Kawasaki disease is a systemic inflammatory disease that manifests as vasculitis, predominantly affecting medium-caliber arteries, in particular, the coronary arteries.1
Diagnostic criteria are based on the presence of fever ≥ 5 days and ≥ 4 of the clinical criteria: erythema or cheilitis of the lips, edema or desquamation of the hands and feet, cervical lymphadenopathy, and polymorphous exanthema and/or conjunctival injection.2 Of the digestive tract manifestations, gastrointestinal bleeding has been reported, but it is a very rare presentation worldwide.
A previously healthy one-year-old boy had disease onset 3 days prior to his arrival at the emergency service, presenting with fever (38.2 °C), conjunctival injection, and diarrheic stools with no mucus or blood, as well as edema in his lower limbs; 24 hours before going to the emergency room, he had melenic stools on 3 occasions. He had no history of self-medication or nonsteroidal anti-inflammatory drug use prior to his symptoms. Laboratory tests were ordered, strikingly revealing severe anemia (hemoglobin 6.1 mg/dl) that was microcytic (73.3 fl) and hypochromic (25.1 pg), as well as platelets at 186 × 103/mcl, a prothrombin time of 10.8%, with an INR of 0.95, and a partially activated thromboplastin time of 30.5 s. Management was started with a blood product transfusion and proton pump inhibitor.
During hospitalization, the patient presented with perioral cheilitis and polymorphous exanthema, meeting the criteria for Kawasaki disease. Complementary test results reported C-reactive protein above 24 mg/l, and ESR 0 mm/h; urinalysis: leukocytes 24 cells/mcl, negative nitrites (sterile pyuria); transthoracic echocardiogram: valvulitis (slight mitral, aortic, and pulmonary insufficiency), and mild pericardial effusion. Immunoglobulin G was administered (2 g/kg/dose), without an antiplatelet agent.
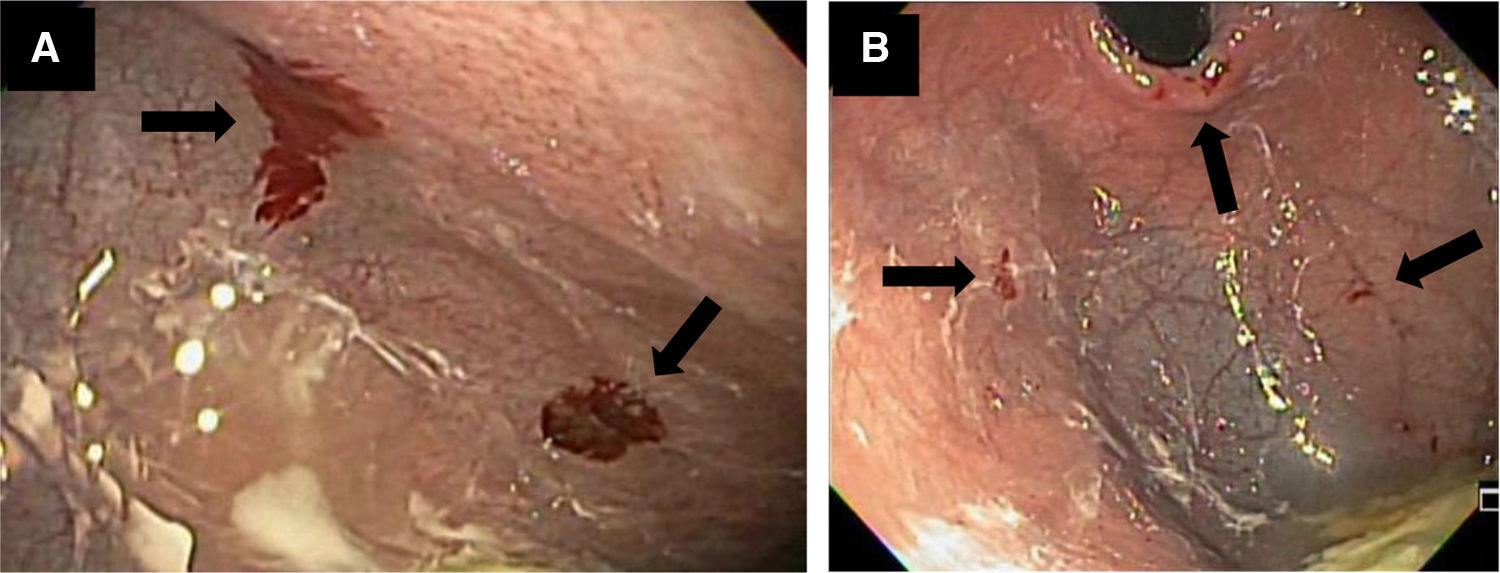
Panendoscopy was performed, as part of the approach to the gastrointestinal bleeding, revealing 6 minor 5 mm ulcers at the level of the gastric body and fundus, 2 with scant bleeding and hyperemic and erythematous mucosa. Biopsies were taken (Fig. 1).
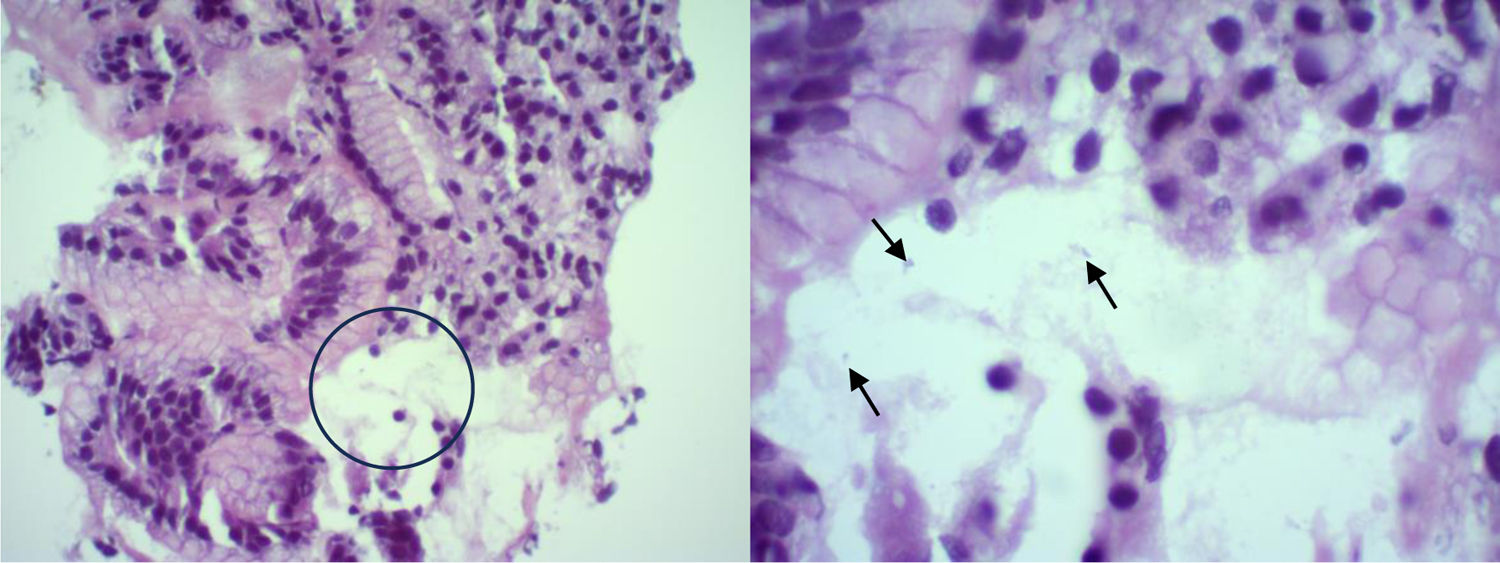
Pathology report: In the gastric body and fundus, the gastric mucosa had a slight increase in lymphocytes and plasma cells; there were areas of superficial erosion and recent bleeding. In the gastric antrum, the mucosa showed a moderate increase in lymphocytes, plasma cells, and the formation of lymphoid follicles. Spiral-shaped bacilli (Helicobacter pylori [H. pylori]) were identified (Fig. 2). In the duodenum, there was a slight increase in lymphocytes and plasma cells.
The patient presented with adequate clinical evolution and was released. At present, he has no symptomatology of H. pylori infection, but the pathology report indicated chronic gastritis data, and so he is receiving eradication therapy as an outpatient. The patient is currently in follow-up at the cardiology and rheumatology services, with no reported events, as well as at the gastroenterology service, where the verification of H. pylori eradication is pending. He has not presented with a new gastrointestinal bleeding episode.
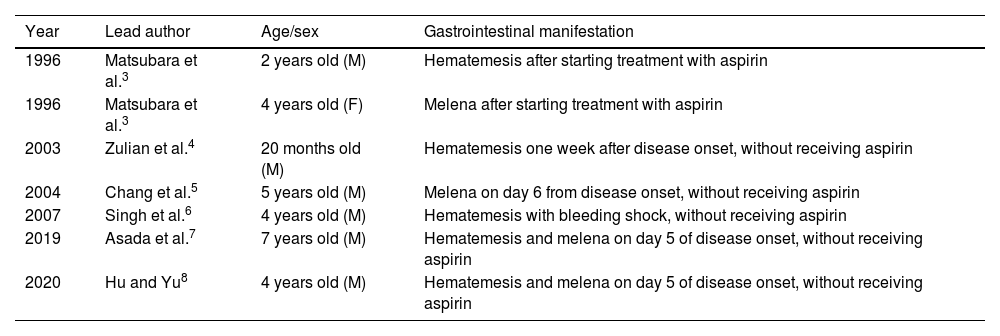
A systematic review of the topic was carried out on the Medscape®, PubMed®, Scopus®, and ScienceDirect® search engines, finding a total of 6 articles that reported gastrointestinal bleeding in Kawasaki disease. Table 1 describes patient clinical characteristics reported in previous years.
Clinical characteristics reported in patients with gastrointestinal bleeding in Kawasaki disease.
| Year | Lead author | Age/sex | Gastrointestinal manifestation |
|---|---|---|---|
| 1996 | Matsubara et al.3 | 2 years old (M) | Hematemesis after starting treatment with aspirin |
| 1996 | Matsubara et al.3 | 4 years old (F) | Melena after starting treatment with aspirin |
| 2003 | Zulian et al.4 | 20 months old (M) | Hematemesis one week after disease onset, without receiving aspirin |
| 2004 | Chang et al.5 | 5 years old (M) | Melena on day 6 from disease onset, without receiving aspirin |
| 2007 | Singh et al.6 | 4 years old (M) | Hematemesis with bleeding shock, without receiving aspirin |
| 2019 | Asada et al.7 | 7 years old (M) | Hematemesis and melena on day 5 of disease onset, without receiving aspirin |
| 2020 | Hu and Yu8 | 4 years old (M) | Hematemesis and melena on day 5 of disease onset, without receiving aspirin |
M: male; F: female.
The latest case of Kawasaki disease with gastrointestinal bleeding prior to anticoagulant therapy was described at the Hangzhou Hospital of the University of Zhejiang, in China, in 2020.8
Gastrointestinal manifestations in this entity are rare, but are important to consider, so that a timely diagnosis can be made and treatment started, thus preventing future complications.
Financial disclosureThis article was financed by the lead author.
Ethical considerationsThis work meets the current bioethical research norms and was not authorized by an ethics committee, given that it contains no information that could identify the patient. Nevertheless, informed consent was obtained from the patient’s guardians.
The authors declare that there is no conflict of interest.