Ulcerative colitis (UC) is a multifactorial and polygenic disease. Interleukin-22 (IL-22) is an immunomodulatory cytokine that belongs to the IL-10 family. Currently, some IL-22 polymorphisms have been associated with inflammatory processes such as rheumatoid arthritis and psoriasis vulgaris, but there are no studies on UC.
AimThe aim of this work was to study the frequency of polymorphisms of IL-22 in Mexican patients with UC.
MethodsWe studied a total of 199 Mexican patients with confirmed UC and 697 healthy controls. All individuals were born in Mexico, at least three family generations earlier. A blood sample was obtained from the UC patients and healthy controls in order to perform DNA extraction and then to determine the frequency of IL-22 polymorphisms (rs2227485, rs2272478, rs2227491).
ResultsNo statistical significance was found in the gene and genotype frequencies of three SNPs of IL-22 (rs2227485, rs2272478, rs2227491) between the UC patients and healthy controls. No association was found between those IL-22 SNPs and clinical features of UC.
ConclusionsThere was no association between IL-22 SNPs (rs2227485, rs2272478, rs2227491) and the development of UC in a Mexican population.
La colitis ulcerosa (CU) es una enfermedad multifactorial y poligénica. La interleucina (IL) 22 es una citocina inmunomoduladora que pertenece a la familia de IL-10. Actualmente, algunos polimorfismos de la IL-22 han sido asociados con procesos inflamatorios, como artritis reumatoide y psoriasis vulgar, sin embargo, no hay estudios en pacientes con CU.
ObjetivoEl objetivo del presente trabajo es estudiar la frecuencia de polimorfismos de la IL-22 en pacientes con CU.
MétodosSe estudió a 199 pacientes mexicanos con diagnóstico confirmado de CU y 697 controles sanos. Todos los individuos de estudio nacieron en México, al igual que sus últimas 3 generaciones. Se obtuvieron muestras de sangre de cada individuo y se extrajo ADN; finalmente, se determinó la frecuencia de polimorfismos en la IL-22 (rs2227485, rs2272478, rs2227491).
ResultadosNo se encontró diferencia significativa en la frecuencia del gen y genotipo de los SNP en la IL-22 (rs2227485, rs2272478, rs2227491) entre pacientes con CU y controles sanos. No se encontró asociación entre los SNP de la IL-22 y las características clínicas de la CU.
ConclusionesAusencia de asociación entre los SNP de la IL-22 (rs2227485, rs2272478, rs2227491) y el desarrollo de CU en población mexicana.
Ulcerative colitis (UC) is a multifactorial disease of unknown etiology. It is considered a polygenic disorder that interacts with immunologic and environmental factors, leading to a chronic and relapsing colonic inflammation characterized by an aberrant immune response.1 The Genome-Wide Association Studies (GWAS) of UC have identified more than 25,000 possible single nucleotide polymorphisms (SNP) for inflammatory bowel disease (IBD) in susceptible regions on several chromosomes such as 1, 3, 4, 5, 6, 7, 10, 12, 14, 16, 19, and X.2,3 It is established that mucosal inflammation is triggered by various cytokines produced by different pathways, such as the Th1, Th2, and recently the Th17 immunological response.4
IL-22 is a 16.7 KDa immunomodulatory cytokine primarily produced by activated T cells and natural killer cells. It belongs to the IL-10 family, which also includes IL-19, IL-20, IL-24, IL-26, IL-28, and IL-29.5 This cytokine acts as an effector of the Th17 pathway in response to IL-23 with pro-inflammatory and anti-inflammatory properties that may be implicated in the pathogenesis of IBD. A previous study reported an increased gene expression of IL-22 in the mucosa from rectal biopsies of patients with active UC.6 The IL-22 gene is a 5.3 Kb region located in the 12q15 loci of the chromosome 12, close to the genes encoding IFN-γ and IL-26.7
Currently, some IL-22 polymorphisms have been associated with inflammatory processes, such as rheumatoid arthritis,8 psoriasis vulgaris,9 multiple sclerosis,10 asthma,11 fungal-bacterial infections, and mice models of IBD infected with Citrobacter rodentium.12 There are no previous studies that evaluate the role of IL-22 SNPs in patients with UC. Thus, the aim of this study was to determine the frequency of IL-22 SNPs (rs2227485, rs2272478, rs2227491) in Mexican patients with UC.
Material and methodsPatients and controlsPatients: One hundred ninety-nine Mexican patients with diagnosis of UC confirmed by histology were studied. These patients were recruited from the Inflammatory Bowel Disease Clinic at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubiran in Mexico City. None of the participants had a family history of IBD.
Both demographic and clinical information were obtained via interview and from medical records. The demographic and clinical variables considered in the analysis were: current age, UC duration, extension of disease (pancolitis, left colitis, or distal colitis), presence of extraintestinal manifestations (EIMs), clinical course of disease, response to medical treatment, and the presence of procto-colectomy. The clinical course of disease was defined as: active then inactive (first episode followed by a long-term remission for more than 5 years), intermittent (fewer than 2 relapses per year), and chronic activity (persistent activity despite medical treatment). Response to medical treatment was categorized as follows: favorable, steroid-dependent (relapse when prednisone was tapered below 15mg/day or 3 months after suspending the steroid); steroid-resistant (persistent activity with prednisone of at least 0.5mg/kg/day); immune modulator-resistant (lack of steroid sparing with azathioprine dose of at least 2mg/kg/day for more than 3 months).
Healthy controls: Six hundred ninety-seven healthy and unrelated individuals that were born in Mexico, as were their last two generations. The individuals had no previous history of any autoimmune disease.
Ethical considerationsThis study was carried out according to the principles in the Declaration of Helsinki. The Ethics and Research Committee of our hospital approved the present study and all participants signed a written statement of informed consent.
Blood sampling and genotypingA 3-ml venous blood sample was obtained from each participant using a vacutainer blood collection tube with 4% EDTA.
DNA extraction was performed from mononuclear cells through a non-enzymatic method, according to Lahiri and Nurnberger.13 DNA quantification was done with an ND-1000 spectrophotometer at a 280nm wavelength. Finally, three SNPs (rs2227485, rs2272478, rs2227491) of the IL-22 gene were genotyped using exonuclease TaqMan genotyping assays on an ABI Prism 7900 HT Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA).
Statistical analysisThe present work is a case-control study and the statistical analysis was computed with IBM SPSS 18.0 software. For each one of the groups (controls and patients), the Hardy-Weinberg equilibrium of the IL-22 polymorphisms was analyzed using the chi-square test. The association analysis for the frequency of a present locus was evaluated with a 2×2 contingency table and the Pearson's chi-square test. Comparisons of allele frequencies between subgroups were performed using the EPI-INFO statistical package (Version 5.0). Significance was set at a p < 0.05.
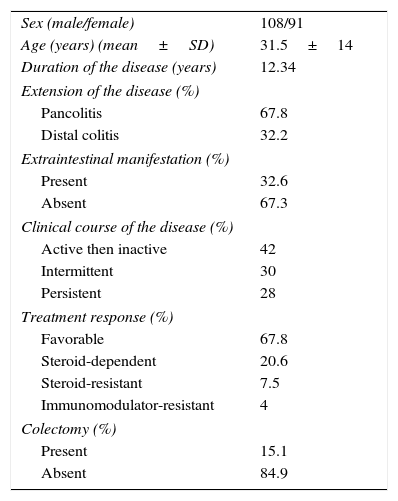
ResultsDemographic and clinical featuresOf the 199 UC patients, 54% were women and 46% were men with a mean age at diagnosis of 31.5 ± 14 years. In the control group, 51% were women and 49% were men, with a mean age of 35±14 years.
Regarding the clinical features, the mean duration of disease was 12.34 years; 67.8% of the patients had pancolitis, 32.2% presented with extraintestinal manifestations, and 15.1% had a proctocolectomy. The clinical course of disease was distributed as follows: 42% of the patients had active disease and then long-term remission; 30% had intermittent activity, and 28% had continuous or persistent activity. The response to medical treatment was favorable in 67.8% of the patients; 20.6% were steroid-dependent; 7.5% were steroid-resistant, and 4% were thiopurine-resistant as shown in Table 1.
Demographic and clinical characteristics of patients with ulcerative colitis.
| Sex (male/female) | 108/91 |
| Age (years) (mean±SD) | 31.5±14 |
| Duration of the disease (years) | 12.34 |
| Extension of the disease (%) | |
| Pancolitis | 67.8 |
| Distal colitis | 32.2 |
| Extraintestinal manifestation (%) | |
| Present | 32.6 |
| Absent | 67.3 |
| Clinical course of the disease (%) | |
| Active then inactive | 42 |
| Intermittent | 30 |
| Persistent | 28 |
| Treatment response (%) | |
| Favorable | 67.8 |
| Steroid-dependent | 20.6 |
| Steroid-resistant | 7.5 |
| Immunomodulator-resistant | 4 |
| Colectomy (%) | |
| Present | 15.1 |
| Absent | 84.9 |
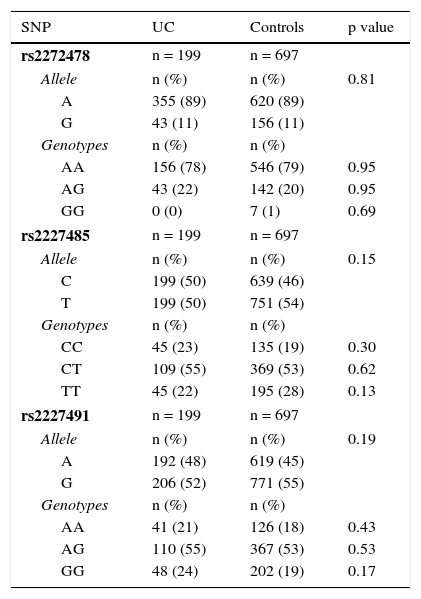
The allele frequency for the rs2272478 SNP was 89% for allele A in both groups, with no statistical difference. With respect to the rs2227485 SNP, the frequency for allele C was 46% in the control group and 50% in the UC patients, and in regard to the rs2227491 SNP, the frequency of allele A was 45 vs 48% in the healthy control group as shown in Table 2.
Allele and genotype frequency of IL-22 gene polymorphisms.
| SNP | UC | Controls | p value |
|---|---|---|---|
| rs2272478 | n = 199 | n = 697 | |
| Allele | n (%) | n (%) | 0.81 |
| A | 355 (89) | 620 (89) | |
| G | 43 (11) | 156 (11) | |
| Genotypes | n (%) | n (%) | |
| AA | 156 (78) | 546 (79) | 0.95 |
| AG | 43 (22) | 142 (20) | 0.95 |
| GG | 0 (0) | 7 (1) | 0.69 |
| rs2227485 | n = 199 | n = 697 | |
| Allele | n (%) | n (%) | 0.15 |
| C | 199 (50) | 639 (46) | |
| T | 199 (50) | 751 (54) | |
| Genotypes | n (%) | n (%) | |
| CC | 45 (23) | 135 (19) | 0.30 |
| CT | 109 (55) | 369 (53) | 0.62 |
| TT | 45 (22) | 195 (28) | 0.13 |
| rs2227491 | n = 199 | n = 697 | |
| Allele | n (%) | n (%) | 0.19 |
| A | 192 (48) | 619 (45) | |
| G | 206 (52) | 771 (55) | |
| Genotypes | n (%) | n (%) | |
| AA | 41 (21) | 126 (18) | 0.43 |
| AG | 110 (55) | 367 (53) | 0.53 |
| GG | 48 (24) | 202 (19) | 0.17 |
SNP: single nucleotide polymorphism; UC: ulcerative colitis.
The finding of the present study was that there was no association between 3 polymorphisms of the IL-22 gene (rs2227485, rs2272478, rs2227491) and the development of UC in Mexican patients. In Western and Northern European populations, there are 27 polymorphisms identified in the IL-22 gene that have been associated with a number of illnesses, such as malaria and autoimmune diseases. Nevertheless, it is important to mention that this is the first study performed on UC patients, In contrast, other studies have found an association between IL-22 polymorphisms and psoriasis vulgaris,14 hepatitis C virus infection,15 and mucosa-associated lymphoma of T cells.16,17 Although the selection of polymorphisms evaluated in the present study was based on immunologic and etiopathologic mechanisms associated with psoriasis vulgaris,14 the evaluation of only 3 IL-22 SNPs is a weakness of this study that could possibly lead to further research with other potential polymorphisms reported in hepatitis, colonic cancer, and lymphoma.15–17The lack of association of IL-22 SNPs and UC in Mexicans patients does not rule out the potential role of other SNPs of IL-22 reported in the above-mentioned diseases; in IBD, IL-22 has the role of a cytokine with both pro-inflammatory and anti-inflammatory effects, depending on the type of tissue target. A previous paper reported that IL-22 has an anti-inflammatory role in an IBD animal model, since the infusion of anti IL-22 proteins delayed epithelial restoration.18 Another study found an increased expression of the IL-22 gene in rectal biopsies from patients with active UC, compared with UC in remission and controls without inflammation. It has been demonstrated that IL-22 participates in the maintenance of the epithelial barrier by inducing the expression of genes regulating proliferation, wound healing, apoptosis, and tight junctions. It also participates in the production of mucins such as MUC1, 3, 10, and 13 and innate antimicrobials peptides such as defensins, Reg family molecules, and S100 proteins.18
The IL-22 receptors include IL-22R1 and IL-10R2, which are heterodimeric proteins with specific distribution in keratinocytes, hepatocytes, and enterocytes,19,20 but not in immune cells. Thus Th17 cells use IL-22 to communicate only with epithelial and stromal cells. Signaling transduction of IL-22R1 activates the MAPK, STAT1, STAT3, and STAT5 pathways through a Jak1/Tyk2 kinase that stimulates the production of antimicrobial agents and tissue repair by inducing proliferation and inhibiting apoptosis. Although IL-22 might have proinflammatory properties, studies have shown that it has a protective role in the liver,21 gut,18 and myocardium.22 The presence of IL-22 and its receptors has been described all along the gastrointestinal tract, including the oral cavity, salivary glands, esophagus, stomach, and colon. The negative regulation of IL-22 is mediated by transforming growth factor beta, so even when it is necessary for TH17 differentiation, it can also act as an inhibitor of IL-22 production through the binding of IRF4 to the promoter region of the IL-22 gene.
Interestingly, the influence of some of the genetic factors may have a geographic distribution. In other words, one specific SNP may predispose to IBD in a given ethnic group, whereas in a different group, it may neither predispose to nor protect against IBD.23–25 In the Mexican population, the inflammatory cytokine polymorphism, IL-15 rs2254514 SNP, was associated with a decreased risk of UC (OR = 0.62, p=0.014) and the IL-15 (rs2254514) genotype CC was associated with early age at diagnosis of UC (OR=3.67, p=0.03).26 In the instance of IL-1β, the rs16944 SNP was associated with the presence of steroid dependence in UC Mexican patients (OR=4.09, p=0.008).27
On the other hand, several SNPs of anti-inflammatory cytokines belong to the IL-10 family, such as the SNPs of IL-20 rs2981573 (p=0.017, OR=0.55, 95% CI=-0.33-0.93), IL-20 rs2232360 (p=0.017, OR=0.55, 95% CI=0.33-0.93),28 IL-19 rs2243188 (p=0.018, OR=0.53, 95% CI=0.32-0.86), and IL-19 rs2243193 (p=0.006, OR=0.48, 95% CI=0.29-0.80) and have been shown to have a protective role in UC.29
In conclusion, there was no association between IL-22 SNPs and the development of UC in the Mexican population.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that the procedures followed conformed to the ethical standards of the responsible committee on human experimentation and were in accordance with the World Medical Association and the Declaration of Helsinki.
Data confidentialityThe authors declare that they have followed the protocols of their work center in relation to the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Yamamoto-Furusho JK, Sánchez-Morales GE, García-Rangel D, Vargas-Alarcón G. Polimorfismos genéticos de interleucina-22 en pacientes con colitis ulcerosa. Revista de Gastroenterología de México. 2016;82:86–90.