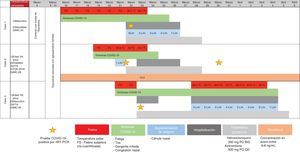
Coronavirus disease 2019 (COVID-19) is a serious respiratory illness caused by the SARS-CoV-2 virus.1 At present, data on COVID-19 in liver transplant (LT) recipients are scarce, and there is controversy about whether their immunosuppressive status is a risk factor or a protective factor for developing severe disease. International teams have published that immunosuppression does not represent a higher risk for developing severe pneumonia2–4 but the mechanism still remains unclear. We report herein the clinical outcome of three family members that had COVID-19 infection, presenting with and without different risk factors that have been described in more severe disease. Paradoxically, the LT recipient presented with a better outcome (Fig. 1), raising the question of the potentially beneficial effect of tacrolimus.
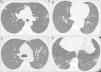
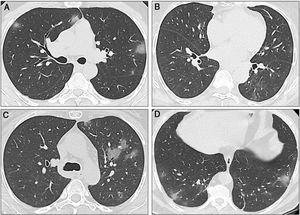
A 41-year-old man, with no past medical history, arrived at the emergency department (ED), with fever and shortness of breath, on March 22. Physical examination revealed fever, oxygen saturation (O2 sat) of 88%, and bibasilar crackles. He was admitted to the hospital. Laboratory tests upon admission reported lymphopenia (complete biologic data are available in Tables 1 and 2). A computed tomography (CT) scan identified bilateral ground-glass opacities (GGOs) and septal thickening (Fig. 2A). The nasopharyngeal swab test for SARS-CoV-2 was positive and treatment with hydroxychloroquine and azithromycin was started. Fever was present during the first two days of hospitalization, and supplemental oxygen delivered by nasal cannula was required for three days. The patient became asymptomatic after the third day and was discharged.
Clinical laboratory test results from all three cases on admission and/or days 3, 5, or 8 of illness.
| Case 1 | Case 2 | Case 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Reference range | Day 0 | Day 3 | Day 3 | Day 5 | Day 0 | Day 5 | Day 8 |
| White cell count (per µL) | 4,000-12,000 | 4,800 | 3,900 | 2,800 | 2,800 | 5,000 | 4,300 | 6,300 |
| Absolute lymphocyte count (per µL) | 1,000-3,900 | 870 | 980 | 870 | 1,000 | 1,100 | 840 | 500 |
| Total bilirubin (mg/dL) | 0.3-1.0 | 0.5 | 0.3 | 0.4 | 0.3 | 0.4 | 0.3 | 0.5 |
| Alanine aminotransferase (U/L) | 7-52 | 24 | 17 | 12 | 9.5 | 19 | 24 | 17 |
| Creatinine (mg/dL) | 0.6-1.2 | 1.1 | 0.8 | 1.1 | 0.8 | 1.5 | 1.4 | 1.6 |
| Lactate dehydrogenase (U/L) | 120-246 | 160 | 193 | N/A | N/A | 160 | 217 | 276 |
| C-reactive protein (mg/dL) | 0.0-1.0 | 0.9 | 0.7 | 0.4 | 0.3 | 1.7 | 10.4 | |
| D-dimer (ng/mL) | 0-500 | 187 | N/A | N/A | N/A | 177 | N/A | 296 |
| Prothrombin time (seconds) | 9.4-12.5 | 12.8 | N/A | N/A | 10.5 | N/A | N/A | N/A |
| Troponin (pg/mL) | < 15 | 1.7 | N/A | 3.8 | 3.1 | N/A | N/A | N/A |
| Creatine phosphokinase (U/L) | 30-223 | 79 | N/A | 61 | 37 | 120 | 114 | N/A |
| Ferritin (ng/mL) | 11-306.8 | 250 | 263 | 141 | 138 | 74 | 128 | 276 |
N/A: not available.
Additional clinical laboratory and microbiologic test results from case 2.
| Measure | Reference range | March 6 | March 23 | March 25 |
|---|---|---|---|---|
| White cell count (per µL) | 4,000-12,000 | 4,800 | 2,800 | 2,800 |
| Absolute lymphocyte count (per µL) | 1,000-3,900 | 2,300 | 900 | 1,000 |
| Absolute neutrophil count (per µL) | 1,900-7,400 | 3,200 | 1,600 | 1,500 |
| Hemoglobin (g/dL) | 13.0-15.7 | 12.4 | 13.6 | 12.9 |
| Platelet count (per µL) | 150,000-450,000 | 212,000 | 174,000 | 155,000 |
| Hematocrit (%) | 38.3-46.7 | 41.0 | 38.5 | 36.2 |
| Sodium (mmol/L) | 136-146 | 136 | 135 | 135 |
| Potassium (mmol/L) | 3.5-5.1 | 4.0 | 4.4 | 4.4 |
| Chloride (mmol/L) | 98-107 | 100 | 102 | 106 |
| Glucose (mg/dL) | 70-99 | 169 | 324 | 166 |
| Creatinine (mg/dL) | 0.6-1.2 | 0.69 | 1.10 | 0.89 |
| Total bilirubin (mg/dL) | 0.3-1.0 | 0.5 | 0.4 | 0.3 |
| Alanine aminotransferase (U/L) | 7-52 | 13 | 12 | 9.5 |
| Albumin (g/dL) | 3.5-5.7 | 3.2 | 3.9 | 3.67 |
| Venous lactate (mmol/L) | 0.5-1. 6 | N/A | 0.7 | N/A |
| C-reactive protein (mg/dL) | 0-1 | 3.9 | 0.67 | N/A |
| Prothrombin time (seconds) | 9.4-12.5 | 10 | 11.4 | N/A |
| Troponin (pg/mL) | < 15 | N/A | 3.8 | 3.1 |
| Creatine phosphokinase (U/L) | 30-223 | N/A | 61 | N/A |
| Ferritin (ng/mL) | 11-306.8 | N/A | 141 | N/A |
| Procalcitonin (ng) | <0.05 | N/A | <0.05 | N/A |
| Tacrolimus (ng/mL) | --- | 7.3 | N/A | |
| Urine culture | Negative | N/A | N/A | Negative |
| Influenza A PCR | Negative | N/A | N/A | Negative |
| Influenza B PCR | Negative | N/A | N/A | Negative |
| Blood cultures | Negative | N/A | N/A | Negative |
| HBV PCR | Negative | N/A | N/A | Negative |
| HCV PCR | Negative | N/A | N/A | Negative |
| CMV PCR | Negative | N/A | N/A | Negative |
| EBV PCR | Negative | N/A | N/A | Negative |
N/A: not available.
Sequential computed tomography scans performed in the three cases. A) Case 1: CT scan shows multiple ground-glass opacities (GGOs) in the upper and lower lobes, with bilateral and peripheral distribution. Some GGOs can also be seen next to the left hilum. These image findings are typical of SARS-CoV-2 pneumonia. B) Case 2: The initial CT scan shows subtle GGOs in the inferior right lobe. In the first follow-up scan, new areas of GGOs are visible in the superior left lobe. C) Case 2: The second CT scan shows that the GGOs increased in size, number, and density. Bilateral involvement is present (not shown). D) Case 3: The CT scan shows a single subpleural GGO. Multiple areas of ground-glass opacities with the classic COVID-19 appearance can be seen, as well as some linear opacities associated with the GGOs.
A 64-year-old woman underwent LT in 2018 due to liver cirrhosis secondary to hepatitis C virus (HCV) that was treated with velpatasvir/sofosbuvir. She had a medical history of type 2 diabetes mellitus (DM2) and arterial hypertension (HTN), and her immunosuppression regimen consisted of tacrolimus (levels: 6-8 ng/mL). On March 21, the patient arrived at the ED with cough, diarrhea, and fever. Laboratory test results showed leukopenia and lymphopenia. A CT scan revealed ground-glass opacities (Fig. 2B). The nasopharyngeal swab test for SARS-CoV-2 was positive. The patient was hospitalized for surveillance and treatment with hydroxychloroquine, azithromycin, and ceftriaxone. Due to drug interaction between tacrolimus and azithromycin, the tacrolimus dose was diminished. Two days after admission, a new CT scan showed progression of pneumonia (Fig. 2C) but no oxygen supplementation was required. The patient was discharged on March 26 (Tables 1 and 2).
Case threeA 60-year-old man with a history of HTN and kidney cancer in remission arrived at the ED on March 23, with a two-day history of fever and cough. Physical examination revealed fever and adequate oxygen saturation. The laboratory test results were not relevant. A CT scan showed subpleural and basal GGOs (Fig. 2D). The nasopharyngeal swab test for SARS-CoV-2 was positive. The patient progressed favorably and was discharged 48 hours after admission. However, on March 28, he returned to the ED with shortness of breath. Vital signs revealed O2 sat at 86% (on room air) and the patient was re-admitted. O2 sat improved after supplemental oxygen was administered via a nasal cannula. Blood tests also showed lymphopenia. A CT scan identified pneumonia progression. Fever was present for 5 days and the patient required oxygen support. He completed 14 days of hydroxychloroquine plus azithromycin and was discharged on April 6.
The three cases that tested positive for COVID-19 reported herein were members of the same family. Case 1 had no risk factors; case 2 had major risk factors and comorbidities, such as 64 years of age, short-term LT (< 2 years), HTN, and DM2, and was under treatment with tacrolimus; case 3 was a 60-year-old man with HTN. When the clinical evolution of the family was compared, the progression of the immunosuppressed patient was more favorable, despite presenting with various adverse clinical factors (Fig. 1).
Two Italian studies have shown that the mortality rate of COVID-19 is not higher in LT recipients. In a study on 200 transplant recipients, including ten current inpatients, D’Antiga et al.2 reported that none of the patients presented with clinical pulmonary disease, despite three testing positive for SARS-CoV-2. Bhoori et al.3 reported the clinical outcomes of 111 long-term LT recipients and 40 short-term recipients (LT < 2 years). Six tested positive for SARS-CoV-2: three were long-term recipients and three were short-term recipients. The COVID-19-related deaths occurred in the three long-term patients on minimal immunosuppressive regimens, as opposed to the recently transplanted, fully immunosuppressed patients,3 suggesting that the risk of severe complications in immunosuppressed patients is not greater than that of the general population. Those findings were recently confirmed in a multicenter international study.4
The aim of the present clinical observation was to show a possible beneficial effect of tacrolimus in patients with COVID-19. The immunosuppressive properties of tacrolimus depend on the formation of a binary complex with FKBP proteins. Those complexes sequester and inhibit calcineurin activity (through the formation of a ternary complex), which is a key player in T cell activation.5,6 The interaction with calcineurin inhibits its phosphatase activity, preventing the nuclear translocation of its substrate, the nuclear factor of activated T cells, and the consequent expression of immunity genes, such as IL-2 and IL-4. In this manner, tacrolimus suppresses the activation of T cells.5,7 In addition, tacrolimus has an antiviral effect by binding to the FKBP proteins, with the subsequent inhibition of their peptidyl-prolyl isomerase activity, whose enzymatic activities are posited to promote coronavirus replication.6,8,9
The present proof of concept observation states that liver transplantation recipients may not have worse outcomes, when compared with other patients that have COVID-19 risk factors. We recognize that the intrinsic limitation of this case report is the small sample size, but its originality lies in the fact that it provides evidence of the mechanisms underlying the potentially beneficial effect of immunosuppressants. Said clinical observation must be confirmed in multicenter trials and real‐life cohorts. Additional data are needed to better define the group of patients that would receive a strong benefit from immunosuppressive strategies, and in turn, provide better management of transplant recipients through said strategies, during this epidemic.
Ethical disclosuresThe present scientific letter fulfills the current bioethical research regulations. It was authorized by the ethics committee of the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”. The patients cannot be recognized or identified through the images or data presented in the article.
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this research.
The authors declare that patient anonymity was preserved at all times, and that patient consent was obtained for the publication of the present article.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestIsaac Ruiz has been a speaker for AbbVie.
Ignacio García-Juárez, Alejandro Campos-Murguía, Victor Hugo Tovar-Mendez, and Alejandro Gabutti declare that they have no conflict of interest.
The authors wish to thank Dr. Oscar Arturo Lozano-Cruz, Dr. José Luis Cardenas-Fragoso, Dr. Daniel Alberto Carrillo-Vazquez, Dr. Alan G. Contreras, and Dr. Nayelli C. Flores-García. The authors also wish to thank Dr. Quentin Nevers and Flora Donati for the critical rereading of the manuscript.
Isaac Ruiz is a recipient of fellowship grants from the CHUM Foundation, Montreal, Canada.
Please cite this article as: García-Juárez I, Campos-Murguía A, Tovar-Méndez VH, Gabutti A, Ruiz I. Evolución clínica en un receptor de trasplante de hígado con la COVID-19: ¿Un efecto benéfico del tacrolimus? Revista de Gastroenterología de México. 2020;85:437–442.