Colombia has high incidence levels of gastric cancer that can be explained by the genetic variability of Helicobacter pylori (H. pylori). Our aim was to establish the relation of the H. pylori CagA and VacA genotypes to dysplasia and gastric cancer, in a high-risk population.
Material and methodsA case-control study was conducted on 202 patients from a high-risk cancer zone. Patients with dysplasia and gastric cancer (cases) and patients with nonatrophic gastritis (controls) were included. Endoscopic sampling and histologic classification were carried out according to the Sydney system and the Lauren classification. Genetic information was obtained through polymerase chain reaction on paraffin blocks. The measures of association of the variables of interest were evaluated in bivariate and multivariate models. A P<0.05 was considered statistically significant and the SPSS version 25 program was employed.
ResultsAge above 50 years (OR: 23.76; CI: 8.40-67.17; P=0.000) and the VacA s1m1 genotype (OR: 6.18; CI: 1.25-30.51; P=0.025) were associated with higher risk for developing dysplasia and gastric cancer. The CagA+ genotype was not found to be a risk factor for developing those pathologies (OR: 1.02; CI: 0.39-2.62; P=0.965).
ConclusionsThe H. pylori VacA genotypes are markers for the development of gastric cancer. That information could be used to create a risk index in a predictive model to optimize the healthcare of higher-risk patients.
Colombia exhibe elevadas tasas de incidencia de cáncer gástrico que podrían explicarse por la variabilidad genética del H. pylori. Nuestro objetivo fue establecer la relación de los genotipos CagA y VacA de H. pylori con el cáncer y la displasia en una población de alto riesgo.
Material y métodosEstudio de casos y controles en 202 pacientes procedentes de una zona de alto riesgo de cáncer. Se incluyeron pacientes con cáncer gástrico y displasia, y pacientes con gastritis no atrófica (controles). El muestreo endoscópico y la clasificación histológica se realizaron de acuerdo al sistema de Sídney y la clasificación de Lauren. La información genética se obtuvo mediante reacción en cadena de la polimerasa sobre bloques de parafina. Se evaluaron las medidas de asociación de las variables de interés en modelos bivariados y multivariados. Un valor de p < 0,05 se consideró significativo. Se empleó el programa SPSS® versión 25.
ResultadosLa edad mayor a 50 años (OR: 23,76; IC: 8,40-67,17; p = 0,000) y el genotipo VacA s1m1 (OR: 6,18; IC: 1,25-30,51; p = 0,025) se asociaron con mayor riesgo de desarrollar cáncer gástrico y displasia. El genotipo CagA+ no se mostró como un factor de riesgo para desarrollar estas enfermedades (OR: 1,02; IC: 0,39-2,62; p = 0,965).
ConclusionesLos genotipos VacA de H. pylori son marcadores para el desarrollo de cáncer gástrico. Esta información se podría utilizar para crear un índice de riesgo en un modelo predictivo para optimizar la atención de los pacientes con mayor riesgo.
Gastric tumors are the main cause of death by cancer worldwide. According to GLOBOCAN data for 2018, there were 1,033,701 new cases of stomach cancer, with 782,685 deaths attributed to the disease1. Two-thirds of stomach cancers occur in developing countries and Colombia has one of the highest incidence rates of the disease2. However, within the country, there are notable differences in its incidence and mortality. The coastal areas have low incidence rates, whereas they are markedly high in the Andes region and the Southwest of the country. The reasons for those differences are poorly understood, which is why some authors have called it the “Colombian enigma”3.
According to the Lauren classification, gastric cancer can be classified as diffuse and intestinal, with important differences in relation to epidemiology and association with premalignant lesions and Helicobacter pylori (H. pylori)4,5.
In the intestinal histotype, the most important risk factor is H. pylori infection. That bacterium can produce a maladaptive response in the gastric mucosa that induces atrophy, metaplasia, dysplasia, and finally, cancer6. Nevertheless, only some patients with H. pylori infection develop cancer, leading to the postulation that genetic variability of the bacterium modulates the risk of gastric carcinogenesis. For example, the CagA+ and VacA/s1m1 genotypes have been associated with greater risk, when compared with the CagA– and VacA/s2m2 genotypes7,8.
The majority of studies have suggested an association of the CagA and VacA s1m1 genotypes with the development of cancer, but that has been more difficult to prove in Colombia, given the small number of studies on the association between cancer and bacterial genotypes9–11. The focus of those studies has been mainly descriptive, in which allelic and genotypic frequencies are specified, reaching contradictory conclusions. Taking into account the differences in incidence of cancer in different regions of Colombia, we posit the hypothesis that the genetic variability of H. pylori favors the appearance of dysplasia and cancer. Our aim was to evaluate the association of the CagA and VacA genotypes of H. pylori with dysplasia and gastric cancer, through an analytic focus that studies the interaction of the variables in bivariate and multivariate models.
Material and methodsAn analytic unmatched case-control study was conducted on patients with a histopathologic diagnosis determined from gastric biopsies at the “Compañía de Patólogos del Cauca” laboratory and the Department of Pathology of the Universidad del Cauca, located in Southwest Colombia. According to information from the National Administrative Department of Statistics of Colombia, the standardized mortality rates for gastric cancer in the department of Cauca are the highest in the country, at 14.7/100,000 inhabitants per year.
Through convenience sampling, our cohort included patients with a microscopic diagnosis of dysplasia and gastric adenocarcinoma (cases), patients with a histologic diagnosis of nonatrophic gastritis (controls), age 18 years and older, and patients seen at diagnostic centers during 2011. Inclusion criteria were patients with a histopathologic determination of H. pylori infection, corroborated by molecular biology tests. Exclusion criteria were patients that previously presented with gastric cancer, a history of cancer, treatment for H. pylori infection, immunodeficiencies, and the presence of bacterial coinfection determined through polymerase chain reaction (PCR) tests.
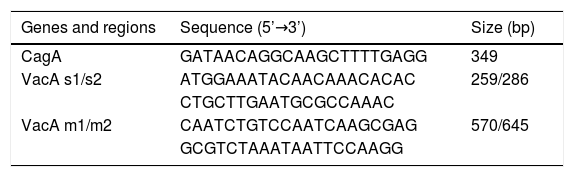
Gastric biopsies were obtained through gastroscopy by highly experienced gastroenterologists. After an 8-h fast by the patients, topical anesthesia was applied and 5 biopsies were taken (one from the greater curvature of the antrum, one from the lesser curvature of the antrum, one from the greater curvature of the corpus, one from the lesser curvature of the corpus, and one from the incisura angularis). The biopsy specimens were fixed in 10% formalin, stained with hematoxylin-eosin, and classified using the Sydney system12. To control information bias, the histopathologic diagnoses were corroborated by a second pathologist and the histopathologic diagnosis of H. pylori infection was confirmed through PCR testing on paraffin blocks using the Chelex® technique (No. C7901; Sigma, St. Louis, MO, USA). The CagA and VacA genes were amplified through PCR, according to the method described by Sugimoto et al.13. The PCR mixtures were prepared with 50 ng of genomic DNA, 100 μmol dNTPs, 2.5 μl of 10X PCR buffer, 1.0 mM MgCl2, 1 U of Taq DNA polymerase (No. M1665; Promega, Madison, WI, USA), and 30 pmol of each of the primers, as shown in Table 1. Denaturalization began at 95 °C for one minute, followed by 35 denaturalization cycles at 94 °C for one minute, hybridization at 52 °C for one minute, extension at 72 °C for one minute, and a final extension step at 72 °C for 10 min. The products were analyzed by electrophoresis in 2% agarose gel at 80 volts for 40 min. The Colombian Instituto Nacional de Cancerología provided the H. pylori strains that were used as the controls. Table 1 shows the primers employed.
Because the age variable had normal distribution and the variance homogeneity hypothesis was proven, the differences between means of the groups were evaluated using the Student’s t test. Differences in proportions were evaluated using the chi-square test. The odds ratio (OR) and its p value were utilized to evaluate the effect of each variable of interest on the response variable (dysplasia/gastric cancer). To assess possible interaction or confounding factors, a multivariate logistic regression test was performed. Statistical significance was set at a p < 0.05 and a 95% confidence interval (CI). The SPSS version 25 program was employed.
Ethical considerationsThe study participants signed statements of informed consent. Ethical approval was provided by the Research Committee of the Universidad del Cauca.
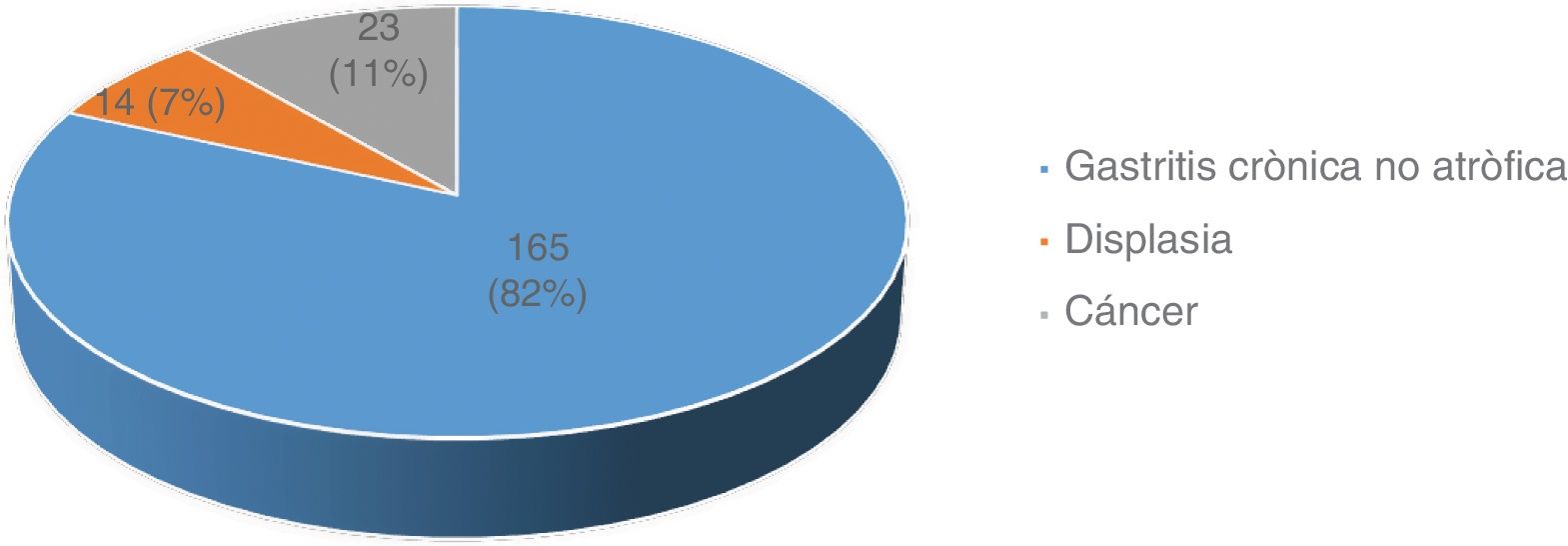
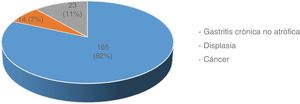
ResultsFrom a total of 654 patients diagnosed at referral centers, 202 were included in the study. Fig. 1 shows patient distribution by diagnosis.
Due to the fact that dysplasia and cancer are more frequent after 50 years of age, the patients were categorized into two group (patients 18 to 50 years of age and patients above 50 years of age).
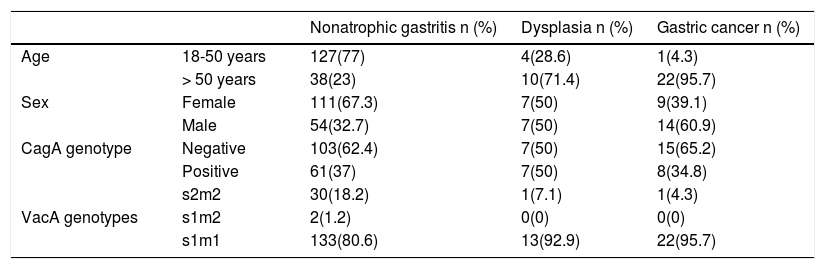
Table 2 shows the distribution of patients by age, sex, and bacterial genotypes.
Distribution of the variables of interest in the study groups.
| Nonatrophic gastritis n (%) | Dysplasia n (%) | Gastric cancer n (%) | ||
|---|---|---|---|---|
| Age | 18-50 years | 127(77) | 4(28.6) | 1(4.3) |
| > 50 years | 38(23) | 10(71.4) | 22(95.7) | |
| Sex | Female | 111(67.3) | 7(50) | 9(39.1) |
| Male | 54(32.7) | 7(50) | 14(60.9) | |
| CagA genotype | Negative | 103(62.4) | 7(50) | 15(65.2) |
| Positive | 61(37) | 7(50) | 8(34.8) | |
| s2m2 | 30(18.2) | 1(7.1) | 1(4.3) | |
| VacA genotypes | s1m2 | 2(1.2) | 0(0) | 0(0) |
| s1m1 | 133(80.6) | 13(92.9) | 22(95.7) |
The chi-square test was used to evaluate the differences between the diagnostic groups, and it showed significant differences between sex and age categories (p = 0.000), but no significant differences between frequencies of the CagA genotype (p = 0.175) or the VacA genotypes (p = 0.364).
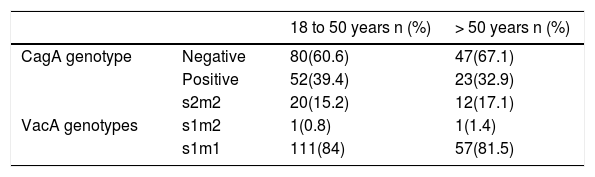
Because the study was unmatched, and to evaluate the distribution of the genotypes between the age groups, the proportions of genotypes in the age categories were compared, finding that the distribution of the genotypes did not vary significantly by age (chi-square test for the VacA genotypes [p = 0.835]; chi-square test for the CagA genotype [p = 0.610]) (Table 3).
Finally, the patients with dysplasia and gastric cancer were grouped together and compared with the reference category (nonatrophic gastritis), obtaining a 4.4:1 control to dysplasia/cancer ratio. To evaluate the age variable, a normality test was performed for the control group (n = 165) through the Kolmogorov-Smirnov test (p = 0.200). For the dysplasia/cancer group (n = 37), normality was proven through the Shapiro-Wilk test (p = 0.700). Once the normal distribution of the data was established, the homogeneity of variance was proven with the Levene’s test, based on the mean, obtaining a value of p = 0.745. With that information, the decision was made to continue the analyses using parametric tests.
The Student’s t test showed statistically significant differences between the mean age of the control group (41 years) and the mean age of the patients with gastric cancer and dysplasia (62 years) (p = 0.000). For the association analyses, the reference categories were age below 50 years, female sex, the CagA– genotype and the VacA s2m2 genotype. Table 4 shows that categorized age and male sex were associated with an increase in the risk for presenting with gastric cancer and dysplasia in the bivariate and multivariate models. Even though the CagA and VacA genotypes showed no significant associations with cancer in the bivariate model, they were introduced in a first logistic regression model, given their association with cancer, described in the literature. The CagA variable showed no significant changes in any of the models, and so was excluded from the final regression (crude OR for CagA+: 4.68, CI: 0.28-77.74, p = 0.282; adjusted OR: 1.02, CI: 0.39-2.62, p = 0.965. Table 4 corresponds to the final and more parsimonious regression model.
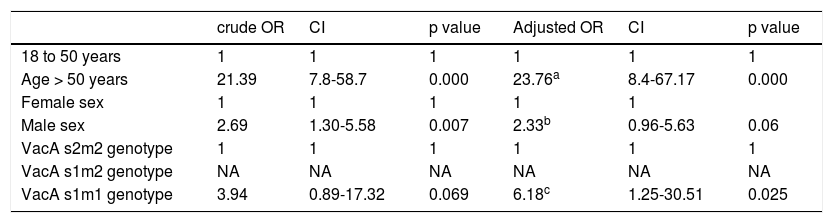
Measures of association for age, sex, and alleles.
| crude OR | CI | p value | Adjusted OR | CI | p value | |
|---|---|---|---|---|---|---|
| 18 to 50 years | 1 | 1 | 1 | 1 | 1 | 1 |
| Age > 50 years | 21.39 | 7.8-58.7 | 0.000 | 23.76a | 8.4-67.17 | 0.000 |
| Female sex | 1 | 1 | 1 | 1 | 1 | |
| Male sex | 2.69 | 1.30-5.58 | 0.007 | 2.33b | 0.96-5.63 | 0.06 |
| VacA s2m2 genotype | 1 | 1 | 1 | 1 | 1 | 1 |
| VacA s1m2 genotype | NA | NA | NA | NA | NA | NA |
| VacA s1m1 genotype | 3.94 | 0.89-17.32 | 0.069 | 6.18c | 1.25-30.51 | 0.025 |
CI: confidence interval; NA: not applicable; OR: odds ratio.
In the present study, a greater risk for presenting with gastric cancer, dysplasia in patients over 50 years of age was observed. Other authors have reported similar conclusions14,15. Those findings are consistent with the theory of carcinogenesis in multiple steps because they suggest that the atrophy-metaplasia-dysplasia-cancer sequence is related to cellular aging. Taking into account that patients with dysplasia –a recognized premalignant lesion– were included in the present study provides relevant information for knowing the factors associated with gastric carcinogenesis, suggests that the genetic variants of H. pylori could utilized be as risk markers in patients with premalignant lesions, such as, dysplasia.
Likewise, those findings underline the importance of prioritizing endoscopic and histologic studies in patients above 50 years of age, in whom cancer and dysplasia are increasingly more prevalent.
In relation to sex, we found significant differences when the study groups were compared by crude rate calculations. The association of male sex with gastric cancer has been reported by several authors16,17, concurring with the results of our study. Possible explanations are greater exposure to carcinogens, such as alcohol and tobacco, in men18,19, and the apparent protective effect of estrogens in women20. However, the modification in the effect in the multivariate model was striking, with p values and confidence intervals very close to the null hypothesis. Those findings suggest that age would play a more determining role than sex in the risk for developing dysplasia or cancer, in the study population.
Although the association of the VacA genotypes with cancer has been questioned by some authors21, in general, the majority of studies have suggested that the VacA/s1m1 genotype and the CagA genotype condition a greater risk for the development of premalignant lesions and cancer22–24. In the present study, we showed that the s1m1 genotype was prevalent in both the patients with gastric cancer and the controls. Nevertheless, the strains with the s1m1 genotype were higher in the patients with dysplasia and cancer, whereas the strains with the s2m2 genotype were higher in the controls. The association of the s1m1 genotype with gastric cancer in the logistic regression model was interesting. Thus, the multivariate analysis suggested that the VacA s1m1 genotype increased the risk for developing dysplasia and cancer in patients above 50 years of age (OR: 6.18, CI: 1.25-30.51, p = 0.025). That information is congruent with the multifactorial nature of gastric cancer and highlights the importance of analyzing variables in multivariate models that enable the confounding or interactive effect of the factors possibly involved in gastric carcinogenesis to be evaluated.
In addition, our results could be useful in the effort to predict the risk of progression to cancer. For example, it is known that only 0.8% of the patients with atrophy and 3.9% of the patients with dysplasia end up developing cancer14,25. However, it is difficult to try and predict which patients will fall into those percentages. Based on our results, we can suggest that patients with the s1m1 genotype are a special risk group, in whom more periodic endoscopic and histopathologic follow-up would be justified.
The pathogenic potential of the VacA/s1m1 genotype can be explained through different mechanisms, such as the capacity to induce cellular vacuolization, mitogen-activated protein kinase (MAPK) activation, and immune system inhibition26,27. Those mechanisms would presumably facilitate the neoplastic transformation in the gastric mucosa28.
The number of Colombian articles on bacterial genotypes and cancer is small. Yamaoka et al. compared the VacA/s1m1 genotype distribution in four countries. Even though they showed that the s1m1 subtype predominated in Colombia, they found no statistically significant associations with the risk for cancer29. In contrast, Citelly et al. showed that the s1m1 genotype was present in 81% of the patients with cancer and only in about 40% of the patients with gastritis, with said difference having statistical significance10. In the study by Sicinschi et al., they compared the numbers of the VacA/s1m1 strains in a high-risk area and a low-risk area for gastric cancer, located in the department of Nariño (Colombia). Those authors concluded that the cytotoxic genotype was more frequent in the high-risk area (p = 0.044)11. Regarding the CagA genotype, a higher risk for developing cancer or dysplasia in the patients with the CagA+ genotype was not shown in our study, which was similar to the results reported by Trujillo et al.9 but different from those reported by Citelly et al. and Sicinschi et al. Those studies had contradictory conclusions, did not calculate the magnitude of association, and were focused on populations that were culturally and geographically different from our study population. To the best of our knowledge, the present study is the first analytic study to calculate the risk for developing dysplasia and adenocarcinoma by evaluating the genotypic variants of H. pylori in a population from the department of Cauca.
It is important to underline the presence of relatively wide confidence intervals (especially for the age variable) in our results that were due to the fact that only five cases of dysplasia and cancer were documented in patients under 50 years of age, and because the strict application of the inclusion criteria conditioned an important reduction in the number of study patients. Nevertheless, we believe that, given the p values, the limits of the confidence intervals, and the calculations obtained in the multivariate model, the conclusion can be made that age and the VacA s1m1 genotype are variables that parsimoniously explain the risk for developing dysplasia and cancer. To obtain accurate calculations and more precise confidence intervals, future studies should include a larger number of patients with cancer and other factors of interest (e.g., BabA genotypes, salt intake, dietary habits, among others) should be included in the multivariate modeling.
In Colombia, despite finding geographic zones with a similar prevalence of H. pylori infection, disparate incidence rates of cancer have been documented, a phenomenon called the “Colombian enigma” by certain researchers3. The genetic variability of H. pylori has been suggested as an explanation of said enigma. Even though the findings of our study appear to lend support to that theory, certain limitations must first be taken into consideration. Our study population was taken from a single geographic region. To determine whether the genetic variability of H. pylori has an influence on the incidence of cancer in the different geographic areas, comparative genotyping studies should be conducted in zones of high risk and low risk for cancer. The study by Trujillo et al.9 employed such a similar methodological design but found no significant differences between bacterial genotypes in two regions of Colombia with contrasting risks for cancer. Therefore, we also suggest that the selection of patients in future studies be complemented with a genetic ancestry analysis, whenever the Colombian population chosen is genetically diverse. The studies by Kodaman et al. have demonstrated the importance of the ancestral composition of the populations and the co-evolutionary relation to bacterial infection30.
In conclusion, it is plausible to state that male patients above 50 years of age are a group at higher risk for gastric cancer. Said risk can be potentiated by the presence of cytotoxic variants of VacA. Therefore, stricter medical follow-up is justified in patients with the VacA/s1m1 genotype. We believe this information can be used for the development of a risk index for gastric cancer, to enable opportune diagnosis of the disease and optimize the use of medical resources in the population at greater risk.
Financial disclosureThe present work was financed by the Programa de Salud de Colciencias de Colombia, project code 1103-519-29123.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank the Human and Applied Genetic Research group of the Universidad del Cauca for their support in the development of this study.
Please cite this article as: Carlosama-Rosero YH, Acosta-Astaiza CP, Sierra-Torres CH, Bolaños-Bravo HJ. Genotipos de Helicobacter pylori asociados con cáncer gástrico y displasia en pacientes de Colombia. Revista de Gastroenterología de México. 2022;87:181–187.