling that includes risk factors for infection and reinfection, disease progression, treatment regimens, and potential harm-reducing strategies is essential. Studies have shown that the addict population faces many barriers that promote poor adherence to treatment and treatment failure. Other factors associated with the chaotic lifestyle can also result in a poor perception of medical treatment, creating obstacles to communication that favor inferior treatment adherence and abandonment due to inadequate doctor-patient communication. Practical strategies in treating injection drug users involve multidisciplinary management, adequate adverse effect management, education, and counselling.221 In addition, it is necessary to include programs dedicated to the pharmacologic management of addiction or to needle and syringe provision to prevent reinfection.222
Subjects with injection drug addictions are a high-risk group for infection with and an elevated prevalence of hepatitis C. That population group also faces the greatest barrier to having access to treatment and clinical research trials. There is evidence, albeit scant, that the therapeutic response to the new direct acting antivirals is similar to that of the pegIFN and ribavirin regimens in injection drug users, when compared with the general population, with the only difference being better treatment adherence. Nevertheless, the studies have small samples and no statistical power for generalizing or extrapolating their results, thus more research on the injection drug-using population is needed. 218,223
Liver transplant in patients that are injection drug usersThe current most common indication for liver transplantation is liver disease due to hepatitis C virus infection and one of the main risk factors for that infection is injection drug use. Comparative population group studies have shown that persons with a past history of injection drug use that required liver transplantation due to cirrhosis of the liver did not have substance use relapse. The retransplantation risk was the same as that in the rest of the population, with similar post-transplantation progression to fibrosis, and patient and graft survival. Therefore, a history of drug addiction should not be an exclusion criterion and transplantation should be considered a therapeutic option in that population.224
There are liver transplantation centers in which, out of fear of or as a precautionary measure against addiction relapse and consequent failure of the liver transplant, one of the pre-transplantation requisites is that subjects are not actively using methadone or receiving another opioid replacement therapy. However, a review conducted by researchers at the Mount Sinai Medical Center included 36 subjects using methadone at the time of liver transplantation and found that only one of the patients had drug use relapse, and graft survival was similar to that of the national average. Based on those data, there is no contraindication for transplantation in patients with active methadone use.225
In another 4-year case series that included 33 patients with liver transplantation and stable methadone use, 4 patients (11%) had heroin use relapse. However, that did not affect survival or graft complications, thus permitting the recommendation that, until there is evidence to the contrary, the stable use of methadone should not be a contraindication for liver transplantation.226
Recommendations- •
Anti-HCV antibody determination should be carried out in active injection drug users (IDUs), and if negative, repeated every 6-12 months (A1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
IDUs should receive sterile injection material and have access to opioid replacement therapy as part of the integrated harm reduction programs, even in prisons (B1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
- •
All patients should be offered pre-treatment education and counseling that includes: HCV transmission routes, risk factors for fibrosis progression, treatment regimens, risk for reinfection, and potential strategies for harm reduction (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
IDUs should be counseled to abstain from alcohol consumption (A1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
- •
IDUs should be counseled to abstain from marijuana consumption (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
HCV treatment should be considered in an individualized manner for IDUs and administered by a multidisciplinary team (A1).
Level of agreement: in complete agreement 95%.
In agreement with minor reservations: 5%.
- •
A history of injection drug use and recent drug use at the start of treatment are not associated with reduced SVR and the decision to treat or not to treat should be made in an individualized manner (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Drug and alcohol users or other patients with social problems and/or a history of psychiatric disease, and those that consume drugs during treatment, are at risk for lower treatment adherence and less probability of achieving SVR. Thus, they should be closely supervised during treatment and receive multidisciplinary support (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
An evaluation of the safety and efficacy of the new antiviral regimens in IDUs is necessary (C1).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
- •
IDUs undergoing opioid replacement therapy should receive an IFN-free regimen (B1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
- •
Antiviral regimens for IDUs are the same as those for the rest of the infected population. Dosing adjustments for methadone or buprenorphine are not necessary, but signs of toxicity or opioid withdrawal should be monitored. More data on the regimens that include daclatasvir are needed in that group of patients (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
If indicated, liver transplantation should be considered as a therapeutic option in patients with a history of injection drug use (B1).
Level of agreement: in complete agreement 91%.
In agreement with minor reservations: 9%.
- •
Opioid replacement therapy is not a contraindication for liver transplantation and does not need to be adjusted (B1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
Hemoglobinopathies and bleeding disordersBeta thalassemia is a hemoglobinopathy that is more frequently associated with chronic hepatitis C virus infection, as is sickle cell anemia. Both require frequent transfusions and present more rapidly with liver damage, due to concurrent iron overload.227 Treatment with pegIFN plus ribavirin is not recommended because it can induce the development of anemia and increase the need for transfusions.228 There are few studies using DAAs for the treatment of those patients, but there is no factor that contraindicates antivirals in those patients.
In the C-EDGE IBLD study, 12-week treatment with grazoprevir plus elbasvir with no ribavirin was administered to 107 patients with beta thalassemia and sickle cell anemia with genotype 1a, 1b, and 4 infections. Twenty-four percent of the patients had cirrhosis of the liver. SVR12 was 93.5% for the study population. Reports of cases of treatment with sofosbuvir plus simeprevir have been described in patients with thalassemia, and studies with larger populations are being developed.229
Hemophilia and von Willebrand disease are bleeding disorders that until a few years ago required constant transfusions, with a high risk for HCV transmission. Progression of liver damage from HCV is similar in the 2 diseases. Treatment of hepatitis C virus in patients with hemophilia is not different from treatment in non-hemophiliacs. In the C-EDGE IBLD study arm for patients with hemophilia and von Willebrand disease, 91% SVR was achieved with the combination of grazoprevir plus elbasvir.230 In another study, the combination of ledipasvir/sofosbuvir plus ribavirin was analyzed in 14 patients with bleeding disorders, and 100% SVR12 was reported.231 Finally, in a real-life study that included 18 patients, mainly with the combination of ledipasvir/sofosbuvir, with and without ribavirin for 8 or 12 weeks, in which some of the patients were treatment-experienced, SVR12 was 94%. It was concluded that real-life use of IFN-free therapies was safe and effective in patients with bleeding disorders.232
Recommendations- •
Indications for HCV therapy are the same in patients with or without hemoglobinopathies (A1).
Level of agreement: in complete agreement 95%.
In agreement with minor reservations: 5%.
- •
Patients with hemoglobinopathies should be treated with an IFN-free regimen, with no ribavirin (B1).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
- •
Antiviral regimens are the same in patients with or without hemoglobinopathies (B1).
Level of agreement: in complete agreement 96%.
In agreement with minor reservations: 4%.
- •
When ribavirin use is indispensable, close surveillance is recommended (B2).
Level of agreement: in complete agreement 100%.
In agreement with minor reservations: 0%.
CommentsIn the treatment of hepatitis C virus, the addition of DAAs has changed the perspective for patients, resulting in extraordinary rates of success and treatment tolerance. However, there are still some challenges to be met, such as having regimens and combinations that are effective, regardless of genotype or the presence of advanced liver damage. The present document only considered the drugs that were available at the time of the consensus meeting, but the reader should be aware of the incorporation of other promising alternatives recently approved by the FDA for simplifying management decisions. Nevertheless, they are not contemplated in the present consensus guidelines, given that they are not yet available in Mexico, nor did they undergo the screening and voting processes described in the methodology of the present document.
We find it relevant to comment on the following two emerging options. The combination of sofosbuvir-velpatasvir-voxilaprevir, developed by the Gilead company, is the first pangenotypic option available in a single tablet at a fixed dose that includes medications from three different classes of antivirals for HCV. It can be given for 8 weeks in all cases, with the exception of patients with genotype 1a, in whom it must be given for 12 weeks. In addition, that regimen fills an important position in relation to patients that have had DAA treatment failure, given that the presence of resistance in NS5A, NS3, or NS5B does not appear to influence the possibilities of achieving SVR, which has been reported at 96% in that group of patients.233 Unfortunately, the presence of the protease inhibitor, voxilaprevir, does not allow that option to be an alternative in patients with moderate-to-severe liver disease in Child class B or C.
Furthermore, the combination of glecaprevir-pibrentasvir is now being prescribed in the United States. It was developed by the AbbVie company, and is the first pangenotypic NS3/4A protease inhibitor-NS5A inhibitor combination regimen. It is a strong ribavirin-free option for the majority of patients with HCV, even those with no cirrhosis, with kidney disease, or with HIV infection, and is administered for 8 weeks. Because it includes a protease inhibitor, that drug is not an option in patients with decompensated cirrhosis in Child class B or C. In registry studies, SVR ranged from 98-100% with 8 or 12-week regimens in patients with genotypes 1, 2, 5, or 6 and there was very little virologic relapse during or after treatment. 234 The glecaprevir-pibrentasvir combination is the least expensive of the DAAs, a relevant fact in the economic context of Mexico. It costs up to 50% less, compared with the other antivirals available, and therefore its expansion and consequent transformation of the worldwide treatment panorama can be anticipated.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Aiza-Haddad I, Ballesteros-Amozurrutia A, Borjas-Almaguer OD, Castillo-Barradas M, Castro-Narro G, Chávez-Tapia N, et al. Consenso Mexicano para el Tratamiento de la Hepatitis C. Revista de Gastroenterología de México. 2018;83:275–324.