Celiac ganglia (CG) can be seen by endoscopic ultrasound; they play an important role in pain management and are a potential site for extrapancreatic tumor neural invasion.
AimsTo evaluate the frequency of CG visualization during endoscopic ultrasound examination and to evaluate the feasibility of this technique to identify extrapancreatic tumor neural invasion in patients with pancreatic lesions.
MethodsWe retrospectively reviewed all endoscopic ultrasound studies performed between November 2007 and June 2010. Images of the celiac region were presented to an endosonographer, who reported the presence or absence of CG.
ResultsWe included 31 cases. CG were identified in 14 (45%) cases. Average size was 10mm (range 4-25mm) by±1mm (range 1-7mm). In 2 cases, fine needle aspiration biopsy was performed and reported nerve cell bodies; in one case malignant cells were seen.
ConclusionsCG were identified in 45% of the cases. Fine needle aspiration biopsy can detect unanticipated extrapancreatic tumor neural invasion in pancreatic malignancies.
Los ganglios celíacos (GC) pueden ser visualizados por ultrasonido endoscópico; juegan un papel importante en el manejo del dolor y son un sitio potencial de invasión tumoral neural extrapancreática.
ObjetivosEvaluar la frecuencia de visualización de los GC durante el examen de ultrasonido endoscópico, así como la factibilidad de este para identificar la invasión tumoral neural extrapancreática en pacientes con lesiones pancreáticas.
MétodosRevisamos retrospectivamente todos los estudios de ultrasonido endoscópico que se llevaron a cabo desde noviembre de 2007 a junio de 2010. Las imágenes de la región celíaca se presentaron a un endosonografista, quien reportó la presencia o ausencia de GC.
ResultadosIncluimos 31 casos. Se identificaron GC en 14 (45%) de los casos. El tamaño promedio fue de 10mm (rango 4-25mm) por±1mm (rango 1-7mm). En 2 casos se llevó a cabo una biopsia por aspiración con aguja fina y se reportaron cuerpos de células neurales; en un caso se observaron células malignas.
ConclusionesSe identificaron GC en el 45% de los casos. La biopsia por aspiración con aguja fina puede detectar invasión tumoral neural extrapancreática inesperada en neoplasias pancreáticas.
The celiac plexus contains the celiac ganglia (CG), which have been of anatomical and clinical interest in chronic pancreatitis and pancreatic cancer due to their role in pain management1,2 and as a potential site for tumor invasion.3
The celiac plexus is located at the level of T12 and L1, anterior to the diaphragmatic crura, medial to the adrenal glands, and close to the aorta between the origin of the celiac trunk and the superior mesenteric artery.4
The CG can be visualized in up to 80% of the endoscopic ultrasound (EUS) examinations. However, this number varies among publications, depending on endosonographer experience, type of echoendoscope used, and on patient-related factors. The CG are best seen with curved linear array echoendoscopes and in female subjects with no prior history of abdominal surgery.5
Visualization of CG by EUS6 has allowed for direct injection into individual CG to perform blocks or neurolysis in patients with pancreatic disorders suffering from intractable pain.5,7] In addition, extrapancreatic neural invasion (EPNI) involving CG in cases with pancreatic malignancy has been identified.8
During EUS examination, the CG appear as oblong or round hypoechoic structures, with jagged edges containing hyperechoic foci or strands. Their average size ranges from 2 to 20mm.7
We report herein the frequency of visualization of CG by EUS using the previously published endosonographic features5–7 and the identification of microscopic EPNI by EUS-guided fine needle aspiration (FNA) in patients with pancreatic lesions.
MethodsWe retrospectively reviewed our database for patients that had been referred for EUS examination of pancreatic lesions between November 2007 and March 2010. We identified 81 cases, but the images from 50 cases were lost during maintenance of our storage system, so 31 cases with available images or video clips from the celiac region were finally included; clinical and demographic data were recorded. A single expert endosonographer (MPL) performed all the studies using a linear-curve array (GF-UC140P-AL5; Olympus America Inc, Center Valley, PA) echoendoscope.
All patients gave their informed consent for the procedure and received sedation with propofol under strict monitoring by an anesthesiologist.
The presence of CG was determined by identifying characteristic endosonographic patterns according to previous descriptions,6,8,9 located on the sides of the emergence of the celiac artery and/or if FNA from that structure showed nerve cell bodies without lymphocytes.
Images and videos from each case were coded and sorted randomly and shown to the endosonographer, who was blinded to the exam report and findings; he reported the presence or absence of CG, in addition to providing a detailed description of the observed structures.
In order to avoid recall bias, we did not include images from cases performed within a time frame of 6 months before the study period.
ResultsIndications for EUS were pancreatic masses with a final diagnosis of adenocarcinoma (18 cases, 58%) and pancreatic cysts with EUS characteristics suggestive of side branch intraductal papillary mucinous neoplasm without worrisome characteristics (13 cases, 42%).
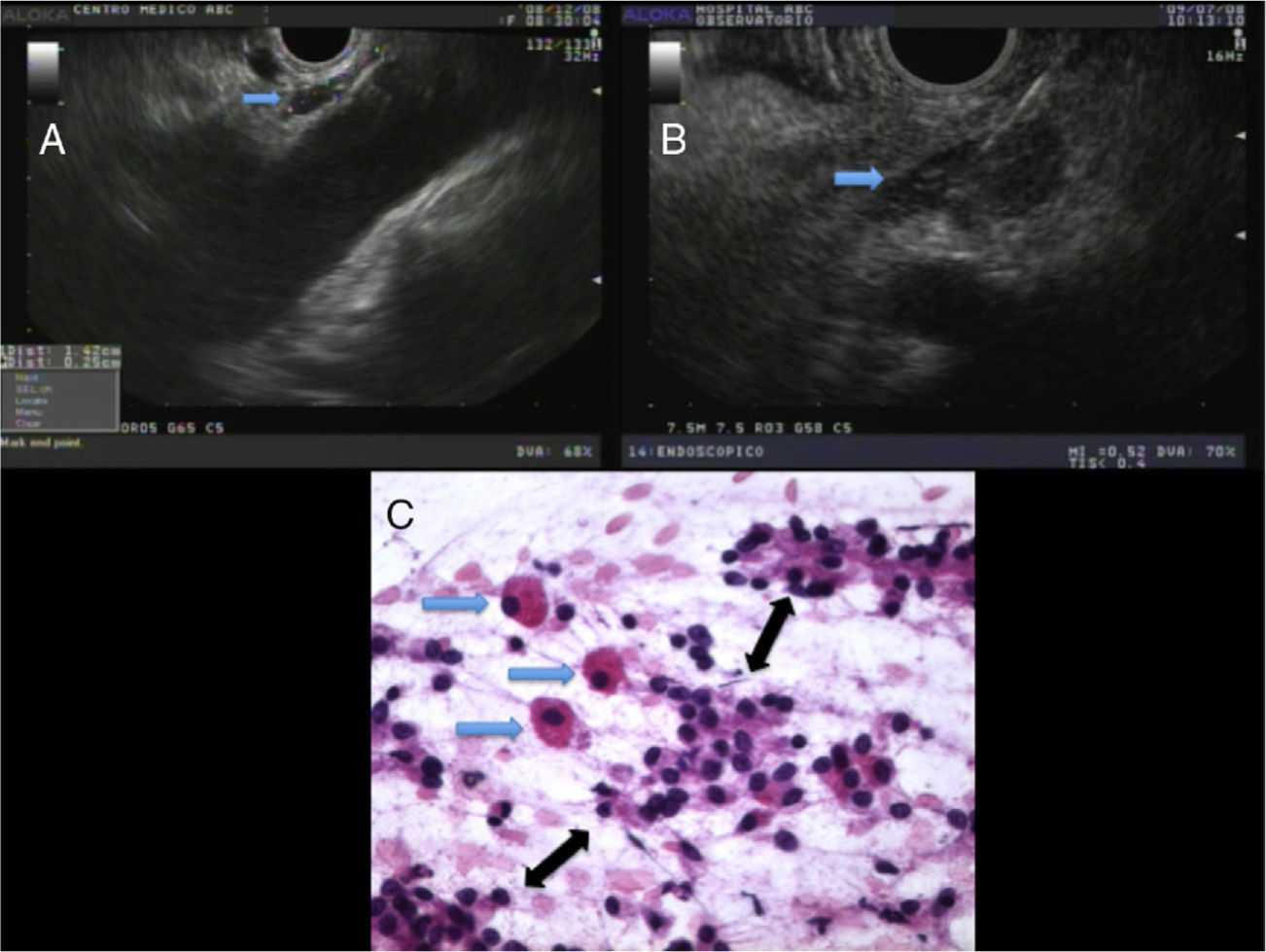
Celiac gangliaStructures consistent with CG were visualized between the celiac artery and left adrenal gland in 14 cases (45%); eight in benign cases and 6 in malignant ones. They appeared as elongated, slim structures that were mostly hypoechoic, with a slight hyperechoic center and irregular borders (fig. 1A). The average size of the axis was 10mm in length±5mm (SD) (range 4-25mm) and the minor axis was 3mm±1mm (SD) (range 1mm-7mm).
A The blue arrow shows normal-looking celiac ganglia. B The blue arrow shows an enlarged CG. Fine needle aspiration biopsy was performed due to suspicion of a metastatic celiac lymph node. C An enlarged CG cytologic examination showed a group of three large, discohesive ganglion-like cells (blue arrows) neatly dispersed among small groups of adenocarcinoma cells (black arrows). In a trained team setting, this kind of image can be confidently interpreted as neoplastic infiltration into the nerve ganglia (hematoxylin & eosin, x400).
In two pancreatic mass cases, to exclude tumor metastasis, FNA biopsy was performed on structures that, due to their EUS appearance, were initially suspected of being celiac lymph nodes (fig. 1B). FNA caused transient pain, characterized by patient movement and an increase in heart rate.
An in situ cytologic examination showed the presence of neural bodies with no lymphocytes and surrounded by malignant cells in one case (fig. 1C), and only neural bodies with no lymphocytes or malignant cells in another case.
DiscussionAbdominal pain related to chronic pancreatitis or pancreatic cancer is usually secondary to anatomic and mechanical abnormalities;1,2 in cases without pancreatic anatomical changes, pain usually results from inflammatory or neoplastic infiltration of intrapancreatic and extrapancreatic pain afferents, including the celiac plexus. 3,8
EUS allows visualization of the celiac plexus ganglia in up to 80% of cases; in our study, we visualized it in approximately 45% of the cases. This could be related to detection errors from losing around 50 cases due to electronic and computer eventualities, using only a linear array echoendoscope, the fact that only one endosonographer participated in the study, and that we based our findings on descriptions given by other authors,6,7 rather than on histology that could confirm that we were actually observing CG. FNA of these structures has precise indications: to perform intraganglionic block or neurolysis; neither of these was the indication in any of our cases.
Nevertheless, 2 cases with diagnosis of pancreatic cancer underwent CG FNA biopsy; this was performed under the initial suspicion of metastatic disease to what appeared to be celiac lymph nodes. Surprisingly, cytologic examination revealed abundant neuronal bodies in both cases and accompanying malignant cells in one patient. In the two cases, noises and movement of the patient, which ceased upon needle withdrawal, accompanied FNA. These clinical findings with the corresponding cytology are signs of nerve structure puncture. 3,8
The importance of visualizing the CG during EUS has been the subject of debate. Multiple reports,6,7 including a meta-analysis9 of patients with abdominal pain secondary to chronic pancreatitis or pancreatic cancer, reported that EUS-guided neurolysis is an effective and safe method with better pain control rates, compared with radiological methods; one of the studies included in the meta-analysis performed intraganglionic celiac block and neurolysis in some patients with promising results; this led to the idea that intraganglionic injection could be associated with better results, compared with the current technique, although numbers and follow-up were limited.5
Invasion and metastasis are key components of cancer progression. The most common tumor dissemination paths are blood and lymphatic vessels. A distinct and overlooked path is perineural invasion (PNI). PNI, defined as the presence of cancer cells in the perineural space, is frequently used by pancreatic and prostate cancer and is associated with local recurrence and pain.3,8
Taking into consideration the reports suggesting that the celiac plexus can be a potential site of metastasis in pancreatic cancer, as well as the fact that EPNI occurs more frequently than suspected, and is associated with poor prognosis and a higher risk of local tumor recurrence,10 suggesting that EPNI detection could modify its management, are all reasons why the identification and sampling of the CG should be considered during the staging process of pancreatic cancer.3,8
After the preliminary report of EPNI in pancreatic cancer detected by EUS,8 this is the second study to report similar findings, although in a single case. This limited information makes it difficult to determine the clinical relevance not only of locating the celiac ganglia by EUS, but also of considering it as part of the staging process in pancreatic cancer. Whether EPNI represents late stages of the disease or can predict a higher rate of local recurrences in pancreatic cancer remains unknown, but provocative, and deserves to be explored in larger well-designed studies.
ConclusionsUsing previously published EUS characteristics, we identified CG in 45% of the cases. EUS CG visualization and FNA could identify unsuspected EPNI in pancreatic cancer. The clinical and prognostic significance of identifying CG and EPNI in pancreatic cancer needs to be clarified in larger studies with a prospective design.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Peláez-Luna M, Borbolla-Arizti J, Herrera-Lozano A, Baquera-Heredia J. Frecuencia de visualización de ganglios celíacos por ultrasonido endoscópico y su potencial en la evaluación de invasión neural en pacientes con lesiones pancreáticas. 2013;78:251–254.