Patients with intestinal failure are unable to maintain adequate nutrition and hydration due to a reduction in the functional area of the intestine. Different strategies have the potential to benefit these patients by promoting intestinal autonomy, enhancing quality of life, and increasing survival.
AimsTo describe the clinical characteristics of children with intestinal failure and disease progression in terms of intestinal autonomy and survival.
Materials and methodsA retrospective study was conducted, evaluating 33 pediatric patients with intestinal failure that were hospitalized within the time frame of December 2005 and December 2013 at a tertiary care referral center. Patient characteristics were described upon hospital admission, estimating the probability of achieving intestinal autonomy and calculating the survival rate.
ResultsPatient median age upon hospital admission was 2 months (interquartile range [IQR]: 1-4 months) and 54.5% of the patients were boys. Intestinal autonomy was achieved in 69.7% of the cases with a median time of 148 days (IQR: 63 - 431 days), which decreased to 63 days in patients with a spared ileocecal valve. Survival was 91% during a median follow-up of 281 days (IQR: 161 - 772 days).
ConclusionsMedical management of patients with intestinal failure is complex. Nutritional support and continuous monitoring are of the utmost importance and long-term morbidity and mortality depends on the early recognition and management of the associated complications.
Los pacientes con falla intestinal son incapaces de mantener una adecuada nutrición e hidratación debido a una reducción en el área intestinal funcional. La implementación de diferentes estrategias tiene el potencial de beneficiar a estos pacientes en términos de favorecer la autonomía intestinal, aumentar la calidad de vida y elevar la supervivencia.
ObjetivosDescribir las características clínicas de niños con falla intestinal, así como la evolución en términos de autonomía intestinal y supervivencia.
Materiales y métodosEstudio retrospectivo en el que se evaluó a 33 pacientes pediátricos con falla intestinal, hospitalizados entre diciembre del 2005 y diciembre del 2013 en una institución de alta especialidad. Se describieron las características de los pacientes al ingreso hospitalario, se estimó la probabilidad de lograr la autonomía intestinal y se calculó la tasa de supervivencia.
ResultadosLa mediana de edad al momento de ingresar al hospital fue 2 meses (rango intercuartílico [RIC]: 1-4 meses) y el 54.5% de los pacientes fueron de sexo masculino. El 69.7% de los casos lograron la autonomía intestinal con una mediana de tiempo de 148 días (RIC: 63-431 días), la cual disminuyó a 63 días cuando los pacientes tenían presencia de válvula ileocecal. La supervivencia fue del 91% durante una mediana se seguimiento de 281días (RIC: 161-772 días).
ConclusionesEl manejo médico de los pacientes con falla intestinal es complejo. El soporte nutricional y la monitorización continua son de vital importancia y la morbimortalidad a largo plazo depende del reconocimiento y manejo precoz de las complicaciones asociadas.
Intestinal failure in pediatrics can be defined as the reduction of the intestinal mass below the minimum necessities for maintaining adequate digestion and absorption of nutrients and fluids necessary for growth.1,2
A number of diseases are recognized as being responsible for this entity, and the most common is short bowel syndrome (SBS). Others are structural abnormalities of the enterocyte, inflammatory alterations, severe food allergies, autoimmune enteropathies, and severe bowel transit disorders.3
Adequate nutrient absorption and growth involve a process of intestinal adaptation that will depend on the extension, functionality, and motility of the intestinal remnant. The clinical course of these patients for achieving intestinal adaptation is long and complex. The maximum intestinal adaptation time estimated in adults is 2 years, but in children it can last longer than 3 years.4,5The treatment of these children is based on the provision of adequate nutrition: total parenteral nutrition (TPN) plus enteral nutrition (EN) when possible, thus promoting intestinal adaptation. It also requires the prevention and/or treatment of the complications derived from the underlying disease or secondary to prolonged TPN use.6
The multidisciplinary programs focused on the integrated treatment of these patients has dramatically changed the survival of these children over the last decades.7–9 The care of these patients at our hospital is guided by a group of professionals under a protocol that has the possibility of individualizing each case and it continues to evolve as new evidence is brought to light.
The aim of this study was to describe the clinical characteristics, such as progression in terms of intestinal autonomy and survival, in a group of pediatric patients with intestinal failure treated at a tertiary care referral center.
Population and methodsA retrospective study was carried out that included 33 pediatric patients hospitalized within the time frame of December 2005 and December 2013 at the Hospital Pablo Tobón Uribe, an advanced specialty institution and center of intestinal rehabilitation and transplantation, located in the city of Medellín, Colombia.
The study included patients under 18 years of age presenting with intestinal failure and undergoing TPN, evaluated by the pediatric nutritional support group of the hospital. There were no exclusions.
The probability of patients achieving intestinal autonomy was estimated. Intestinal autonomy was defined as the capacity to develop structural and physiologic changes following intestinal resection and that is seen upon the suspension of parenteral nutrition, 100% tolerance of nutritional requirements through enteral feeding, and maintenance of the expected level of growth.10
All the information was gathered from the medical histories of the patients and so informed consent from the families was not required. Before the collection of data, a pilot test was conducted on a simple random sample of 10% of the initial population in order to test and adjust the process and the data-gathering instrument.
Statistical analysisThe qualitative variables were expressed through frequencies and percentages and the quantitative variables through median and interquartile range (IQR). The latter variables showed abnormal distribution through the Shapiro-Wilk test.
The probability of achieving intestinal autonomy during treatment time was estimated using the Kaplan-Meier method and comparisons were made with the log-rank test. The event was to achieve intestinal autonomy and the censured cases were the deaths, transplantations, or patients for whom there was no follow-up.
In all cases, statistical significance was set at a p<0.05 and the Windows SPSS version 19.0 software program was used.
ResultsThe study included 33 children, and 54.5% were boys. There was a predominance of boys under the age of 2 years, representing 75.8% of the study population. A total of 18.2% of the patients arrived at the hospital in the neonatal period and 6% were older than 2 years of age. The median age upon hospital admission was 2 months (IQR: 1-4 months). The median hospital and outpatient follow-up of these patients was 281 days (IQR: 161 - 772 days).
The majority of patients underwent intestinal resection the first month of life (84.8%), and the 5 primary causes were: intestinal atresia (30.3%), necrotizing enterocolitis (NEC) (24.2%), intestinal atresia plus gastroschisis (12.1%), intestinal invagination (12.1%), and volvulus or malrotation (9.1%).
Due to the fact that some of the patients were referred from other hospitals, the classification of the extent of resection was available in only 26 patients; 73.1% of them presented with large intestinal resection (residual intestine between 40 and 100cm), 19.2% with massive resection (residual intestine residual under 40cm), and 7.7% with ultra-short bowel syndrome (residual intestine under 15cm). The colon was spared in all 33 patients and the ileocecal valve was spared in 8 patients (Table 1).
Initial characteristics of the study population.
| Population total | |
|---|---|
| n=33 | |
| Age in months upon hospital arrival, median (IQR) | 2 (1 - 4) |
| Sex, n (%) | |
| Female | 15 (45.5) |
| Male | 18 (54.5) |
| Age at the time of resection, n (%) | |
| First month of life | 28 (84.8) |
| Between the first month and one year of life | 3 (9.1) |
| Between one and 5 years of life | 1 (3.0) |
| After 10 years of life | 1 (3.0) |
| Extent of intestinal resection, n (%) | |
| Large (residual intestine between 40 and 100 cm) | 19 (57.6) |
| Massive (residual intestine under 40 cm) | 5 (15.2) |
| Ultra-short bowel syndrome (residual intestine under 15 cm) | 2 (6.1) |
| No datum | 7 (21.2) |
| Cause of resection, n (%) | |
| Intestinal atresia | 10 (30.3) |
| Necrotizing enterocolitis | 8 (24.2) |
| Intestinal invagination | 4 (12.1) |
| Intestinal atresia and gastroschisis/Hirschsprung's disease | 4 (12.1) |
| Volvulus and malrotation | 3 (9.1) |
| Motility disorder | 2 (6.1) |
| Hirschsprung's disease | 1 (3.0) |
| Gastroschisis | 1 (3.0) |
| Residual intestine in cm, median (IQR) | 60 (35 - 95) |
| Presence of ileocecal valve | 8 (24.2) |
| Presence of colon | 33 (100) |
The predominant type of support during treatment in the hospital was mixed (TPN and EN) in 75.8% of the cases, followed by TPN plus oral nutrition, exclusive TPN (6.1%), exclusive EN (3.0%), and exclusive oral nutrition (3.0%). It should be mentioned that the cases that received exclusive EN or oral nutrition were referred from other hospitals in which they had received prolonged TPN.
Enteral nutrition or oral nutritionThirty-one hospitalized patients received food through the gastrointestinal tract in the form of enteral nutrition or oral nutrition. In 61.3% of the patients this manner of feeding was begun through a catheter with continuous infusion and the rest of the patients had oral nutrition. The initial hospital formula used was based on free amino acids in 67.7% of the patients, on an extensively hydrolyzed formula (EHF) in 19.3%, and on a polymeric formula in 12.9%. A total of 48.4% of the patients did not tolerate the first formula provided by the hospital, but this did not seem to be related to the type of administration (intolerance to continuous infusion: 47.4%, oral nutrition: 50%).
Parenteral nutritionTPN was administered to 31 of the 33 patients during their hospital stay. Although the other 2 patients had received TPN at some point during their illness, it was suspended before their admission to our hospital. Median TPN duration at our institution was 68 days (IQR: 21-138 days). The TPN cycling period was 2 to 12h in accordance with the conditions of the patient and was carried out in 8 cases.
Three patients had outpatient parenteral nutrition.
Intestinal failure progressionDuring the median follow-up of 281 days (IQR: 161-772 days), 69.7% of the patients achieved intestinal autonomy, 15.1% were released from the hospital without having achieved autonomy, and 9.1% died during the process due to septic shock (2 cases) and bowel obstruction with deteriorating condition (one case). During the treatment, the multidisciplinary team also determined that 2 patients (6.1%) should undergo bowel transplantation (Table 2).
Progression according to the extent of intestinal resection.
| Variable/extent of resection | Large | Massive | Ultra-short bowel syndrome | Undetermined |
|---|---|---|---|---|
| Number of patients | 19 | 5 | 2 | 7 |
| Days with TPNa, median (IQR) | 60 (18-83) | 364 (239-452) | 198 (134-263) | 42 (8-68) |
| Intestinal adaptation, n (%) | 15 (78.9) | 1 (20) | 0 (0) | 7 (100) |
| Days to achieve intestinal autonomy, median (IQR) | 148 (81-314) | 431 | -- | 41(32-80) |
| Survival | 17 (89.5) | 5 (100) | 1(50) | 7(100) |
| Transplantationb | 0 | 2 (40) | 0 | 0 |
IQR: interquartile range; TPN: total parenteral nutrition
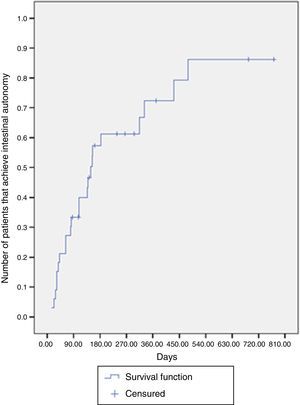
The median time for achieving intestinal autonomy was 148 days (IQR: 63-431 days). Six months after treatment, the probability of reaching autonomy was 61.2% and at one year after treatment it was close to 72.3% (Fig. 1).
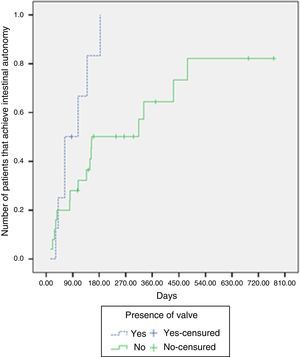
Intestinal adaptation was achieved in 87.5% of the patients with an intact ileocecal valve, whereas it was achieved in 64% without an ileocecal valve. The median time required for intestinal adaptation in patients with an ileocecal valve was 63 days (IQR: 41-139 days), compared with 154 days (IQR: 81-479 days) in the patients without one. There were significant differences upon comparing the likelihood of adaptation according to the presence or absence of an ileocecal valve (log-rank test, p=0.029) (Fig. 2).
The probability of adaptation according to the initial type of EN administration (continuous infusion vs oral nutrition) was compared and showed no significant differences (log-rank test, p=0.68).
DiscussionThe management of patients with intestinal failure is complex, requiring highly qualified personnel from different specialties that form part of an intestinal rehabilitation program, in order to achieve the primary aim of enteral autonomy. The advances of the last few years in the diagnosis and treatment of intestinal failure have improved the survival rate of children with this pathology. Optimum management of these patients includes both intestinal rehabilitation and enhanced quality of life.11
Even though the results of our study show that the main causes of intestinal failure in our population concur with studies conducted in Europe and the United States,1214 it was surprising that the first 2 causes in a French study were gastroschisis and volvulus,13 and NEC and gastroschisis in one from the United States,12,14 whereas in our population it was intestinal atresia and NEC. This could be related to regional differences in regard to genetic susceptibility, the possibility of early access to hospital centers with neonatal intensive care units, and differences in the South American health systems from those of Europe and the United States.
The work protocol of the Hospital Pablo Tobón Uribe includes adaptation stimulation through EN, beginning with continuous infusion and progressing to boluses and oral nutrition. One of the topics that is still a subject of debate among clinicians in regard to nutritional support in children with intestinal failure is the manner of nutrition administration (continuous or with boluses) and the composition of the formula (polymeric, elemental, or semi-elemental).11,15 Some authors have demonstrated that continuous infusion administration could increase the absorption percentage of lipids, proteins, and energy, compared with bolus administration, by maximizing transport protein saturation.15,16 In our study, EN through continuous infusion was initially used in a little over half of the patients (61.3%), and oral nutrition was begun in the other 38.7% due to the fact that the patients were admitted with this feeding regimen and they had no history of intolerance. No differences were found upon comparing the probability of intestinal autonomy and the type of initial administration.
With respect to the ideal formula for beginning enteral feeding, better tolerance with the use of free amino acids has been described in animal models with SBS17 and case reports in humans.18 However, other studies showed no differences in the absorption of proteins, permeability, and intestinal adaptation with the use of an EHF or a whole-protein formula.15,19 In our case series, formulas based on free amino acids were used in 67.7% of the patients, whether indicated by the gastroenterologist or because of prior intolerance to EHF and/or maternal breast milk.
In relation to TPN use, the median duration at our hospital was 68 days (IQR: 21-138 days). Both the United States and European literature reported a median duration of 229 days (IQR: 129-408 days) in a group of patients from Boston7 and 292 days (IQR: 146-438 days) in a Finnish study.20 It is important to point out that in our study we were not able to determine the number of days of TPN prior to admission to our hospital and therefore the TPN time of those patients could actually be longer than that reported.
Taking into account that prolonged hospital stay can affect psychomotor development and quality of life, outpatient TPN should be considered as soon as possible in children with severe intestinal failure.13 Of the total of patients that received TPN, 9.7% had outpatient management, a lower figure than that reported in the French study in which that strategy was used in 40.7% of the patients.13 This underlines the local need for improving health programs for at-home care and parenteral nutrition.
Enteral autonomy continues to be the fundamental aim of intestinal rehabilitation programs. According to a 2013 systematic review, the percentage of patients that achieved autonomy varied from 42 to 84% through different rehabilitation programs, during a follow-up period of 12 to 50 months.9 Our study showed that 69.7% of the patients achieved intestinal autonomy, a result concurring with that reported in the systematic review and in more recent studies, such as one conducted in 2015 by Demehri et al. that showed intestinal autonomy in 64.3% of the patients.14
The median time necessary to achieve intestinal autonomy was 148 days. Some studies show a median of 7621 and 117 days22, indicating the variability involved and the dependence on anatomic factors, intestinal remnant function, and complications that can arise during progression.23 For example, in our study, the presence of the ileocecal valve was shown to be a factor that contributed to intestinal autonomy achievement (log-rank test, p=0.029). A Canadian study reported that the presence of the ileocecal valve was a significant predictor for adaptation time in the univariate analysis and despite the fact that it was not included in the final multivariate model, it was shown to be an important predictor for the intestinal adaptation result in various models.14,24,25
The survival rate was 91% in the 33 patients that had a median 9-month follow-up period. This result was also in accordance with other small cohort studies that demonstrated survival rates of 89% (48/54)7 and even 100% (31/31), when standardized care protocols were applied.22 Other studies with longer follow-up periods reported survival rates of 85%14 and up to 71% in a cohort of 114 patients with a median 7-year follow-up.13 A systematic review published in 2013 stated that the mortality rate varied from 6.4 to 37.5% among different multidisciplinary programs and the wide mortality range could be partially explained by differences in the baseline characteristics of the patients. For example, the programs that reported high mortality rates tended to be conducted at tertiary care referral centers that covered large geographic areas, suggesting the possibility of bias in the results.9 The systematic review also pointed out that the cause of death was the same in all the programs9 and the first cause was sepsis, as was the case in regard to our patients.
Although follow-up after intestinal transplantation was not an aim of the present study, it should be mentioned that the 2 patients that underwent transplantation died 42 and 240 days after the procedure. Those deaths were secondary to cardiorespiratory infarct during control endoscopy. The death of a third patient was due to sepsis caused by multiresistant Klebsiella pneumoniae.
Our study has the limitations characteristic of retrospective analyses that do not allow the desired control of the nature and quality of the measurements. Information on the days of TPN and management carried out before admission to our hospital was impossible to collect from secondary sources.
In conclusion, the management of patients with intestinal failure is complex and should be performed by an multidisciplinary team, as demonstrated in the literature, given that it improves survival and quality of life in these patients.7–9,22,23 In our study, 69.7% of the patients with intestinal failure treated by the pediatric nutritional support group achieved intestinal adaptation and there was a survival rate of 91%, figures that are comparable with other intestinal rehabilitation programs. Treatment of this pathology is a challenge for the different specialists involved that must always direct their work towards the achievement of intestinal rehabilitation and enteral autonomy. According to our results, opportune referral to a specialized center is highly recommended and has a positive influence on attaining adequate intestinal autonomy in the majority of patients.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for this study.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Contreras-Ramírez MM, Giraldo-Villa A, Henao-Roldan C, Martínez-Volkmar MI, Valencia-Quintero AF, Montoya-Delgado DC, et al. Evolución en niños con falla intestinal en un hospital de referencia en Medellín, Colombia. Revista de Gastroenterología de México. 2016;81:21–27.