Posttransplantation diabetes mellitus (PTDM) is a serious long-term complication that has a negative impact on graft and patient survival. The purpose of the present study was to describe the incidence of PTDM in a Mexican cohort and evaluate its association with a previous family history of diabetes (FHD).
MethodsA retrospective single-center cohort study was conducted on patients undergoing liver transplantation (LT). The primary outcome was time from LT to PTDM. The diagnosis of PTDM was established using the ADA criteria. A mediation analysis that used adjusted Cox regression models and considered pretransplant prediabetes a mediator was performed, to determine the total effect and direct effect of FHD on PTDM.
ResultsA total of 152 patients were included, with a median follow-up time of 41 months; 19.2% (n = 29) had pretransplant diabetes. During the follow-up time, 15% of patients developed PTDM (n = 23), with an incidence rate of 4.71 cases/100 person-years. PTDM was significantly higher in patients with FHD, compared with those with no FHD (8.72 cases/100 person-years vs 2.04 cases/100 person-years, respectively; p = 0.001). The adjusted hazard ratio of PTDM for FHD was 4.14 (95% CI 1.60–10.7), p = 0.005) and 3.48 (95% CI 1.35–9.01, p = 0.010), when further controlled for pretransplant prediabetes.
ConclusionThe occurrence of PTDM was similar to that reported in most international studies. As with type 2 diabetes, family history plays an important role in the development of PTDM, even after accounting for pretransplant prediabetes. Patients with FHD should undergo a stricter metabolic program.
La diabetes mellitus posterior a trasplante (DMPT) es una complicación grave de largo plazo que tiene un impacto negativo sobre el injerto y la sobrevida del paciente. El objetivo del presente estudio fue describir la incidencia de la DMPT en una cohorte mexicana y evaluar su asociación con el antecedente familiar de diabetes (AFD).
MétodosSe realizó un estudio de cohorte retrospectivo unicéntrico de pacientes sometidos a trasplante hepático (TH). El desenlace primario fue el tiempo entre el TH y el desarrollo de DMPT. El diagnóstico de DMPT fue establecido utilizando los criterios de la ADA. Se realizó un análisis de mediación que utilizó modelos de regresión de Cox ajustados y se manejó la prediabetes pretrasplante como mediador, para determinar el efecto total y el efecto directo del AFD sobre la DMPT.
ResultadosSe incluyó a un total de 152 pacientes, con una mediana de seguimiento de 41 meses; 19.2% (n = 29) presentaron diabetes pretrasplante. Durante el tiempo de seguimiento, 15% de los pacientes desarrollaron DMPT (n = 23), con una tasa de incidencia de 4.71 casos/100 personas-año. La DMPT fue significativamente más elevada en pacientes con AFD en comparación con aquellos sin AFD (8.72 casos/100 personas-año vs 2.04 casos/100 personas-año, respectivamente; p = 0.001). El cociente de riesgo ajustado para el desarrollo de DMPT en los pacientes con AFD fue 4.14 (IC 95% 1.60–10.7; p = 0.005) y 3.49 (IC 95% 1.35–9.01; p = 0.010), cuando se controló por prediabetes pretrasplante.
ConclusiónLa incidencia de DMPT fue similar a la reportada en la mayoría de los estudios internacionales. Al igual que con la diabetes tipo 2, el AFD juega un papel importante en el desarrollo de la DMPT, incluso después de considerar la prediabetes pretrasplante. Los pacientes con AFD deben someterse a un programa metabólico más estricto.
Liver transplantation (LT) has become the main treatment for a wide range of acute and chronic liver diseases. Over the last 30 years, advances in perioperative medicine, surgical techniques, and immunosuppression have substantially improved survival.1,2 Due to this improvement, attention has recently shifted towards long-term complications of LT. Posttransplantation diabetes mellitus (PTDM) is a well-described and serious metabolic complication associated with reduced graft function, risk of infections, graft rejection, and worse patient survival. During long-term follow-up after LT, up to 30% of deaths are attributable to cardiovascular causes, such as metabolic syndrome or diabetes.3,4 A broad variation in the prevalence of PTDM has been reported at different centers due to a lack of consensus on its definition and diagnosis timing, ranging from 9% to 63.3%.5–7
PTDM is defined as the development of posttransplant diabetes in a previously nondiabetic patient and is now established according to the American Diabetes Association/World Health Organization (ADA/WHO) criteria.8 Several factors have been associated with its development, both modifiable and nonmodifiable. The nonmodifiable factors include age, ethnicity (Black and Hispanic populations), family history of diabetes, etiology of cirrhosis (nonalcoholic fatty liver disease), and prediabetes, whereas the modifiable factors include obesity, posttransplant immunosuppression, hypomagnesemia, and viral infections, such as hepatitis C virus and cytomegalovirus (CMV).7,9–11 The prevalence of diabetes mellitus is high in the Mexican population, reaching up to 13.7%, according to the 2016 National Health and Nutrition Examination Survey (ENSANUT, the Spanish acronym).12 Ethnicity is thought to play a role in the development of diabetes and PTDM.13 For example, Native American ancestry has been associated with an increased risk of type 2 diabetes. In addition to lifestyle, diet, and healthcare access, said association may be related to genetic factors.14 However, it remains unclear if the pathogenesis of PTDM and type 2 diabetes share the same pathophysiology. Even though the presence of PTDM has also been linked to B-cell dysfunction secondary to immunosuppression, studies have shown that it shares common genetic factors with type 2 diabetes.15–17 Currently, there are no reports of PTDM in the Mexican population, hence, the purpose of this study was to determine the incidence of PTDM in a Mexican cohort and evaluate its association with family history of type 2 diabetes mellitus (FHD).
Materials and methodsWe performed a retrospective cohort study, including patients that underwent orthotopic LT, within the time frame of 2015–2019, at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, a tertiary care hospital in Mexico City. Patients with a previous history of diabetes mellitus and those that died or were lost to follow-up, within the first six months after LT, were excluded. Based on the FHD, we classified patients into 2 groups: with FHD and without FHD. We considered FHD, when parents or siblings were affected by type 2 diabetes. The primary aim was to determine whether patients with FHD had a higher incidence of PTDM and the primary outcome was time to PTDM diagnosis. Secondary outcomes were time to graft rejection, time to CMV infection, and the need for re-transplantation. Data were collected from the pretransplant clinical charts and follow-up visits through electronic records. PTDM was defined according to the American Diabetes Association (ADA) and included the following: A fasting plasma glucose (FPG) level of 126 mg/dl (7.0 mmol/l) or higher, a 2 h plasma glucose level of 200 mg/dl (11.1 mmol/l) or higher during a 75 g oral glucose tolerance test, random plasma glucose of 200 mg/dl (11.1 mmol/l) or higher in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, or a hemoglobin A1c (HbA1c) level of 6.5% (48 mmol/mol) or higher. Patients that had transient hyperglycemia and did not meet the ADA criteria after 6 months post-LT were not recorded as PTDM cases.
The following data were collected from pretransplant clinical charts: age, sex, body mass index (BMI), FHD (first-degree relatives), history of prediabetes, history of hypertension, Child-Pugh score, MELD score, MELD-Na score, history of hepatocellular carcinoma, liver disease etiology, and blood group. The presence of PTDM, according to ADA diabetes criteria and immunosuppression information (use of basiliximab, calcineurin inhibitors, sirolimus, and steroids, as well as time on steroids and prednisone dosage for patients on steroids for more than one year), were collected from posttransplant outpatient visits and/or recurrent hospitalization records. For patients that received tacrolimus, the average and median tacrolimus levels within the first 6 months posttransplant were also collected. Data collected from organ donors were age, sex, BMI, presence of hepatic steatosis through biopsy, and blood group. The study was conducted in accordance with the Declaration of Helsinki and met the current regulations on bioethical research; it was also authorized by the ethics committee of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (CAI-3743-21-21-1).
Statistical analysisThe categorical variables are presented in frequency and percentages, whereas the numerical variables are summarized in mean and standard deviation or median and interquartile range (IQR), as appropriate. Baseline characteristics were compared between patients with and without FHD, using a chi-square test, if categorical, or a t-test allowing for heteroscedasticity, if numeric.
Overall PTDM cumulative incidence at 1, 2, 3, and 4 years posttransplant and FHD-related PTDM incidence were calculated, using the Kaplan-Meier method, and they were compared using the log-rank test. The unconditional and conditional total effect of FHD on developing PTDM was estimated using an unadjusted and an adjusted Cox regression model, respectively. The adjusted model controlled for age, BMI, and liver disease etiology, which were considered a priori confounders. In addition, because prediabetes is a condition that precedes diabetes, diabetes pretransplant was treated as a mediator, albeit a partial mediator because patients might develop prediabetes posttransplantation and PTDM afterwards (see diagram below). A mediation analysis using the Baron and Kenny method was performed to verify that pretransplant prediabetes was a partial mediator and to estimate the direct unconditional and conditional effect of FHD on developing PTDM.18 The total effect of FHD on developing PTDM includes the causal pathway throughout prediabetes before LT (indirect effect) and that which does not include prediabetes before LT (direct effect).
Finally, as an exploratory analysis, baseline characteristics and immunosuppression features were assessed as predictors for PTDM, using univariate proportional hazard Cox regression models. Variables that were statistically significant at the 0.10 level in the univariate analysis were included in a multivariate proportional hazard Cox regression model.
For all Cox regression models, the proportional hazards assumption was assessed, using the Schoenfeld residuals. The statistical analysis was performed with R version 4.0.3. The confidence level was established as 0.05 at 2 tails.
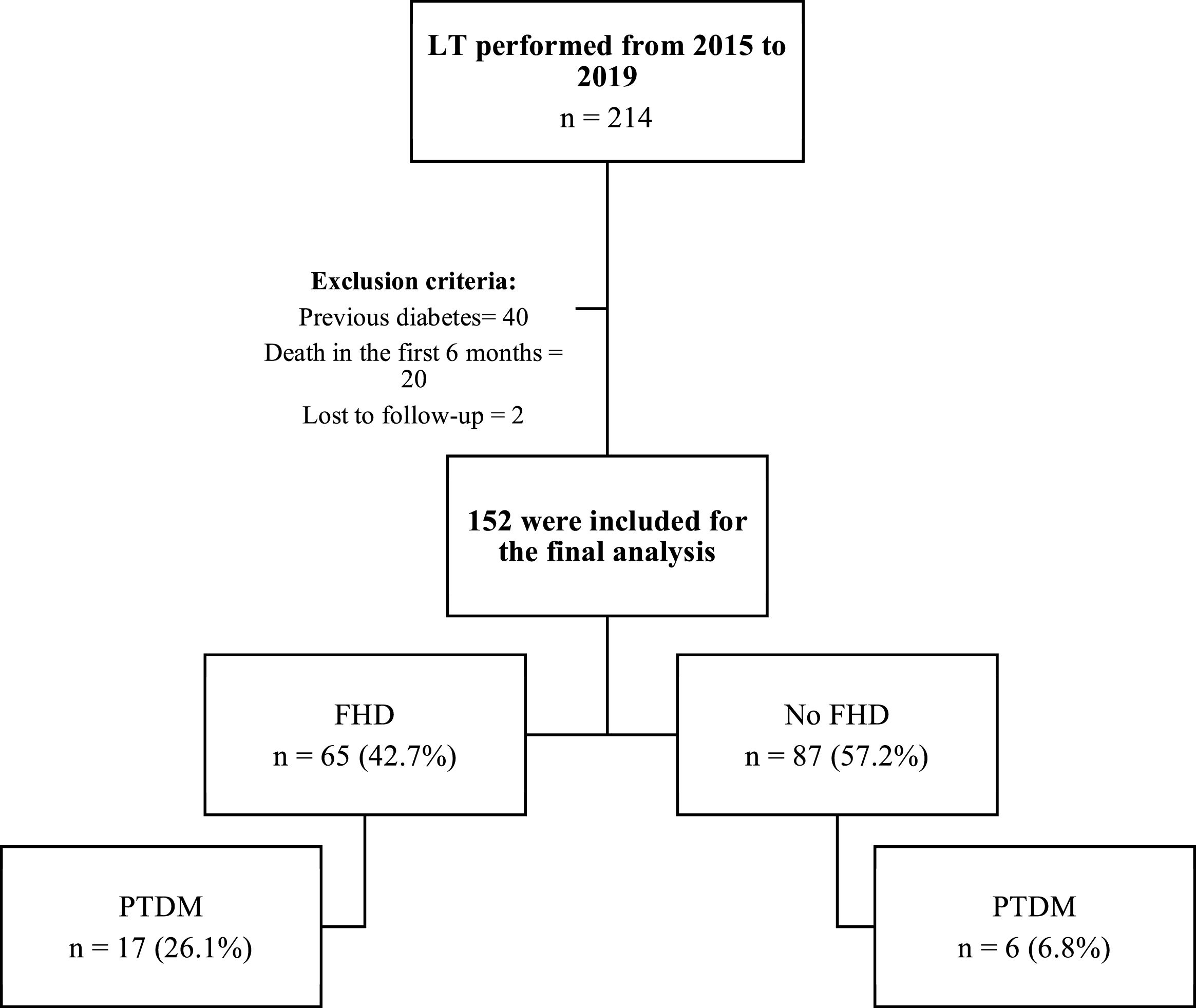
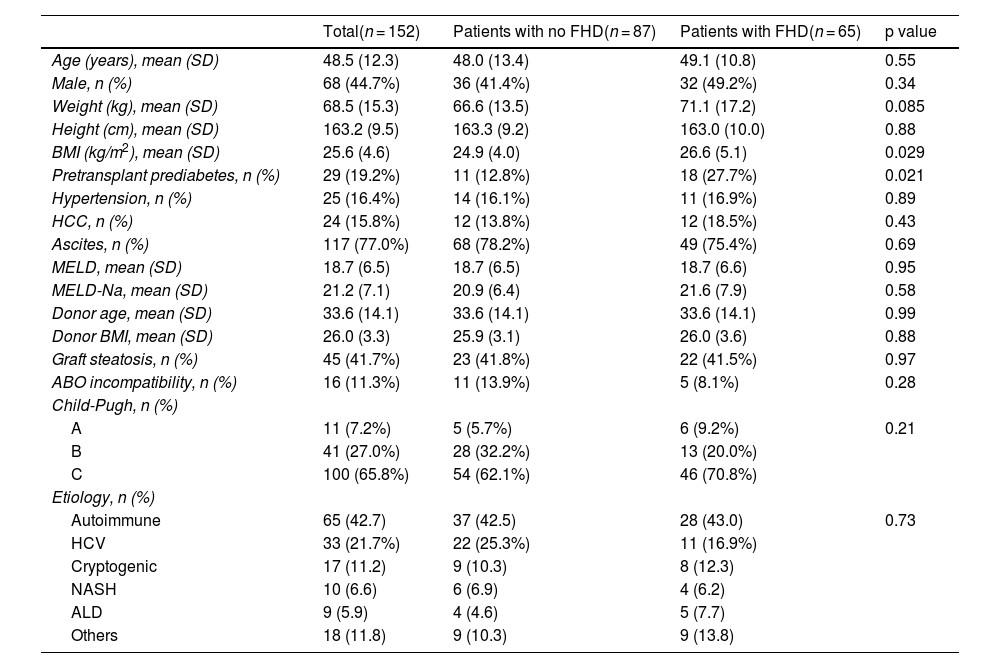
ResultsA total of 214 orthotopic LTs were performed during the established period, of which 152 fulfilled the selection criteria (Fig. 1). Baseline clinical characteristics are shown in Table 1. Patients with FHD had a higher BMI (mean of 26.6 ± 5.1 kg/m2 in patients with FHD vs 24.9 ± 4.0 kg/m2 in those without FHD, p = 0.029) and a higher prevalence of pretransplant prediabetes (27.7% of patients with FHD vs 12.8% of those without FHD, p = 0.021), but the remaining characterististics were similar between the 2 groups. There were no statistically significant differences regarding donor BMI, donor age, the presence of graft steatosis, or ABO incompatibility.
Baseline clinical characteristics.
| Total(n = 152) | Patients with no FHD(n = 87) | Patients with FHD(n = 65) | p value | |
|---|---|---|---|---|
| Age (years), mean (SD) | 48.5 (12.3) | 48.0 (13.4) | 49.1 (10.8) | 0.55 |
| Male, n (%) | 68 (44.7%) | 36 (41.4%) | 32 (49.2%) | 0.34 |
| Weight (kg), mean (SD) | 68.5 (15.3) | 66.6 (13.5) | 71.1 (17.2) | 0.085 |
| Height (cm), mean (SD) | 163.2 (9.5) | 163.3 (9.2) | 163.0 (10.0) | 0.88 |
| BMI (kg/m2), mean (SD) | 25.6 (4.6) | 24.9 (4.0) | 26.6 (5.1) | 0.029 |
| Pretransplant prediabetes, n (%) | 29 (19.2%) | 11 (12.8%) | 18 (27.7%) | 0.021 |
| Hypertension, n (%) | 25 (16.4%) | 14 (16.1%) | 11 (16.9%) | 0.89 |
| HCC, n (%) | 24 (15.8%) | 12 (13.8%) | 12 (18.5%) | 0.43 |
| Ascites, n (%) | 117 (77.0%) | 68 (78.2%) | 49 (75.4%) | 0.69 |
| MELD, mean (SD) | 18.7 (6.5) | 18.7 (6.5) | 18.7 (6.6) | 0.95 |
| MELD-Na, mean (SD) | 21.2 (7.1) | 20.9 (6.4) | 21.6 (7.9) | 0.58 |
| Donor age, mean (SD) | 33.6 (14.1) | 33.6 (14.1) | 33.6 (14.1) | 0.99 |
| Donor BMI, mean (SD) | 26.0 (3.3) | 25.9 (3.1) | 26.0 (3.6) | 0.88 |
| Graft steatosis, n (%) | 45 (41.7%) | 23 (41.8%) | 22 (41.5%) | 0.97 |
| ABO incompatibility, n (%) | 16 (11.3%) | 11 (13.9%) | 5 (8.1%) | 0.28 |
| Child-Pugh, n (%) | ||||
| A | 11 (7.2%) | 5 (5.7%) | 6 (9.2%) | 0.21 |
| B | 41 (27.0%) | 28 (32.2%) | 13 (20.0%) | |
| C | 100 (65.8%) | 54 (62.1%) | 46 (70.8%) | |
| Etiology, n (%) | ||||
| Autoimmune | 65 (42.7) | 37 (42.5) | 28 (43.0) | 0.73 |
| HCV | 33 (21.7%) | 22 (25.3%) | 11 (16.9%) | |
| Cryptogenic | 17 (11.2) | 9 (10.3) | 8 (12.3) | |
| NASH | 10 (6.6) | 6 (6.9) | 4 (6.2) | |
| ALD | 9 (5.9) | 4 (4.6) | 5 (7.7) | |
| Others | 18 (11.8) | 9 (10.3) | 9 (13.8) |
ALD: alcohol-related liver disease; BMI: body mass index; FHD: Family history of diabetes; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; MELD: model for end-stage liver disease; MELD-Na: model for end-stage liver disease-sodium; NASH: non-alcoholic steatohepatitis; SD: standard deviation.
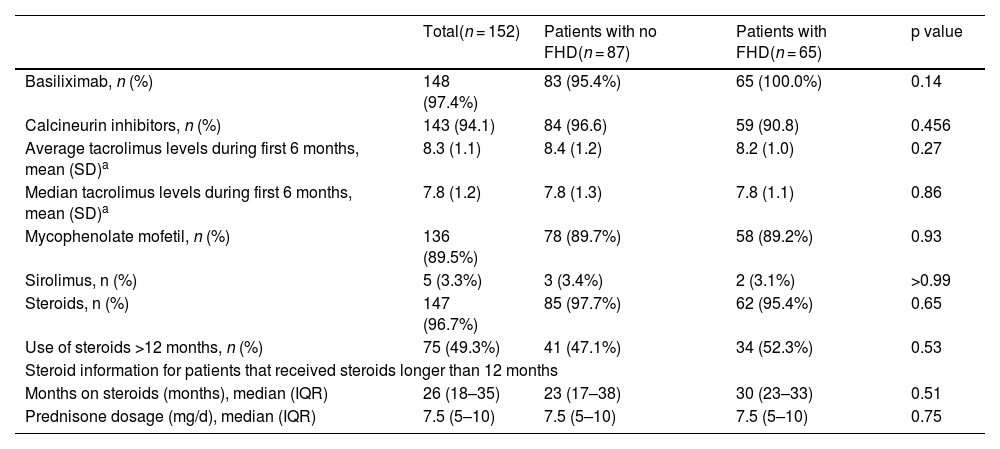
With respect to immunosuppression, the most common scheme was basiliximab induction, along with calcineurin inhibitors, mycophenolate mofetil, and steroids. There were no differences in the immunosuppression schemes, tacrolimus levels, time on steroids, or prednisone dosage between the 2 groups (Table 2).
Immunosuppression schemes.
| Total(n = 152) | Patients with no FHD(n = 87) | Patients with FHD(n = 65) | p value | |
|---|---|---|---|---|
| Basiliximab, n (%) | 148 (97.4%) | 83 (95.4%) | 65 (100.0%) | 0.14 |
| Calcineurin inhibitors, n (%) | 143 (94.1) | 84 (96.6) | 59 (90.8) | 0.456 |
| Average tacrolimus levels during first 6 months, mean (SD)a | 8.3 (1.1) | 8.4 (1.2) | 8.2 (1.0) | 0.27 |
| Median tacrolimus levels during first 6 months, mean (SD)a | 7.8 (1.2) | 7.8 (1.3) | 7.8 (1.1) | 0.86 |
| Mycophenolate mofetil, n (%) | 136 (89.5%) | 78 (89.7%) | 58 (89.2%) | 0.93 |
| Sirolimus, n (%) | 5 (3.3%) | 3 (3.4%) | 2 (3.1%) | >0.99 |
| Steroids, n (%) | 147 (96.7%) | 85 (97.7%) | 62 (95.4%) | 0.65 |
| Use of steroids >12 months, n (%) | 75 (49.3%) | 41 (47.1%) | 34 (52.3%) | 0.53 |
| Steroid information for patients that received steroids longer than 12 months | ||||
| Months on steroids (months), median (IQR) | 26 (18–35) | 23 (17–38) | 30 (23–33) | 0.51 |
| Prednisone dosage (mg/d), median (IQR) | 7.5 (5–10) | 7.5 (5–10) | 7.5 (5–10) | 0.75 |
FHD: Family history of diabetes; IQR: interquartile range; SD: standard deviation.
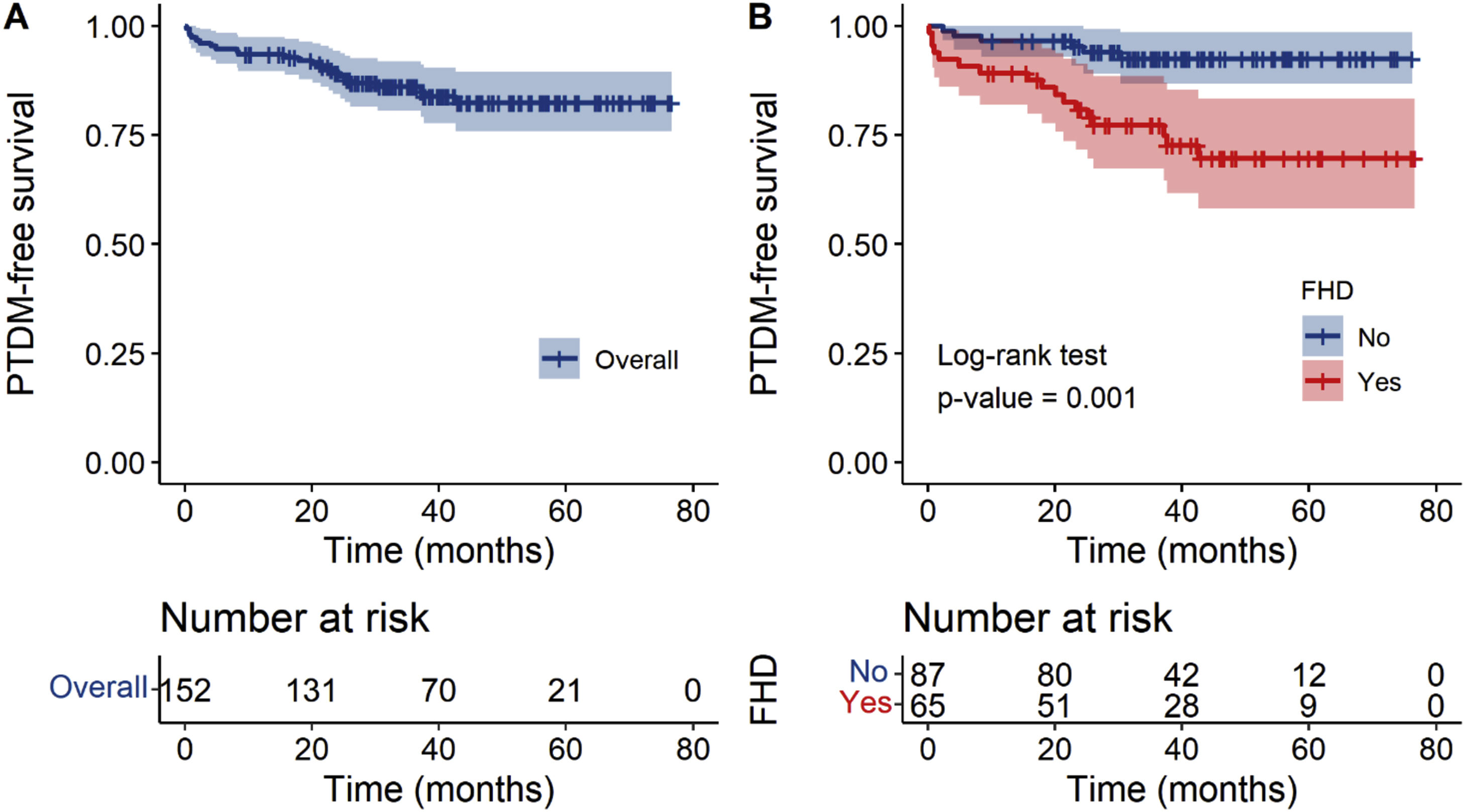
The median follow-up was 41 months (IQR 31–55) and did not differ between patients with and without FHD (42 months vs 40 months, p = 0.6). During that time, 15% of patients developed PTDM (n = 23); 26.1% of those patients had FHD, and 6.8% did not. Fig. 2 shows the overall PTDM-free survival after LT and stratified by FHD status. The overall cumulative incidence of PTDM at 1, 2, 3, and 4 years posttransplant was 6.6, 10.8, 14.0, and 17.6%. The average incidence rate was 4.71 cases/100 person-years. The incidence rates were significantly higher in patients with FHD, compared with those without FHD (10.8% vs 3.4% at 1 year, p = 0.086; 19.2% vs 4.7% at 2 years, p = 0.008; 22.8% vs 7.5% at 3 years, p = 0.013; 30.3% vs 7.4% at 4 years, p = 0.001; and 8.72 cases/100 person-years vs 2.04 cases/100 person-years, p = 0.001, respectively). Regarding the other posttransplant outcomes, graft rejection occurred in 29 (19%) patients, with an incidence rate of 5.58 person-years, and CMV infection in 28 (18%) patients, with an incidence rate of 6.09 person-years.
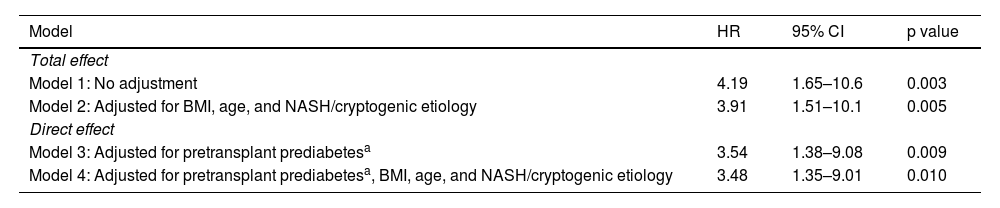
Total and direct effect of family history of diabetes on posttransplantation diabetes mellitusThe unadjusted hazard ratio of PTDM for FHD (exposed vs unexposed) was 4.19 (95% CI 1.65–10.6, p = 0.003), and 4.14 (95% CI 1.60–10.7, p = 0.005), when controlled for age, BMI, and liver disease etiology. In the mediation analysis, the pretransplant prediabetes-adjusted hazard ratio of PTDM for FHD (exposed vs unexposed) was 3.54 (95% CI 1.38–9.08, p = 0.009), and 3.48 (95% CI 1.35–9.01, p = 0.010) when further controlled for age, BMI, and liver disease etiology. The FHD-adjusted hazard ratio of PTDM for pretransplant prediabetes vs no prediabetes before transplantation was 3.19 (95% CI 1.38–7.35, p = 0.007) and 2.57 (95% CI 1.05–6.27, p = 0.038), when further controlled for age, BMI, and liver disease etiology. The unadjusted and adjusted hazard ratio estimates for the exposure of FHD are shown in Table 3. In both the unadjusted and adjusted models, the direct effect of FHD on developing PTDM was 84% of the total effect, which supports the hypothesis that pretransplant prediabetes is only a partial mediator between FHD and PTDM.
Total and direct effect of family history of diabetes in posttransplantation diabetes mellitus.
| Model | HR | 95% CI | p value |
|---|---|---|---|
| Total effect | |||
| Model 1: No adjustment | 4.19 | 1.65–10.6 | 0.003 |
| Model 2: Adjusted for BMI, age, and NASH/cryptogenic etiology | 3.91 | 1.51–10.1 | 0.005 |
| Direct effect | |||
| Model 3: Adjusted for pretransplant prediabetesa | 3.54 | 1.38–9.08 | 0.009 |
| Model 4: Adjusted for pretransplant prediabetesa, BMI, age, and NASH/cryptogenic etiology | 3.48 | 1.35–9.01 | 0.010 |
BMI: body mass index; CI: confidence interval; FHD: family history of diabetes; HR: hazard ratio; NASH: nonalcoholic steatohepatitis; PTDM: posttransplantation diabetes mellitus.
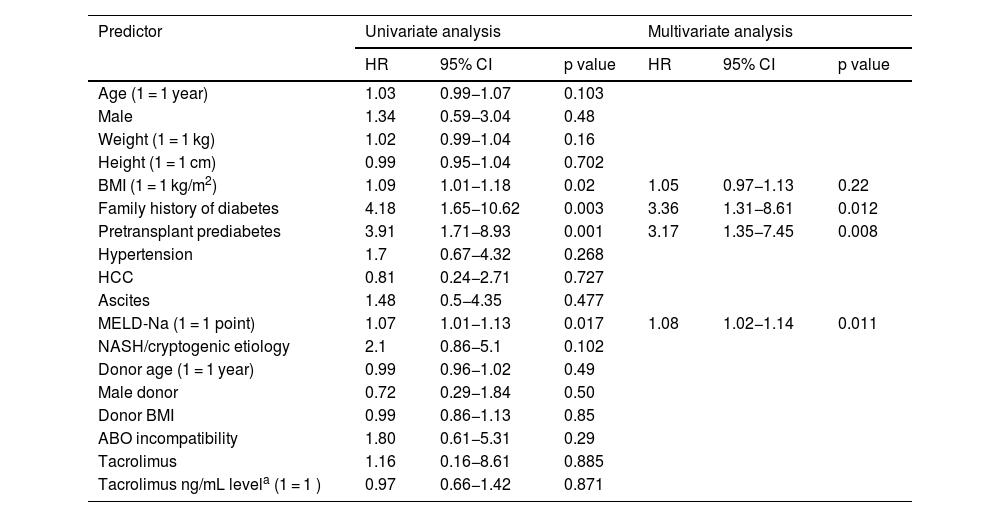
Table 4 summarizes the exploratory analysis of predictors for developing PTDM. In the univariate analyses, higher body mass index values, FHD, pretransplant prediabetes, higher MELD scores, and higher MELD-Na scores were associated with a higher risk of PTDM. The MELD score was not included in the multivariate analysis because it is highly correlated with the MELD-Na score. In the multivariate analysis, FHD, pretransplant prediabetes, and the MELD-Na score remained associated with PTDM. The predictor with the highest hazard ratio for PTDM was FHD. When controlled for the other covariates in the multivariate model, the hazard of developing PTDM in subjects that had FHD was 3.36 times higher (95% CI 1.31−8.61, p < 0.012) than the corresponding hazard in those without FHD.
Potential predictors for posttransplantation diabetes mellitus.
| Predictor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age (1 = 1 year) | 1.03 | 0.99−1.07 | 0.103 | |||
| Male | 1.34 | 0.59−3.04 | 0.48 | |||
| Weight (1 = 1 kg) | 1.02 | 0.99−1.04 | 0.16 | |||
| Height (1 = 1 cm) | 0.99 | 0.95−1.04 | 0.702 | |||
| BMI (1 = 1 kg/m2) | 1.09 | 1.01−1.18 | 0.02 | 1.05 | 0.97−1.13 | 0.22 |
| Family history of diabetes | 4.18 | 1.65−10.62 | 0.003 | 3.36 | 1.31−8.61 | 0.012 |
| Pretransplant prediabetes | 3.91 | 1.71−8.93 | 0.001 | 3.17 | 1.35−7.45 | 0.008 |
| Hypertension | 1.7 | 0.67−4.32 | 0.268 | |||
| HCC | 0.81 | 0.24−2.71 | 0.727 | |||
| Ascites | 1.48 | 0.5−4.35 | 0.477 | |||
| MELD-Na (1 = 1 point) | 1.07 | 1.01−1.13 | 0.017 | 1.08 | 1.02−1.14 | 0.011 |
| NASH/cryptogenic etiology | 2.1 | 0.86−5.1 | 0.102 | |||
| Donor age (1 = 1 year) | 0.99 | 0.96−1.02 | 0.49 | |||
| Male donor | 0.72 | 0.29−1.84 | 0.50 | |||
| Donor BMI | 0.99 | 0.86−1.13 | 0.85 | |||
| ABO incompatibility | 1.80 | 0.61−5.31 | 0.29 | |||
| Tacrolimus | 1.16 | 0.16−8.61 | 0.885 | |||
| Tacrolimus ng/mL levela (1 = 1 ) | 0.97 | 0.66−1.42 | 0.871 | |||
BMI: body mass index; HCC: hepatocellular carcinoma; MELD-Na: model for end-stage liver disease-sodium; NASH: non-alcoholic steatohepatitis.
To the best of our knowledge, this is the first study of PTDM in the Mexican population. During a median follow-up time of 41 months, we found that 15% of our patients developed PTDM, with an incidence rate of 4.71 cases/100 person-years and a cumulative incidence at 1, 2, 3, and 4 years posttransplant of 6.6, 10.8, 14.0, and 17.6%, respectively. Using the same diagnostic criteria, other studies have reported higher percentages of PTDM (18%–31%).7,19 Unfortunately, those analyses did not report the incidence rate or the cumulative incidence at a fixed time after transplantation. The incidence rate or cumulative incidence at a fixed time are better measures for comparing PTDM development across different studies because their follow-up times are usually different.
In our study, we expected a higher incidence of diabetes because of the high burden of diabetes in Mexican-mestizo patients. Previous reports indicate that Hispanics have an increased risk of PTDM,13,20 but a higher incidence was not found in our cohort. One explanation for our results could be the 6-month interval, during which we did not record cases of transitory hyperglycemia as PTDM. A 6-month time period of sustained hyperglycemia after LT appears to be adequate for making the diagnosis.21 Studies with a short follow-up time report a higher proportion of PTDM because they include transitory hyperglycemia cases, whereas longer follow-up periods yield lower estimates.3 We also chose that approach because sustained PTDM is associated with negative graft and patient survival and because up to 57% of nondiabetic patients present with transitory hyperglycemia in the first months post-LT.22 In a retrospective study from the OPTN/ UNOS liver transplant database that included 20,172 patients, PTDM occurred in 26.4% of recipients, with a median follow-up time of 22.8 months. However, the ADA definition was not used, the cohort was made up of older patients, and the majority of cases (82.1%) were diagnosed within one year, possibly including transitory hyperglycemia.23
Regarding the immunosuppression scheme used, a lower risk of PTDM has been reported in patients treated with basiliximab induction, antimetabolite therapy, and early steroid withdrawal.19 This approach is used in our center, but due to a high prevalence of autoimmune liver disease (42%) in our cohort, early steroid withdrawal was not possible in a considerable number of patients. That situation could be explained by the fact that our institution is a referral center for autoimmune disease.
Our study provides evidence that FHD is a risk factor for PTDM. When controlled for potential confounders, we found that the risk of developing PTDM was 4.14 times higher in patients with FHD (a first degree relative), compared with those without FDH, suggesting a genetic predisposition. Moreover, 84% of said association was preserved after additionally adjusting for pretransplant prediabetes. As noted above, our mediation analysis confirmed that pretransplant prediabetes is a partial mediator for the relationship between FHD and PTDM. Theoretically, prediabetes should be a full mediator, but because prediabetes was assessed in the pretransplant period, the pretransplant prediabetes variable was no longer a full mediator; therefore, some patients might transition to prediabetes after transplantation and eventually to diabetes.
Regarding our exploratory analysis of other factors associated with PTDM, BMI was statistically significant in the univariate analysis, but not in the multivariate analysis. Evidence on BMI is inconclusive; some studies have found a positive association between BMI and the risk of PTDM,11,24 whereas others have not.21,25 The association found in some studies may have been confounded by covariates that were not considered in the analysis (confounders). In addition to FHD, in our exploratory analysis, prediabetes and higher MELD-Na scores were also associated with developing PTDM. Although pretransplant prediabetes was associated with PTDM, it did not eliminate the effect of FHD on developing PTDM, and an exclusive focus on pretransplant prediabetes could be insufficient.26 Lastly, although older donors, ABO incompatibility, and graft steatosis have previously been associated with a higher risk of PTDM,9,27 we found no differences in the donor characteristics between the 2 groups, nor were they associated with developing PTDM.
In relation to posttransplant outcomes, graft rejection and CMV infection have been associated with an increased risk of PTDM.28,29 In a post-hoc analysis of our data, using an adjusted Cox regression model with CMV infection as a time-varying predictor and controlling for age, BMI, pretransplant prediabetes, MELD-Na, and cirrhosis etiology, we found that graft CMV infection was associated with an increased risk of developing PTDM (aHR of 6.40, 95% CI 2.07–19.8, p = 0.001). Regarding genetics, some studies have reported a 2-to-6-fold increase in the risk of type 2 diabetes in patients with FHD.30,31 The association between single nucleotide polymorphisms and the risk of PTDM has been documented in multiple studies.32 However, no genetic profile was carried out on patients with PTDM and a positive FHD in our cohort.
The strengths of the present study include a long follow-up time, a considerable sample size, a recent cohort of LT recipients, the use of the ADA criteria to define PTDM, and a standardized immunosuppression protocol that is up to date with current guidelines. Another strength is our focus on evaluating FHD as a risk factor for PTDM. Despite previous studies stating that family history represents a risk for PTDM,7,33 they are exploratory and examine diverse risk factors. By a priori choosing family history as our exposure of interest and adjusting for potential confounders, we performed a confirmatory analysis, rather than merely an exploratory one (subject to random high bias). Our analysis also included a mediation analysis to quantify the direct effect of FHD on PTDM, by eliminating the effect through prediabetes. The main limitations of our study include its retrospective and single-center design, the lack of HbA1c levels close to the transplantation time (to better quantify the prediabetes level), and the lack of a genetic profile of our patients.
ConclusionIn the Mexican population, a positive FHD might increase the risk of developing PTDM. Although there is a high national prevalence of diabetes, the incidence of PTDM was similar to that found in previous international reports. LT patients with a positive FHD should undergo a stricter metabolic program, including lifestyle modifications and early steroid withdrawal, to prevent PTDM.
Financial disclosureNo specific grant from any funding agencies in the public, commercial, or not-for-profit sectors were received regarding this research.
We wish to thank and acknowledge all the members of the Hepatology and Liver Transplant Unit staff for the patient care and monitoring throughout these years.