Viral hepatitis, which appears most frequently at birth or during childhood, is a disease whose transmission routes include tears, bile, sexual fluids, sweat, milk, urine, feces, and saliva. The aim of the present study was to analyze the specificity of the immunochromatographic and ELISA diagnostic tests for hepatitis B surface antigen and compare them with PCR testing.
Materials and methodsThe study sample was made up of 140 men and 60 women referred to the Urmia Medical University hospital to undergo PCR testing for HBV diagnosis. The ELISA test was performed using the Pioneer Medicine Company kit (Tehran, Iran).
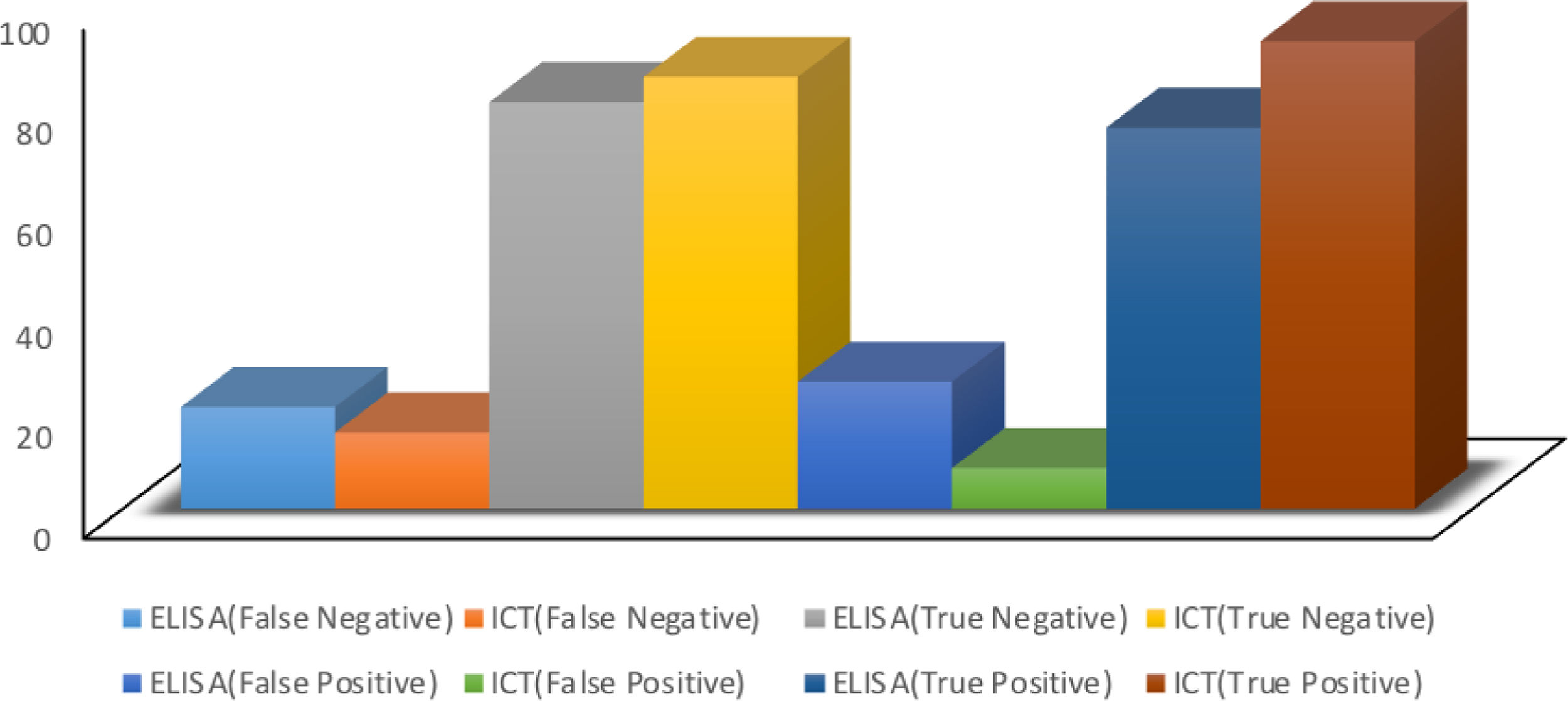
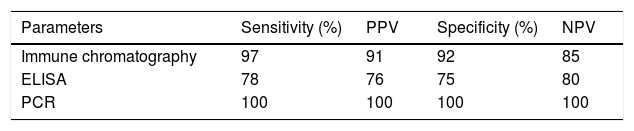
ResultsThe results of the HBs-Ag rapid test and the ELISA test were compared with the PCR test. The HBs-Ag rapid test had 97% sensitivity and 91% specificity, whereas the ELISA test had 78% sensitivity and 76% specificity.
Discussion and conclusionAccording to our results, the immunochromatographic test was accurate for diagnosing HBs-Ag in blood and the ELISA test had acceptable sensitivity and specificity, compared with PCR testing.
La hepatitis viral aparece más frecuentemente al nacer o durante la infancia y es una enfermedad cuyas vías de transmisión incluyen lágrimas, bilis, fluidos sexuales, sudor, leche, orina, heces y saliva. El objetivo del presente estudio fue analizar la especificidad de la inmunocromatografía y la prueba ELISA para el antígeno de superficie de hepatitis B y compararlas con la prueba PCR.
Materiales y métodosLa muestra estuvo compuesta por 140 hombres y 60 mujeres referidos al hospital de la Escuela de Medicina de la Universidad de Urmia para diagnóstico de VHB por medio de prueba PCR. La prueba ELISA fue realizada con el kit de la Pioneer Medicine Company (Teherán, Irán).
ResultadosLos resultados de la prueba rápida HBsAg y de la prueba ELISA fueron comparados con la prueba de PCR. La prueba rápida de HBsAg tuvo una sensibilidad del 97% y especificidad del 91%, mientras que la prueba ELISA tuvo una sensibilidad del 78% y especificidad del 76%.
Discusión y conclusiónDe acuerdo con nuestros resultados, la prueba de inmunocromatografía fue precisa para diagnosticar HBsAg en sangre y la prueba ELISA tuvo sensibilidad y especificidad aceptables en comparación con la prueba de PCR.
The presence of hepatitis B surface antigen (HBs-Ag), hepatitis B virus (HBV), and DNA indicators implies that the disease is infectious HBV. High levels of HBV and DNA and low levels of aminotransferases indicate a poor immune system response to the virus. High levels of the IgM type of anti-hepatitis C virus (HBC) and HBs-Ag in the serum or plasma of patients indicate either acute hepatitis or reactivation of chronic HBV infection, and low levels indicate previous immune infections.1–3 Serum levels of liver enzymes are the most important biochemical factors in diagnosing chronic HBV infection.4 The enzyme-linked immunosorbent assay (ELISA), during which the reaction takes place, based on a primary antigen-antibody interaction, is the most widely used serologic method for identifying HBV antigens and antibodies in developed countries. Improvements in diagnostic methods provide new opportunities for enhancing screening, referral, and treatment. Previous studies on HBV infection have focused on immunologic responses, surveillance of cirrhosis, and treatment. A large number of methods categorized under the enzyme immune assay (EIA) procedure benefit from antibodies that can function as identifying factors.5 The disadvantages of the ELISA test are that it is time-consuming, requires sophisticated equipment and trained technicians, and requires a continuous supply of electricity. Mutations in antigen and antibody molecules reduce the diagnostic sensitivity of that method.6 Patients negative for HBs-Ag are carriers of mutations in the S gene region, incapacitating the ability to produce HBs-Ag. Because of their high sensitivity and availability, quantitative EIA methods are the most frequently used tests in referral hospitals and blood transfusion centers. The immunochromatographic test, a qualitative test for showing HBs-Ag present in serum, plasma, and blood, is used when there is no possibility of conducting an EIA.7,8 Polymerase chain reaction (PCR) methods are applied as the gold standard in most research and advanced medical diagnostic centers. The process of DNA replication is used to quantitatively evaluate the presence of HBV, by isolating the viral DNA and replicating it with oligonucleotide primers.9,10 Considering the broad utilization of diagnostic tests in medical procedures, understanding the concept of diagnostic test evaluation indices is very important. The aim of the present study was to compare immunochromatography, PCR, and ELISA, to determine which was the superior testing method in medical practice.
Materials and methodsThe serum from 200 patients (140 men and 60 women, 25 to 60 years of age) admitted to the Urmia University of Medical Sciences Hospital, within the time frame of November 2013 and December 2014 and stored at minus 70 °C in the Clinical Biochemistry Department, and the experiments carried out in January 2015, were used in the present study. Viral nucleic acid DNA was purified for DNA from the patient serum samples, carried out using the Magcore Cat. No. MVN 400-03 kit, with a magnetic device. The extraction and preparation of hepatitis virus DNA for real-time PCR tests were carried out using a new gene kit (HBV RQ Ref. #HBV0913A-V3.2) and a Rotor gene machine (PCR device, Australia). Fluorescein amidite (FAM) was measured by the progress of sigmoid curves for positive patient samples, according to the amount and number of viruses per unit/mL volume. All samples containing between 10,000 IU/mL and 100,000 IU/mL were ultimately considered positive, and the results of the PCR-negative cases were evaluated as negative samples. The positive and negative PCR cases were divided into two groups. Serum samples underwent HBs-Ag testing by immunochromatography and ELISA procedures.
The ELISA procedureThe Pioneer Medicine Company kit (Tehran, Iran), registration number 93002, was employed in the present study. The ELISA tests were performed after the incubation process and positive-negative case controls. An ELISA Reader Awareness Device (USA) was used to read the tests and record the optical density of the ELISA wells, at a 450-nm wavelength versus the 630-nm reference, to achieve more precise results. Optical densities (ODs) below 0.15 for negative cases and above 0.8 for positive cases were considered the cutoff values for optical density absorbance.
The rapid method: immunochromatographyThe Ab Core Cassette from the Rojan Azma Company, with reference information REF: Ab-3101, was used in the present study. Serum samples (0.075 ml) were placed in the special site of the cassette, and the results were observed qualitatively. The appearance of two red lines indicated a positive result and one red line indicated a negative result (Fig. 1).
The ability of tests to distinguish healthy subjects from patients is assayed by sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Understanding those values is relatively straightforward.
The authors have attempted to present those concepts, utilizing the current results to facilitate comprehension.
ResultsIn the present study, the sensitivity and specificity of both the immunochromatographic tests and the ELISA tests, for diagnosing HBs-Ag, were analyzed. The results of those two methods were compared with the results of PCR procedures for HBV. The immunochromatographic method achieved a sensitivity of 97% and specificity of 91%, with a 0.92 PPV and a 0.85 NPV, compared with the PCR results. The PCR and immunochromatography data showed high sensitivities, making the immunochromatographic test an acceptable method for diagnosing hepatitis B. The ELISA method achieved 78% sensitivity, 76% specificity, 0.75 PPV, and 0.80 NPV, compared with the PCR results. The results showed the efficiency, high sensitivity, and acceptable specificity of the ELISA method, as well. Based on the data collected, the immunochromatographic method had higher sensitivity and specificity, in comparison with the ELISA method (Tables 1 and 2; Fig. 2).
Hepatitis B is a highly infectious virus worldwide and leads to long-term insidious infection. Hepatitis B infections are more prevalent in endemic regions (over 75%), namely Southeast Asia and Africa, with a lifetime risk of infection, ranging from 60 to 80%. Eastern Europe, the Middle East, and India have shown an intermediate risk of infection from 20 to 60%. In the United States, almost 1.25 million individuals are chronically infected with HBV, and an estimated 78,000 cases of new HBV infections occur yearly, mostly among sexually active young adults, Blacks, Latinos, and intravenous drug users.11,12 It remains a major blood-borne infection, with grave implications for blood recipients, if infected blood is not identified during screening, and is the main etiologic agent in chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma, in developing countries.13,14 The global pandemic of hepatitis B, especially in the context of coinfection with HIV and hepatitis C, is critical. Although each laboratory method used to diagnose hepatitis B has its advantages and disadvantages, no research to date has studied the causes of possible differences in results.15,16 The current results and their practical application are significant because the immunochromatographic test is accurate for diagnosing HBs-Ag in blood and the ELISA test has acceptable sensitivity and specificity, compared with the PCR procedure, considered the gold standard. The detection and diagnosis of HBV infections are based mainly on immunologic assays, among which the ELISA and immunochromatographic tests are the most common. However, rapid diagnostic tests are easier to conduct and impose the minimum amount of discomfort on the patient, because a very small sample volume is required. The current study compared two commonly used methods for diagnosing HBV. The presence of HBs-Ag in the serum of patients was measured using the immunochromatographic and ELISA methods, and the specificity and sensitivity of those procedures were compared with the specificity and sensitivity of the PCR method. The immunochromatographic test had considerably greater sensitivity than the ELISA test, with rates of 91% and 75%, respectively, which was reflected in the better PPV and NPV values of the immunochromatographic test. The current results are in line with those of previous studies, reporting the advantages of immunochromatography over ELISA because of its low cost, ease of use, efficiency, and its rapid test status (Nazir, Yousuf et al., 2019; Asaduzzaman, Milon et al. 2018). HBs-Ag immunochromatographic tests have been reported to have good sensitivity and excellent specificity, compared with laboratory immunoassays.7 Lau et al. declared EIA testing to be the standard method for detecting HBs-Ag and HBe-Ag, with the rapid immunochromatographic test having several advantages.17 PCR testing is known as the gold standard for detecting DNA sequences and quantifying HBV, but if serologic and molecular methods are used together, the diagnostic accuracy for HBV infection increases.18 The sensitivity and specificity of the immunochromatographic test kit was 100%, in serum collected in a Korean study that assessed the performance of a diagnostic kit,19 as in our analysis. Another study reported that 38 out of 660 subjects tested positive for HBS-Ag, using the immunochromatographic method, whereas 71 were positive, using the ELISA.20 In the present study, the immunochromatographic test was an acceptable method for diagnosing hepatitis B, with a good level of sensitivity and specificity. The present study revealed a higher PPV with the immunochromatographic test, along with better efficiency (92%), than the ELISA, in most of the HBV cases. In agreement with the current findings, a “rapid” one-step immunochromatographic, visually read, antigen capture assay used for rapid screening of HBs-Ag was evaluated and showed sensitivity of 97% and specificity of 91%. Reports have also indicated seroprevalence values of 0.82% and 0.91% for the immunochromatographic and ELISA tests, respectively.21 Amini et al. observed that diagnostic immunochromatographic tests for detecting HBs-Ag carried out on serum/plasma and capillary whole blood specimens had good sensitivity and important specificity.
ConclusionThe immunochromatographic test was an acceptable method for diagnosing hepatitis B, with good levels of sensitivity and specificity, compared with the gold standard PCR method. Therefore, we consider it to be a satisfactory method for diagnosing hepatitis B.
Ethical considerationsThis article contains no personal information that could identify the patients. The study samples were archived by the clinical biochemistry department of the Urmia University of Medical Sciences Hospital.
Financial disclosureThe present research, conducted at the Urmia Azad University, was financed by the authors. No financial support was received, in relation to this article.
Conflict of InterestThe authors declare they have no conflict of interest.
Please cite this article as: Navvabi N, Khadem Ansari MH, Navvabi A, Chalipa HR, Zitricky F. Evaluación comparativa de las pruebas de diagnóstico ELISA e inmunocromatografía para detección de HBsAg en infección por VHB confirmada por PCR. Rev Gastroenterol Méx. 2022;87:176–180.