The differential diagnosis of pancreatic cystic lesions (PCLs) includes non-neoplastic lesions and neoplastic epithelial lesions. Given that management is determined by the risk for malignant progression, associated symptoms, and other characteristics, an accurate diagnosis is imperative. The present review attempts to provide a critical path that facilitates the characterization and management of PCLs.
El diagnóstico diferencial de las lesiones quísticas de páncreas (LQP) incluye lesiones no neoplásicas y lesiones epiteliales neoplásicas. El diagnóstico preciso es imperativo, pues su manejo está determinado por el riesgo de progresión maligna, síntomas asociados y otras características. La presente revisión intenta proporcionar una ruta crítica que facilite la caracterización y manejo de la de las LQP.
The differential diagnosis for pancreatic cystic lesions (PCLs) is broad and includes neoplastic epithelial lesions and benign inflammatory lesions. PCLs are largely identified incidentally on imaging studies that are often indicated for symptoms unrelated to the pancreas. Their frequency is estimated at 2–45% in the general population and increases with age1.
The risk for malignant transformation of incidental PCLs (IPCLs) is relatively low (0.01%) but can increase (0.21%) in cysts ≥2 cm, making accurate diagnosis essential for planning their management. Some IPCLs may require no surveillance or treatment at all, whereas others may need periodic evaluation or surgical resection. Selection of the route of management mostly depends on the risk for malignant transformation and the presence of symptoms2–6.
The aim of the present review is to provide a diagnostic path that facilitates the characterization and adequate treatment of IPCLs.
Epidemiologic notesThe true frequency of IPCLs in Mexico is not known. Similar to other case series, after analyzing 170 cases treated at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán we found that intraductal papillary mucinous neoplasm (IPMN) was the most frequent lesion (44.7%), followed by mucinous cystadenoma (MCA) (18.8%), serous cystadenoma (SCA) (14.7%), solid pseudopapillary neoplasm (SPN) (11.8%), pseudocyst (4.1%), neuroendocrine tumor with cystic degeneration (4.1%), and simple cyst (1.8%)4,7. Both IPMN and MCA produce mucin and have a greater risk for malignant transformation. Thus, a simple way to plan the treatment or follow-up strategy is to know whether the lesion produces mucin or not, which can be fairly easily determined by analyzing the cyst fluid, preferably obtained through endoscopic ultrasound (EUS)-guided aspiration.
IPCL characterization calls for a detailed study. Clinical presentation, the morphologic features revealed by imaging methods, such as, contrast-enhanced dedicated pancreatic computed tomography [CT], magnetic resonance imaging [MRI], yield important data on the diagnostic possibilities. Those findings, or magnetic resonance cholangiopancreatography [MRCP]), combined with the cyst fluid analysis, cytologic, histologic studies, enable the accurate diagnosis to be, made, that treatment, follow-up measures will be, based upon. Although some authors favor surgery in all IPCLs, such strategy involves pancreatic resection, which is not exempt from risks, including death8. Accurate diagnosis enables the selection of patients that will truly benefit from surgical treatment9.
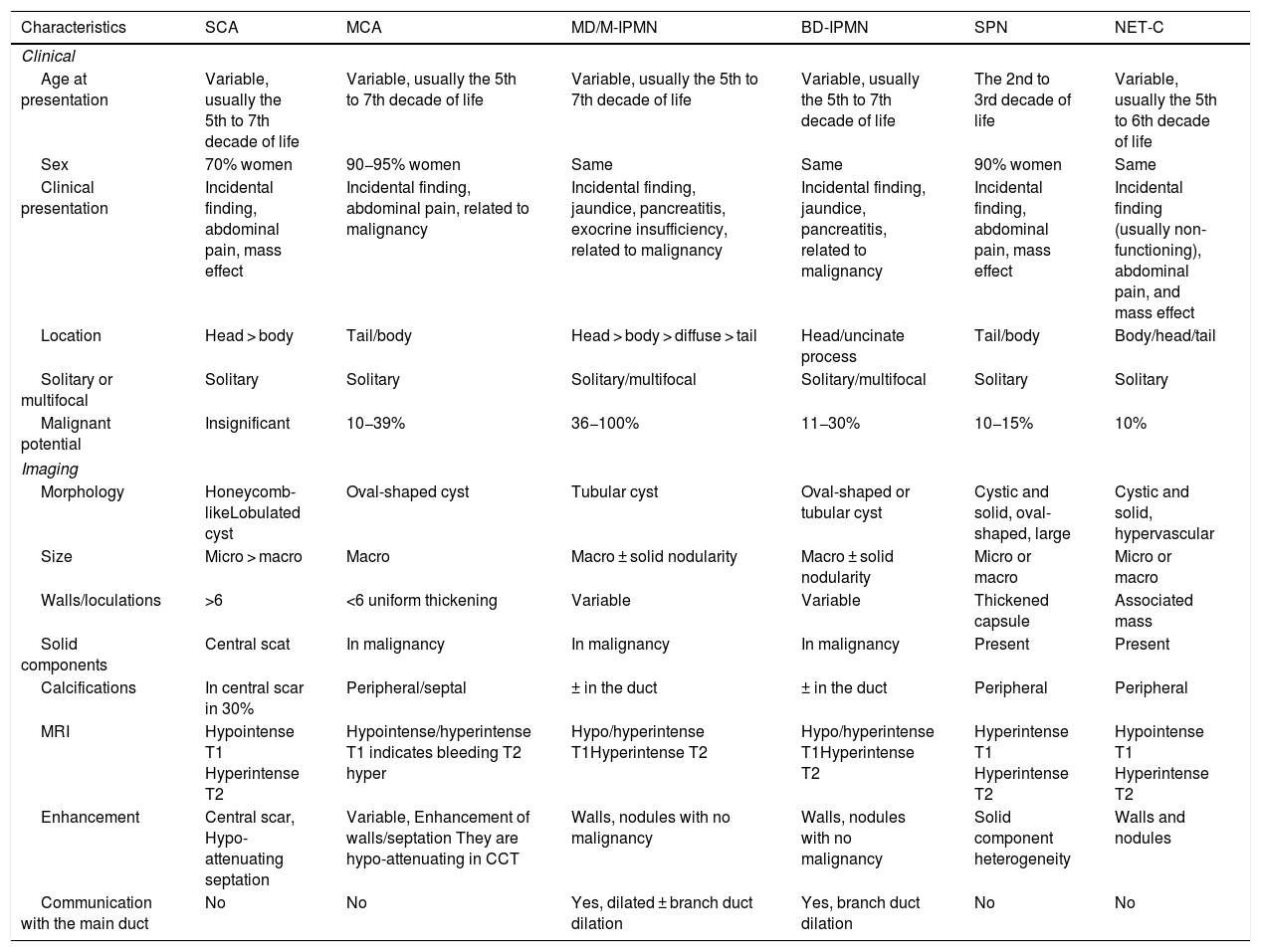
Clinical characteristicsClinical history is paramount. Each type of IPCL has its own clinical characteristics and demographics, such as preference for specific age group, sex, location, particular anatomic features, or specific manifestations (Table 1).
Clinical and imaging characteristics of the pancreatic cystic tumors.
| Characteristics | SCA | MCA | MD/M-IPMN | BD-IPMN | SPN | NET-C |
|---|---|---|---|---|---|---|
| Clinical | ||||||
| Age at presentation | Variable, usually the 5th to 7th decade of life | Variable, usually the 5th to 7th decade of life | Variable, usually the 5th to 7th decade of life | Variable, usually the 5th to 7th decade of life | The 2nd to 3rd decade of life | Variable, usually the 5th to 6th decade of life |
| Sex | 70% women | 90−95% women | Same | Same | 90% women | Same |
| Clinical presentation | Incidental finding, abdominal pain, mass effect | Incidental finding, abdominal pain, related to malignancy | Incidental finding, jaundice, pancreatitis, exocrine insufficiency, related to malignancy | Incidental finding, jaundice, pancreatitis, related to malignancy | Incidental finding, abdominal pain, mass effect | Incidental finding (usually non-functioning), abdominal pain, and mass effect |
| Location | Head > body | Tail/body | Head > body > diffuse > tail | Head/uncinate process | Tail/body | Body/head/tail |
| Solitary or multifocal | Solitary | Solitary | Solitary/multifocal | Solitary/multifocal | Solitary | Solitary |
| Malignant potential | Insignificant | 10−39% | 36−100% | 11−30% | 10−15% | 10% |
| Imaging | ||||||
| Morphology | Honeycomb-likeLobulated cyst | Oval-shaped cyst | Tubular cyst | Oval-shaped or tubular cyst | Cystic and solid, oval-shaped, large | Cystic and solid, hypervascular |
| Size | Micro > macro | Macro | Macro ± solid nodularity | Macro ± solid nodularity | Micro or macro | Micro or macro |
| Walls/loculations | >6 | <6 uniform thickening | Variable | Variable | Thickened capsule | Associated mass |
| Solid components | Central scat | In malignancy | In malignancy | In malignancy | Present | Present |
| Calcifications | In central scar in 30% | Peripheral/septal | ± in the duct | ± in the duct | Peripheral | Peripheral |
| MRI | Hypointense T1 Hyperintense T2 | Hypointense/hyperintense T1 indicates bleeding T2 hyper | Hypo/hyperintense T1Hyperintense T2 | Hypo/hyperintense T1Hyperintense T2 | Hyperintense T1 Hyperintense T2 | Hypointense T1 Hyperintense T2 |
| Enhancement | Central scar, Hypo-attenuating septation | Variable, Enhancement of walls/septation They are hypo-attenuating in CCT | Walls, nodules with no malignancy | Walls, nodules with no malignancy | Solid component heterogeneity | Walls and nodules |
| Communication with the main duct | No | No | Yes, dilated ± branch duct dilation | Yes, branch duct dilation | No | No |
BD-IPMN: branch duct-intraductal papillary mucinous neoplasm; CCT: contrast-enhanced computed tomography; MD/M-IPMN: main duct/mixed-type intraductal papillary mucinous neoplasm; MCA: mucinous cystadenoma; MRI: magnetic resonance imaging; NET-C: neuroendocrine tumor with cystic degeneration; SCA: serous cystadenoma; SPN: solid pseudopapillary neoplasm.
The first step in the diagnostic approach of a PCL is to rule out the possibility of a pseudocyst, which is the most frequent PCL. A pseudocyst occurs at least four weeks after the beginning of an episode of acute pancreatitis. It usually presents as a single cystic lesion, circumscribed by a fibrous wall, often without septations, that may or may not communicate with the pancreatic duct. It contains a slightly viscous or non-viscous fluid, with high levels of amylase (>250 mg/dl), insignificant or null levels of carcinoembryonic antigen (CEA), and abundant inflammatory cells10.
Importantly, some pancreatic cystic neoplasms (PCNs) present with episodes of abdominal pain or acute pancreatitis. This has been described in IPMNs, with an estimated frequency that ranges between 13 and 35%. In those cases, the differential diagnosis is more difficult, but pseudocysts never produce mucin, and so mucin-associated markers, such as CEA levels, tend to be absent.
Patients with recurrent acute pancreatitis or chronic pancreatitis sometimes present with pancreatic atrophy and varying degrees of ductal involvement, with dilation of the main duct and branch ducts that cause pseudocysts, generally associated with periods of acute exacerbation or retention cysts due to ductal obstruction from lithiasis or strictured zones.
Those ductal changes, though common in advanced stages of chronic pancreatitis, can also be seen in IPMNs; either those arising in the main duct (MD-IPMN) or branch ducts (BD-IPMN). The mucin produced by them may cause ductal obstruction, leading to pain and episodes of pancreatitis that can eventually lead to chronic obstructive pancreatitis. In a subset of patients with changes consistent with chronic pancreatitis, PCLs may be due to retention cysts or IPMNs. Some of them may be easy to diagnose, but more information, obtained from cyst fluid analysis, is often required. Other clinical manifestations associated with PCLs that should be documented because they influence treatment decisions include jaundice, abdominal pain, hyporexia, early satiety, nausea, vomiting, or weight loss. Jaundice and pancreatitis are common manifestations of IPMNs, but also of other PCLs, which due to their size, compress adjacent tissues9.
To determine which cystic lesion or lesions should be surgically removed merely through clinical characteristics is no easy task. The collected clinical information helps to narrow down diagnostic possibilities but is usually not sufficient to guide therapeutic decisions.
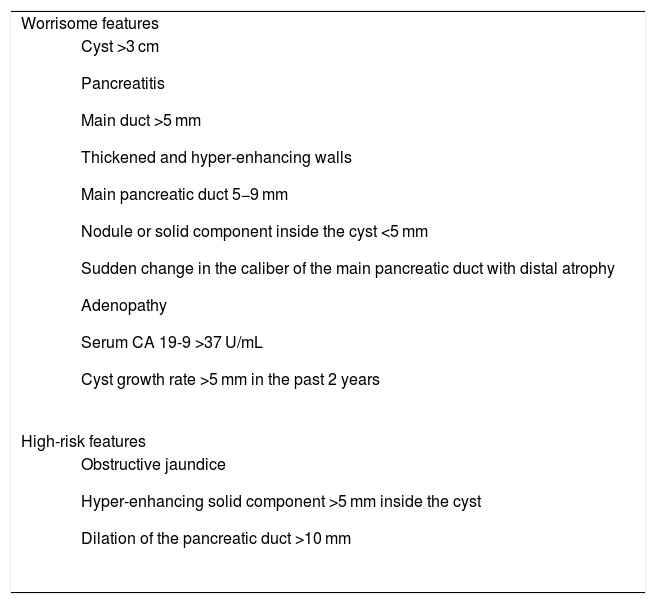
Specific morphologic, imaging, cytologic, and biochemical characteristics modify treatment strategy; their presence aids in assessing the risk for malignant transformation (worrisome features) or the presence of malignancy at the time of evaluation (high-risk features). Such characteristics were initially described for IPMNs but have been extended and applied to the management and diagnosis of the other PCLs (Table 2).
Worrisome and high-risk features in pancreatic cystic lesions.
| Worrisome features |
|
| High-risk features |
|
Imaging methods are essential in the evaluation of IPCLs. Location, number, size, main pancreatic duct dilation or branch ducts communicating with the main pancreatic duct, the presence of septations, locules, nodules, or wall thickening are morphologic features that are key to making a presumptive diagnosis which eventually must be supported by the physicochemical characteristics of the fluid (e.g., viscosity and amylase, glucose, and CEA levels).
Except for the cyst fluid characteristics, other questions can be adequately answered utilizing imaging methods. However, because the majority of IPCLs are discovered on imaging studies indicated for reasons other than diseases of the pancreas, the diagnostic yield of the index study tends to be limited.
Due to its availability and cost, contrast-enhanced dedicated pancreatic CT tends to be the initial imaging study of choice. Utilizing digital reconstruction methods, it can describe the pancreatic ductal system and the interior of the cyst in detail. Nevertheless, MRCP (preferably with secretin stimulation [S-MRCP]), in addition to avoiding radiation exposure, provides better imaging of the soft tissues, as well as better characterization of the relation of the cystic lesions with the main pancreatic duct, identifies septations, nodules, and thickening of the cyst wall2. Currently, both techniques are considered complementary, with a slight preference in specialized centers for S-MRCP (secretin is not available in our country).
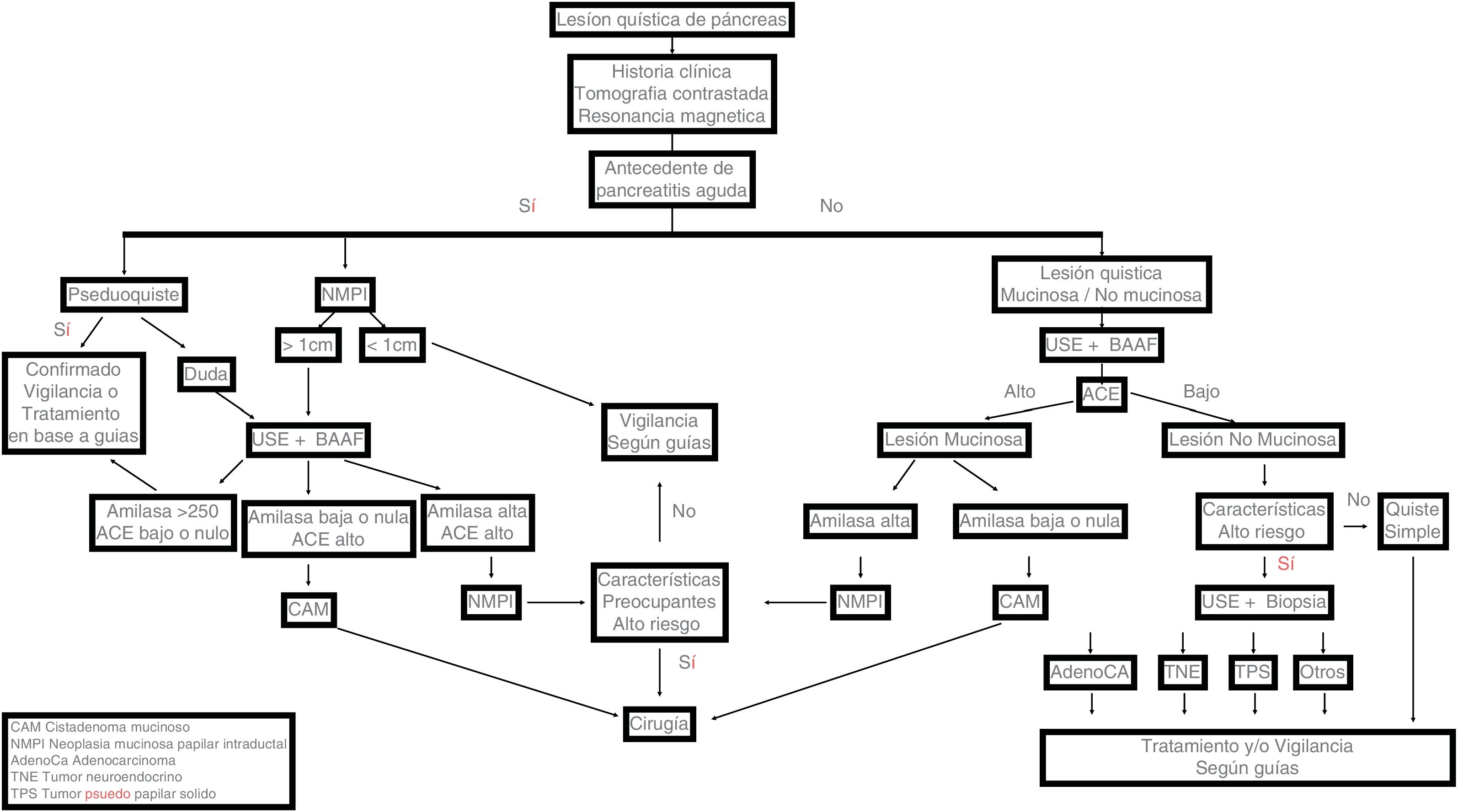
If an IPCL is identified on an abdominal ultrasound or conventional abdominal CT, MRI can adequately complement the information provided by those two methods. It is also the preferred technique for long-term surveillance. If MRI is unavailable, the initial study should be a contrast-enhanced dedicated pancreatic CT (Fig. 1).
Table 3 shows the main imaging characteristics of the different IPCLs.
Performance of imaging modalities for the identification and characterization of neoplastic pancreatic cysts.10
| Lesion identification | Lesion characteristics | |
|---|---|---|
| MDTC | 100% specificity for differentiating SCNs from MCNs.90% specificity for differentiating SCNs from IPMNs.70–81% specificity for SB-IPMNs, showing communication with the main pancreatic duct.86% specificity for showing IPMNs. | 74% sensitivity for detecting septations and 86% sensitivity for detecting communication with the main duct. 74−78% accuracy for differentiating benign IPMNs from malignant IPMNs. 86% specificity for differentiating benign MCNs from malignant MCNs. |
| MRI/MRCP | 91% specificity for identifying IPMNs. | 91% sensitivity for detecting septations and 100% sensitivity for detecting communication with the main duct. 74−75% accuracy for differentiating benign IPMNs from malignant IPMNs. |
| EUS | 76% accuracy for identifying SCNs.84–96% accuracy for identifying MCNs. | 68% accuracy for differentiating benign IPMNs from malignant IPMNs. |
| PET | – | 94% efficacy for differentiating benign cystic lesions from malignant cystic lesions. 97% sensitivity and 91% specificity for differentiating benign IPMNs from malignant IPMNs. |
EUS: endoscopic ultrasonography; IPMNs: intraductal papillary mucinous neoplasms; MDTC: multidetector computed tomography; MCNs: mucinous cystic neoplasms; MRCP: magnetic resonance cholangiopancreatography; MRI: magnetic resonance imaging; PET: positron emission tomography; SB-IPMNs: side-branch intraductal papillary mucinous neoplasms; SCNs: serous cystic neoplasms.
Positron emission tomography is not routinely used because of lack of availability, cost, and diagnostic limitations11.
Table 4 summarizes the diagnostic yield of each imaging modality.
Biochemical and cytologic cyst fluid analyses.
| Parameter | Pseudocyst | SCN | Benign MCN | Malignant MCN | IPMN |
|---|---|---|---|---|---|
| Viscosity | Low | Low | High | High | High |
| Amylase | High | Low | Low | Low | High |
| Glucose | High | High | Low | Low | Low |
| CEA | Low | Low | High | High | High |
| CA19-9 | Low | Low | Intermediate | High | High intermediate |
| Cytology | Histiocytes | Cubic cells with glycogen-rich cytoplasm | Cylindrical mucinous epithelial cells with varying degrees of atypia | Adenocarcinoma cells |
CEA: carcinoembryonic antigen; MCN: mucinous cystic neoplasm; IPMN: intraductal papillary mucinous neoplasm; SCN: serous cystic neoplasm.
EUS is an essential tool in the evaluation of IPCLs. It provides high-resolution images and facilitates the acquisition of cyst fluid and tissue from the interior and wall of the cyst, using fine needles12.
EUS diagnostic efficacy is comparable to that of MRI and contrast-enhanced CT, for characterizing the lesions, as well as demonstrating their communication with the main pancreatic duct. In addition, EUS is superior in detecting multifocal, synchronous, or metachronous lesions and identifying intramural nodules that are considered to represent a high risk for malignancy. In all cases, whenever technically possible, tissue should be taken for histopathologic study, because the EUS image alone cannot confirm or rule out the presence of malignancy13.
EUS diagnostic accuracy depends on the expertise of each operator, which accounts for the reported low interobserver agreement rates9. EUS-guided fine needle aspiration biopsy (FNAB) has 98.6% sensitivity, 50% specificity, and 84%–97.3% diagnostic accuracy.
EUS diagnostic yield and utility can increase through the introduction of new technologies, such as elastography, confocal microscopy, through-the-needle forceps biopsy, and contrast-enhanced EUS. Elastography and intravenous contrast-enhanced EUS aid in differentiating mucus from solid nodules. Mucus tends to present as a smooth, soft structure with well-defined borders and a hyperechoic center with no evident blood flow, compared with a solid nodule, which has a firm (solid/hard) consistency and blood flow12,14,15 (Table 3).
Fluid analysisThe data collected from the aforementioned clinical analysis and imaging techniques make the identification of certain lesions, such as pseudocysts and SCA, relatively easy. Others have yet to be properly differentiated, such as simple cysts and those with a high risk for malignancy, some of which require immediate surgery (MCA, solid pseudopapillary tumor, MD-IPMN), whereas others (BD-IPMN) can be conservatively managed. Thus, it is imperative to know if the cystic lesion produces mucin. To that purpose, cyst fluid analysis (specifically measurement of CEA, whose levels, regardless of the figures, indicate mucin production), is required. The first studies that established a cutoff point of >192 mg/dl, included only MCAs16,17. Later studies and meta-analyses that added other neoplasms (e.g., IPMNs) modified them. Currently, the usefulness of CEA is to determine mucin production, given that no relation between CEA levels and the presence or absence of malignancy has been confirmed11,18.
Cyst fluid can be obtained through EUS-guided fine needle aspiration (FNA), which is a safe procedure, with a low risk (2–3%) for complications6.
In addition to CEA, amylase content within the cyst fluid should be quantified. The combination of those two markers enables the mucinous nature of the PCNs to be established, as well as the indirect inference of whether there is communication with the main pancreatic duct, which aids in differentiating mucinous cystic neoplasms (MCNs) from IPMNs and other lesions (Table 4).
A high amylase level indicates a communication between the cyst and the pancreatic ductal system, which is characteristic of pseudocysts and IPMNs but not of the other PCNs. An amylase level below 250 U/L rules out a pancreatic pseudocyst, with 44% sensitivity and 98% specificity10.
A CEA cutoff point ≥192 mg/dL has 63% sensitivity and 79% diagnostic accuracy for diagnosing MCAs. With respect to IPMNs, those levels are more varied, most likely due to the communication between the IPMN and the main pancreatic duct, whose water and bicarbonate-rich secretion may dilute the content/concentration of CEA. Therefore, CEA levels should be interpreted in conjunction with the clinical data and imaging characteristics17,19.
Recent studies have shown that intra-cystic glucose levels below 50 mg/dL are found in mucin-producing lesions, with high sensitivity (95%) but mediocre specificity (57%), indicating an inherent risk of a high number of false-positive diagnoses. Therefore, it should be considered a potential biomarker under study and interpreted together with CEA and amylase values20–23.
The quantification of the CA 19-9 tumor marker within the cyst fluid offers no benefits, and thus is not recommended. Nevertheless, its determination in serum can be useful when malignant transformation is suspected24.
Considering that cyst fluid analysis by itself is not sufficiently accurate for differentiating between mucinous and non-mucinous PCLs, adding visual evaluation of the fluid has been suggested, especially the “string sign” that is defined as the presence of a string or filament >1 cm in length formed by the cyst fluid that remains completely formed for more than one second, and is highly specific for diagnosing mucinous lesions (58% sensitivity, 95% specificity, 94% positive predictive value, and 60% negative predictive value). However, it does not replace the above-mentioned analysis, given that there is great variability in its definition and interobserver evaluation25. Thus, it should be considered a complementary characteristic that can improve the diagnostic yield, especially when it is positive26. The reported diagnostic accuracy of the combination of CEA, cytology, and the string sign is approximately 94%, compared with the use of each of those components individually (81%, 51%, and 70%, respectively)27.
In addition to material for biochemical analysis, EUS-FNA can also acquire tissue from solid components within the cyst for cytologic and histopathologic study, and identify the risk or presence of malignant transformation, such as dysplasia, carcinoma in situ, or adenocarcinoma.
A meta-analysis showed that the cytopathology analysis of cyst fluid has poor sensitivity (54%) but good specificity (93%) for differentiating mucinous and non-mucinous PCNs28–30.
In the absence of solid components, cyst fluid cytologic analysis has little usefulness, given that the cyst fluid is usually acellular. The clinician should keep in mind that in IPCLs smaller than 1 cm, without high risk or worrisome characteristics, cyst fluid aspiration has limited benefits, given that most of the cysts of that size are benign and the amount of fluid that can be withdrawn is minimal. When CEA cannot be determined in the cyst fluid, biopsy of the tissue surrounding the cyst or from the cyst’s wall, once it has collapsed, can be performed, if considered necessary. For that purpose, the use of fine needle biopsy (FNB) needles, rather than fine needle aspiration needles, increases the diagnostic yield in about 29%, resulting in 87% diagnostic accuracy for detecting malignant or pre-malignant lesions31,32.
The use of miniaturized biopsy forceps (e.g., Moray microforceps; Steris Healthcare, Mento, Ohio, USA, or Spybite forceps; Boston Scientific, Marlborough, MA, USA), which are introduced through a 19-gauge aspiration needle during the EUS-guided aspiration process, enables fragments of the wall or mural nodules of the cyst to be obtained. The technical success rate has been reported at 86–100%, with a diagnostic yield close to 70% and 89% sensitivity for differentiating mucinous lesions from non-mucinous ones33,34.
Confocal microscopy is another technique that is being studied. It consists of introducing an optical fiber that emits a light beam through a low-power light-amplification by the stimulated emission of radiation (the acronym for which is “laser”) that enables high-resolution, magnified microscopic images of the epithelial lining of the cyst. Compared with CEA determination, this technique is superior in differentiating mucinous lesions (64% vs. 96%), as well as in identifying high-grade and low-grade dysplasia, with 87% sensitivity and 100% specificity. Its use is limited because of its high cost, the fact that the entirety of the wall of the cyst cannot be evaluated, and great interobserver variability that places its diagnostic yield at around 46%. More studies are needed, to establish its indications and utility35–37.
Molecular analysisApplying next-generation sequencing techniques to the cyst fluid analysis makes it possible to identify the mucin-producing cysts and even differentiate IPMNs from MCAs. The mutations detected in the KRAS or GNAS genes are very sensitive and specific for diagnosing IPMNs, but not MCAs, whereas the mutations or deletions in the SMAD4, CDKN2A, TP53, PIK3CA, or PTEN genes are associated with advanced neoplasms. A prospective study that included 102 patients that underwent surgical resection found that the combination of KRAS or GNAS gene mutations and TP53, PIK3CA, or PTEN gene alterations had 89% sensitivity and 100% specificity for detecting advanced pancreatic neoplasms. Mutations in the VHL gene occur in 89–100% of SCAs18,30,38,39. The SPINK1 gene has emerged as a new tumor marker, with 93% sensitivity and 89% specificity for differentiating benign PCLs from potentially malignant ones8.
Despite the promising results, further studies are still needed before those molecular tests can be included in the regular diagnostic and treatment guidelines for PCNs7.
TreatmentSimple cysts do not require treatment or surveillance. Pseudocysts should be managed according to their clinical characteristics. The potential for malignant transformation of SCAs is extremely low, but even so, their surveillance is recommended. The indication for surgical intervention in SCA depends primarily on the presence of symptoms rather than tumor size40,41.
Mucinous neoplasms (e.g., MCA and MD-IPMN or mixed-type IPMN) and non-mucinous neoplasms with a high risk for malignancy (e.g., high-grade neuroendocrine tumor with cystic degeneration, adenocarcinoma of the pancreas, solid pseudopapillary tumor) require immediate surgery.
BD-IPMNs and PCLs of uncertain diagnosis but with no evident malignancy, can be treated according to the presence or absence of worrisome features or high-risk features6,18 (Table 2).
SurveillanceThe size and clinical and imaging characteristics of the lesion dictate the periodicity of follow-up and the imaging technique (triple-phase CT, MRI, EUS) to be employed, as well as the need to repeat EUS and guided biopsy or aspiration.
MRI/MRCP is considered the best surveillance tool. In the absence of morphologic and clinical changes, management should remain conservative, with periodic follow-up38.
Based on the low malignant transformation rate of asymptomatic incidental pancreatic cysts (0.12% annually), some guidelines13 recommend a “low intensity” surveillance strategy and even suggest limiting the duration of surveillance to 5 years, in cases in which no changes are detected. There is no information supporting that conduct and the development of malignant neoplasms after 5-year follow-up has been reported to occur in 1–18% of cases of cysts that had remained “stable” during that period42–44.
Once the pros and cons of surveillance have been discussed and clarified with the patient, the decision whether to continue or stop follow-up should be individualized and decided together with the patient’s preferences. Surveillance could be stopped in patients that are not good candidates for surgical treatment (because of the presence of comorbidities, advanced age) or even because the patient is not willing to undergo surgery.
Performing EUS/FNAB during follow-up is justified, if new imaging and clinical worrisome features or high-risk features that would modify the therapeutic conduct appear6,13.
Currently, we consider that the surveillance strategy suggested in the Fukuoka guidelines for IPMNs and in the European Union guidelines for all PCLs are the most adequate6,7,38.
Surgical treatmentThe presence of worrisome features or high-risk features, a cytology suggestive of or positive for malignancy, main pancreatic duct dilatation >1 cm (with no signs of secondary obstruction or chronic pancreatitis), or high suspicion of MD-IPMN or mixed-type IPMN are indications for surgical resection.
The surgical procedure should preferably be performed by experienced surgeons, carried out at referral centers, and offered to patients in good general condition24,45.
Even though surgical indications tend to coincide among the different published guidelines, there are slight variations, given that each of them was developed for specific scenarios. The American Gastroenterological Association guidelines on the managing of asymptomatic and incidental PCLs and the Fukuoka guidelines (from where the worrisome features and high-risk features of the PCLs are taken) deal only with the management of IPMNs and MCAs, whereas the European Union guidelines consider the majority of PCLs and clinical scenarios6,13,38,46 (Table 5).
Surgical indications for intraductal papillary mucinous neoplasms according to the currently available guidelines.
| Guidelines | Absolute indication | Relative indication |
|---|---|---|
| IAP/Fukuoka, 2017, Tanaka et al.38 | JaundiceEnhancing mural nodule ≥5 mm Dilation of the pancreatic duct >10 mmSuspicious cytology or positivity for malignancy | Size of cyst ≥3 cmIncrease in size ≥5 mm/2 yearsEnhancing mural nodule <5 mmIncrease in serum CA 19-9 Pancreatic duct 5−10 mmAcute pancreatitis associated with IPMN Thickened enhanced cyst wall Stricture of the pancreatic duct with distal atrophy Lymphadenopathy |
| European Guidelines, 20186 | JaundiceContrast-enhanced solid cysts ≥5 mmPancreatic duct ≥10 mmPositive cytologySolid tumor | Size of cyst ≥4 cmIncrease in size ≥5 mm/year. Contrast-enhanced solid cysts ≤5 mmIncrease in serum CA19-9 Pancreatic duct 5−10 mmAcute pancreatitis associated with IPMN New-onset diabetes |
| AGA American Guidelines, 2015, Vege et al.13 | Solid cysts and pancreatic duct >5 mm detectable in MRI and EUS Malignancy in cyst cytology |
AGA: American Gastroenterological Association; EUS: endoscopic ultrasonography, IAP: International Association of Pancreatology; IPMN: intraductal papillary mucinous neoplasm; MRI: magnetic resonance imaging.
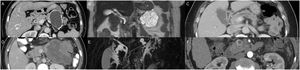
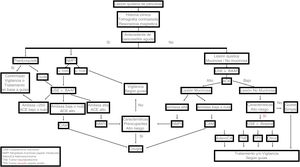
In cases in which surgery is contraindicated or rejected, less invasive treatments, such as the injection of alcohol or chemotherapy into the interior of the cysts and radiofrequency ablation, have been examined. However, none of them are currently a viable option and are still considered experimental therapies. Fig. 2 shows the clinical path for PCL diagnosis.
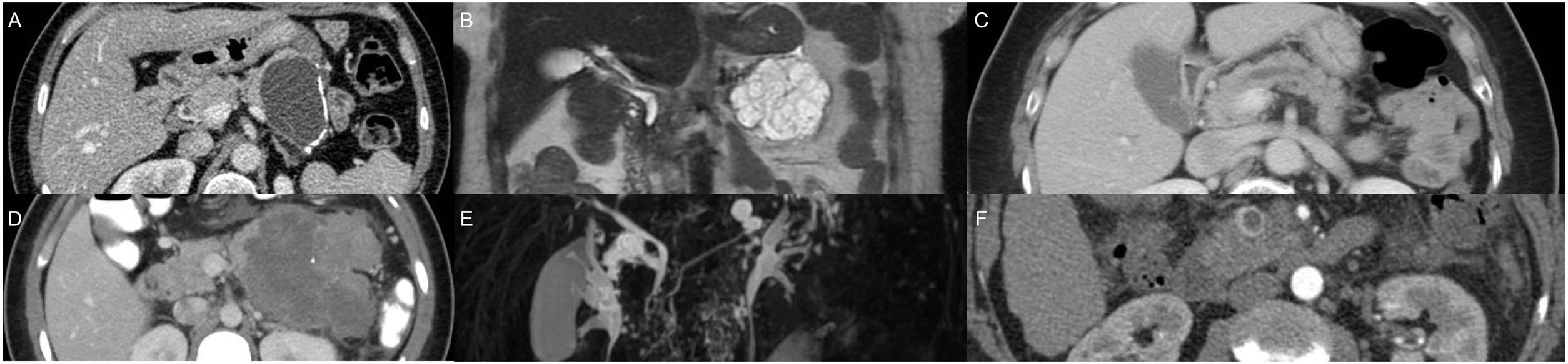
Critical path for the diagnosis and management of pancreatic cystic lesions. (A) MCA: Paucilocular cystic lesion with peripheral calcifications in the tail of the pancreas. (B) SCA: Multilocular microcystic lesion in the tail of the pancreas. (C) MD-IPMN: Segmental dilation of the main pancreatic duct. (D) SPN: Heterogeneous lesion with solid, cystic components and septal calcifications in the body and tail of the pancreas. (E) BD-IPMN: Bilobulated cystic lesion with communication to the main pancreatic duct in the body-tail of the pancreas. (F) NET-with cystic degeneration: Cystic lesion with hyper-contrast-enhancement and thickened wall in the arterial phase, in the head of the pancreas.
The incidence of IPCLs increases with age. The differential diagnosis is extensive and challenging. A structured strategy focused on differentiating lesions that have a low risk or a high risk for malignancy, as well as distinguishing the lesions that can be included in a surveillance program or that require immediate surgery, facilitates treatment and benefits everyone involved in the care of those patients. PCLs should preferably be treated by a multidisciplinary team with experience in their management.
Ethical considerationsThe bibliographic review meets all current good practice norms in research. Given its nature, no clinical records or patient data were utilized that could be identified, therefore, no patient informed consent or review or approval by the institutional research and ethics committee were required.
Financial disclosureNo financial support was received in relation to this study/article.
AuthorshipDr. Lira-Treviño and Dr. Carranza Mendoza equally participated in the development, design, and review of the manuscript and share main authorship, each of them considered the lead author of the manuscript.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Lira-Treviño A, Carranza Mendoza IG, Borbolla Arizti JP, Soriano-Ríos A, Uscanga-Domínguez L, Peláez-Luna M. Lesiones quísticas de páncreas. Diagnóstico diferencial y estrategia de tratamiento. Rev Gastroenterol Méx. 2022;87:188–197.