El genotipo 5 del virus de la hepatitis C (VHC) es sumamente raro; además, existe poca literatura respecto a su tratamiento. Presentamos el caso de un paciente con VHC genotipo 5, que presumiblemente adquirió la enfermedad por medio de transfusión sanguínea en la infancia. Tras 24 semanas de tratamiento, se logró la respuesta virológica sostenida. De acuerdo con la evidencia disponible sobre el tratamiento del VHC genotipo viral 5, este muestra una respuesta similar al VHC genotipo 1. En este caso, el paciente presentaba varios factores pronósticos favorables. La cantidad de información respecto al tratamiento del VHC genotipo viral 5 es mucho menor en comparación con la disponible para el tratamiento de los VHC genotipos 1, 2, 3 y 4, esto debido principalmente a la baja prevalencia que muestra el VHC genotipo 5 en nuestro medio.
Hepatitis C virus (HCV) genotype 5 is extremely rare and there is very little reported on its management in the medical literature. We present herein the case of a patient with HCV genotype 5 that presumably acquired the disease through a blood transfusion during infancy. Sustained virologic response was achieved after 24 weeks of treatment. According to the available information on HCV genotype 5 treatment, it has a similar response to that of HCV genotype 1. Our patient presented with various favorable outcome factors. There is much less reported on the treatment of HCV genotype 5 than there is regarding HCV genotypes 1, 2, 3, and 4. This is mainly due to the low prevalence of genotype 5 in the Mexican environment.
Introduction
Hepatitis C has a worldwide prevalence of approximately 170 to 180 million persons.1 The prevalence rates of hepatitis C for Latin America are estimated to vary from 1 to 2.6%, depending on the region or country that is evaluated.2
The estimated number of Mexicans infected with hepatitis C virus (HCV) above 20 years of age reaches 700,000, with a prevalence of 1.4%. Studies conducted on high risk populations (persons receiving hemodialysis) have shown rates from 6.7 to 10.2%.3
In the study by Márquez-Rosales et al., genotype 1 represented 58.3% of the cases; genotype 2, 30.5%; genotype 3, 8.7%; genotype 4, 1%; and the mixed genotypes, 1.5%; genotype 1b was the most frequent in Mexico.4
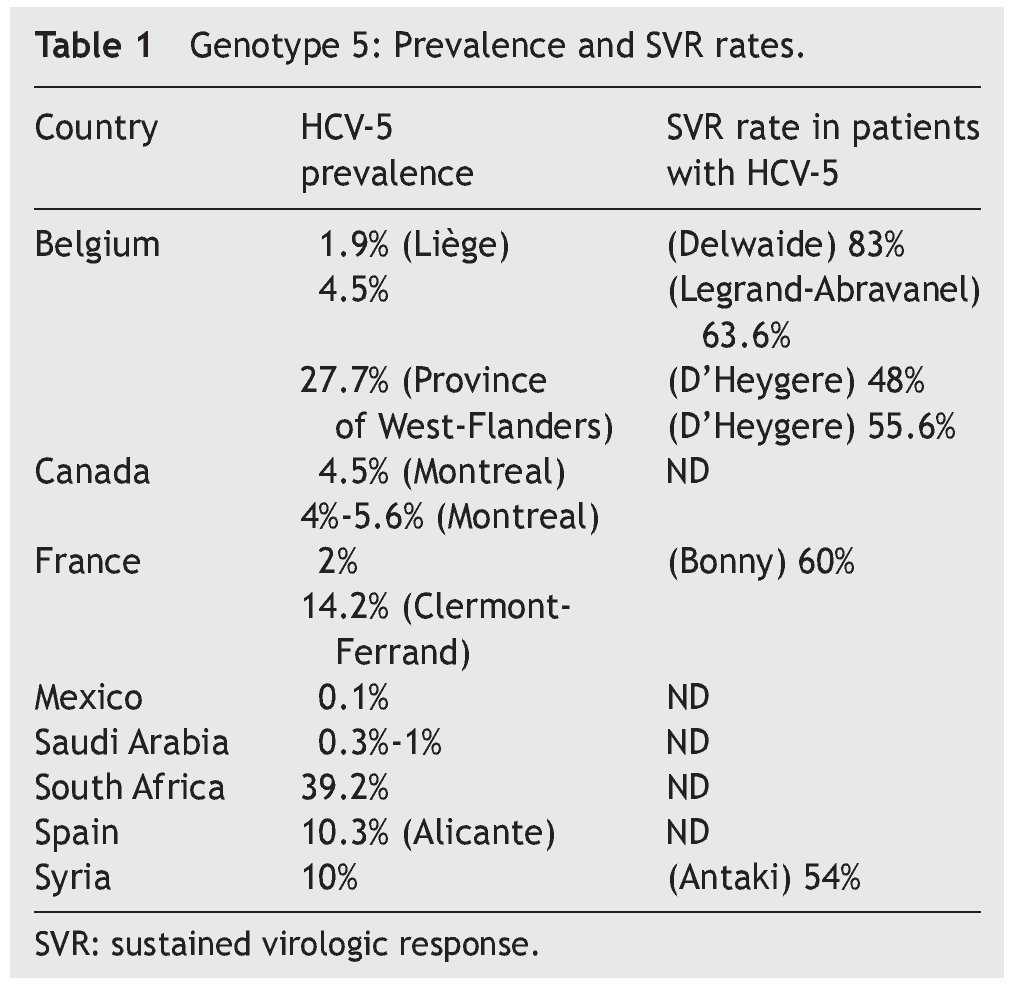
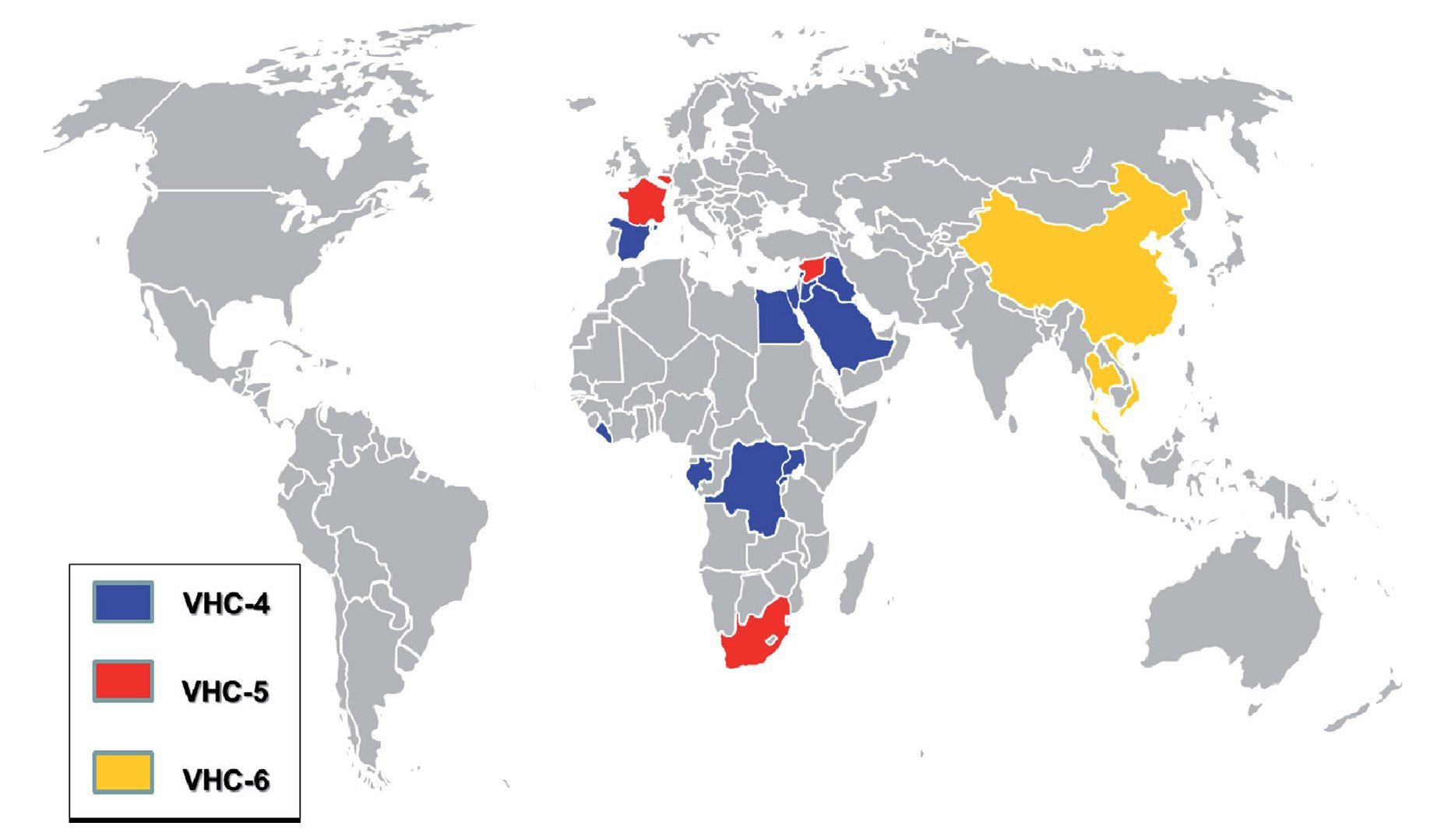
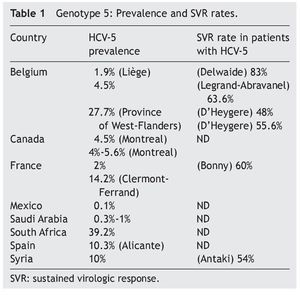
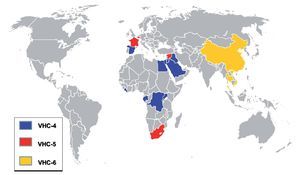
The frequency of the 6 genotypes described varies, depending on the geographic location studied. Little has been reported on HCV-5 and HCV-6 in relation to treatment, due to their low prevalence in the Americas and in Europe. HCV-5 has a very limited distribution and this information is summarized in Table 1. In Mexico, studies have reported a 0.1% prevalence of HCV-5.5,6 (Fig. 1 and Table 1).
Figure 1. Worldwide distribution of genotypes 4, 5, and 6. Map showing the distribution of genotypes 4, 5, and 6 in the countries with the highest reported prevalence. Genotype 4 is blue, genotype 5 is red, and genotype 6 is yellow.
The low frequency of HCV-5 and the consequent scant knowledge of its management have resulted in uncertainty with respect to treating HCV-5 patients. Therefore we decided to share this case and review the current treatment recommendations.
Case presentation
A 27-year-old male student had a past medical history of scarlet fever at 5 years of age, requiring one blood transfusion during his hospitalization in 1986. He was incidentally diagnosed with hepatitis C in 2001 when he attempted to donate blood, and at that moment was identified as a HCV carrier. There was no other relevant history in regard to his condition.
When he arrived for treatment he had no disease symptoms. Upon physical examination he weighed 70 kg, his height was 1.75 meters, and he had no signs of chronic hepatic disease.
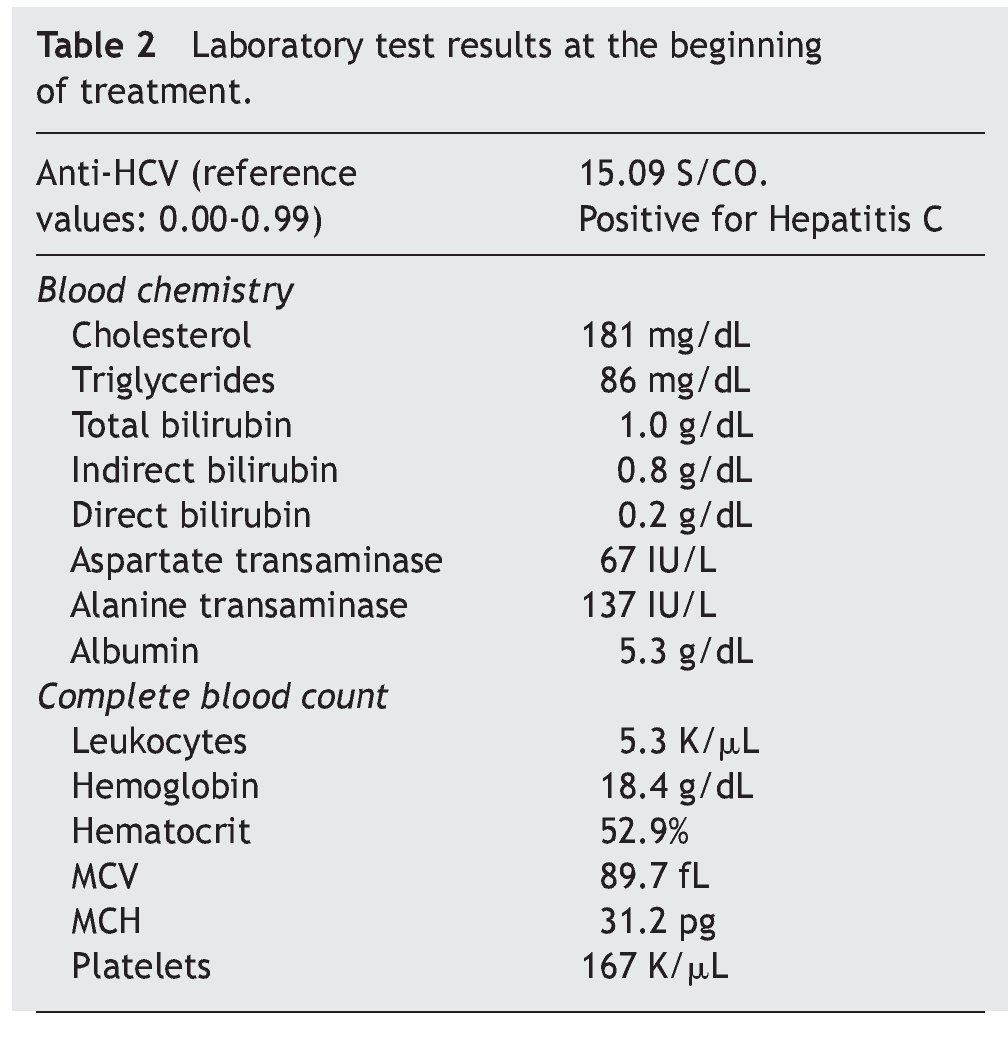
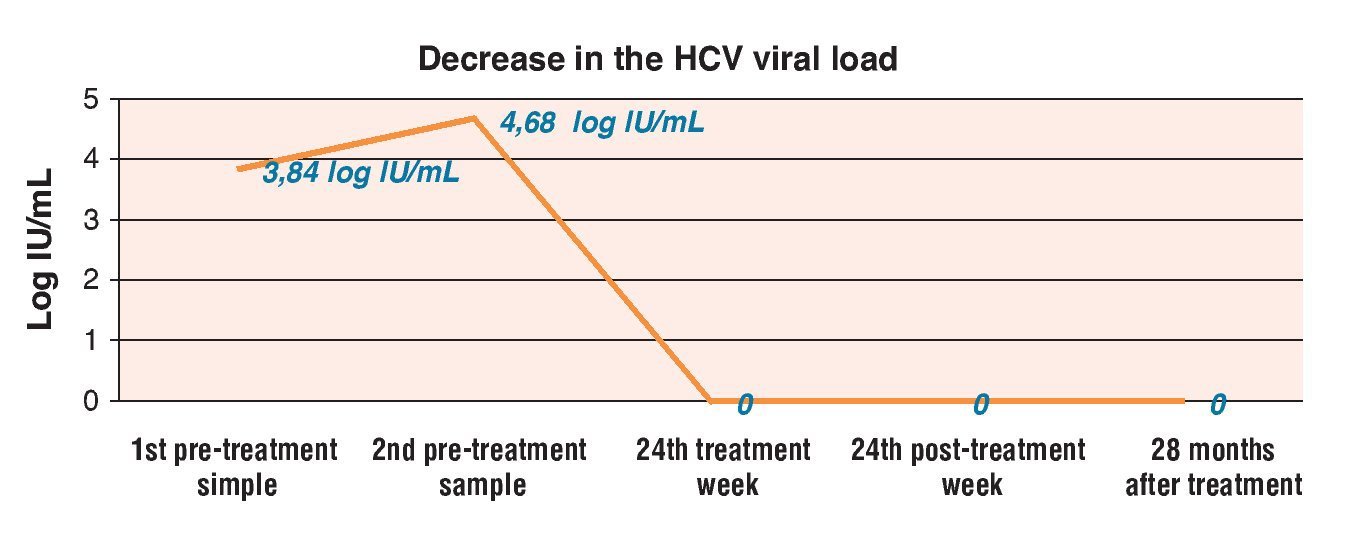
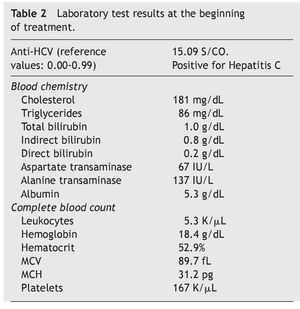
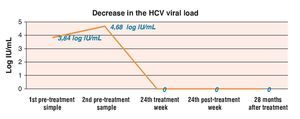
Table 2 and Figure 2 show the laboratory test results.
Figure 2. Decrease in viral load before, during, and after treatment.
The patient was given ribavirin and pegylated interferon-α-2a combination treatment in May of 2009:
1. Pegylated interferon-α-2a: 180 mcg weekly for 24 weeks.
2. Ribavirin 1,000 mg every 24 h for 24 weeks (divided into 400 mg - 200 mg - 400 mg).
He was also given one tablet of folic acid daily as a nutritional complement.
Treatment was well tolerated and carried out as indicated and the patient showed favorable progression with no added symptoms. The only problem reported was a decrease in hemoglobin to 13.7 g/dL with hematocrit of 41.7% at approximately 24 weeks of treatment that did not require erythropoietin.
End-of-treatment response (ETR) was obtained upon management conclusion, as well as sustained virologic response (SVR) with undetectable viral load, which remained the same at the control follow-up visit 2 years after the end of treatment.
Discussion
The SVR rate reported for HCV-5 with the combination treatment for 48 weeks is 60%.15 The most adequate treatment for HCV-5 is currently unknown, due to the low frequency of this disease.16
HCV-5 appears to have higher SVR rates than HCV-1 and slightly poorer ones when compared with the rates reported for HCV-2 and HCV-3. Pang et al. described how treatment response may be due to the adaptive changes the HCV has experienced throughout its history; specifically focusing on HCV-5, it is suspected that its branching took place at some intermediate point — after that of HCV-2 and before that of HCV-1 and HCV-4. Relating the SVR rates to the time at which the genotypes branched (the more recent the separation, the poorer the treatment response), they propose that response is related to the time the genotype has had to adapt to the organism's immune response, before branching.17
Bonny et al. reported a SVR of 60% in 87 HCV-5 patients, contrasting with the SVR of 37% reported for HCV-1 and 63% for HCV-2 and 3.18 Antaki et al. reported a SVR of 54% in 26 HCV-5 patients.
More recently, the meta-analysis conducted by D'Heygere et al. included the results of the BERNAR-1 and BERNAR-2 studies in a total of 48 HCV-5 patients and, as in their previous study, the SVR rates suggested a similar response between HCV-5 and HCV-1 (55.6% and 50%, respectively). This response was clearly inferior to that of HCV-2 and HCV-319 (75%) (p < 0.001 between HCV-5 and HCV-2 and 3) (Table 1).
Antaki et al. found higher SVR rates in the combination treatment using pegylated interferon when compared with interferon20 (67% and 47%, respectively, p = 0.43). This supports the results previously reported by D'Heygere et al.19 describing a SVR of 55% in HCV-5 patients treated with pegylated interferon-α-2a vs. 48% with standard interferon.
The length of treatment duration for HCV-5 has not yet been established, but Antaki et al. found that it did not affect SVR rates (54% with 24 weeks and 54% with 48 weeks, p = 1); these results suggest that 24 weeks may be sufficient.
As we have already mentioned, our patient received 24 weeks of treatment with satisfactory results. Short cycle treatment was chosen because the patient was a student and his health insurance coverage was due to expire at the end of that same 24-week period, and he could not afford to continue treatment. In addition, there was no treatment consensus in 2009, given that there were very few studies on HCV-5 management and their results were controversial. Furthermore, some of them were published after our patient was treated. We must mention that the current accepted conduct is to treat HCV-5 patients with the same regimen described for HCV-1, in other words, pegylated interferon plus a standard dose of 1,000 or 1,200 mg/day of ribavirin according to body weight, and treatment duration of 48 weeks.21
The majority of studies show that HCV-5 treatment response is better than that shown by HCV-1, but its results are lower when compared with those of HCV-2 and HCV-3.22
It should be noted that all of these studies have been conducted on small groups of patients and with differences in the type of interferon employed, doses, treatment duration, hepatic fibrosis stage, treatment-naïve patients, or recurrence, etc.
So far, the direct action antiviral drugs have only been approved for HCV-1 treatment. Further studies on other genotypes are required, including the HCV-5 genotype.23
We also wish to clarify that folic acid administration is not part of the treatment for infection with any HCV genotype. We took this measure to provide our patient with a vitamin complement.
In relation to HCV-5 management and distribution, the focus of our review was to add to the medical bibliography on this genotype, given that there is so little information currently available.
Financial disclosure
No financial support was received in relation to this article.
Conflict of interests
The authors declare that there is no conflict of interest.
Received 23 August 2012;
accepted 26 December 2012
* Corresponding author at:
Boulevard Manuel Ávila Camacho S/N,
Las Margaritas, Tlalnepantla de Baz, Estado de México. Mexico.
Tel.: +52 (55)53976955.
E-mail address: amadel24@hotmail.com (M.A. Rubio-Lezama).