Surgery is the main treatment for gastric cancer. D2 radical gastrectomy is associated with a variable postoperative morbidity and mortality rate worldwide. The aim of the present study was to identify the risk factors associated with the postoperative morbidity and mortality of D2 radical gastrectomy, with curative intent, for gastric cancer.
Materials and methodsA retrospective case series was conducted, in which the medical records were reviewed of patients with gastric cancer that underwent D2 radical gastrectomy, within the time frame of January 2014 and December 2018. Univariate and multivariate analyses were carried out to identify the risk factors related to postoperative morbidity and mortality within 90 days.
ResultsThe percentages of postoperative morbidity and mortality in 691 patients were 23.3% and 3.3%, respectively. In the multivariate analysis, age ≥70 years (OR = 1.85, 95% CI: 1.25−2.76), ASA III-IV (OR = 2.06, 95% CI: 1.28−3.34), total gastrectomy (OR = 1.96, 95% CI:1.19−3.23), and pancreatosplenectomy (OR = 5.41, 95% CI: 1.42−20.61) were associated with greater postoperative morbidity, and age ≥70 years (OR = 4.92, 95% CI:1.78−13.65), lower BMI (OR = 0.81, 95% CI: 0.71−0.92), and hypoalbuminemia (OR = 0.91, 95% CI: 0.85−0.98) were associated with greater mortality in distal and total D2 radical gastrectomy.
ConclusionsD2 radical gastrectomy for gastric cancer was shown to be a safe treatment, with low postoperative morbidity and mortality rates. Age ≥70 years, ASA III-IV, total gastrectomy, and pancreatosplenectomy were factors associated with a higher complication rate. Age ≥70 years, lower BMI, and hypoalbuminemia were mortality predictors in distal and total radical gastrectomy.
La cirugía es el tratamiento principal para el cáncer gástrico. La gastrectomía radical D2 está asociada a morbilidad y mortalidad postoperatoria variable a nivel mundial. El objetivo del estudio fue identificar los factores de riesgo asociados con morbilidad y mortalidad postoperatoria de la gastrectomía radical D2 con intención curativa por cáncer gástrico.
Material y métodosEn este estudio de serie de casos retrospectivo, se revisó las historias clínicas de los pacientes con cáncer gástrico operados de gastrectomía radical D2 desde enero del 2014 a diciembre del 2018. Se realizó un análisis univariado y multivariado para identificar los factores de riesgo relacionados con la morbilidad y mortalidad postoperatoria a los 90 días.
ResultadosEn 691 pacientes, el porcentaje de morbilidad y mortalidad postoperatoria fue del 23.3% y 3.3%, respectivamente. En el análisis multivariado, la edad ≥70 años (OR = 1.85, IC 95%:1.25-2.76), ASA III-IV (OR = 2.06, IC 95%:1.28-3.34), la realización de gastrectomía total (OR = 1.96, IC 95%:1.19-3.23) y pancreatosplenectomía (OR = 5.41, IC 95%:1.42-20.61) se asociaron con mayor morbilidad postoperatoria. Por otro lado, la edad ≥70 años (OR = 4.92, IC 95%:1.78-13.65), un menor IMC (OR = 0.81, IC 95%:0.71-0.92) e hipoalbuminemia (OR = 0.91, IC 95%:0.85-0.98) se asociaron a una mayor mortalidad en la gastrectomía radical D2 distal y total.
ConclusionesLa gastrectomía radical D2 por cáncer gástrico es un tratamiento seguro con una baja morbilidad y mortalidad postoperatoria. La edad ≥70 años, ASA III-IV, la gastrectomía total y la realización de pancreatosplenectomía fueron factores asociados con una mayor tasa de complicaciones. La edad ≥70 años, un menor IMC e hipoalbuminemia son predictores de mortalidad en la gastrectomía radical distal y total.
Gastric cancer is the fifth most common cancer and the third most frequent cause of cancer mortality worldwide.1 In Peru, where most patients are diagnosed with locally advanced disease, gastric cancer is the main cause of cancer mortality.2 Radical gastrectomy with D2 lymphadenectomy, the mainstay treatment for gastric cancer, is a complex surgical procedure requiring a multidisciplinary team for intraoperative and postoperative management.3,4 D2 radical gastrectomy was introduced and systematically adapted by surgeons at our medical center, who were trained at the National Cancer Center Japan in the 1990s, and said technical procedure has been used as the gold standard treatment for gastric cancer ever since. Several epidemiologic studies have reported variable postoperative morbidity and mortality rates in relation to gastric cancer across the globe.5–8 D2 radical gastrectomy is most frequently associated with nonsurgical postoperative complications,9,10 but it can also involve surgical complications, the most common of which is anastomotic leak.7 Age, patient comorbidities, hemoglobin levels, albumin levels, type of gastrectomy, and multivisceral resection have been identified as risk factors for postoperative morbidity and mortality after gastrectomy.11–13 The aim of the present study was to identify the risk factors associated with postoperative morbidity and mortality in patients with gastric cancer that underwent distal or total D2 radical gastrectomy with curative intent at the Instituto Nacional de Enfermedades Neoplásicas (INEN) in Lima, Peru.
Materials and methodsStudy design and patient selectionA retrospective case series was conducted at the Abdominal Surgery Department of the Instituto Nacional de Enfermedades Neoplásicas (INEN) in Lima, Peru, through a review of the medical records of patients diagnosed with gastric cancer and treated with distal or total radical gastrectomy with D2 lymphadenectomy with curative intent, within the time frame of January 2014 and December 2018.
Eligible patients were those ≥18 years of age that had preoperative and postoperative histologic diagnoses of gastric carcinoma, treated with D2 distal or total radical gastrectomy, with curative intent. Other eligibility criteria included open surgery or laparoscopy; elective surgery; clinical and pathologic stages I, II, and III (according to the AJCC Cancer Staging System [8th edition]); and surgery with R0 resection.
Key exclusion criteria included treatment with proximal gastrectomy; palliative surgery or emergency surgery; histologic diagnosis of lymphoma, GIST, NET, or diseases other than gastric carcinoma; clinical or pathologic stage IV (according to the AJCC Cancer Staging System [8th edition])14; R1 or R2 resection; and medical records with incomplete data (unreported laboratory exams, unreported comorbidities, and incomplete surgical reports).
Preoperative assessmentThe preoperative assessment consisted of upper gastrointestinal endoscopy with gastric biopsy, complete blood count, coagulation profile, hepatic and renal function tests, and routine psychologic, cardiologic, and nutritional evaluation. The preoperative imaging study was a chest-abdomen-pelvis computed tomography scan. Patients presenting with comorbidities were evaluated by a specialist. Based on the preoperative assessment, the risk factors of age, sex, American Society of Anesthesiologists (ASA) physical status classification grade, body mass index (BMI), preoperative hemoglobin, preoperative albumin, diagnosis or history of hypertension, diabetes, chronic obstructive pulmonary disease (COPD), coronary heart disease, and heart failure were analyzed. No patient received neoadjuvant chemotherapy, which was only available at our institution until after the study period.
Surgical procedureAll surgeries were performed by abdominal oncologic surgeons at the Department of Abdominal Surgery. The selection of gastrectomy was based on tumor location, and resection margins were established according to the Japanese Gastric Cancer Guidelines.15 The extent of lymphadenectomy in distal radical gastrectomy included lymph nodes 1, 3, 4sb, 4d, 5, 6, 7, 8a, 9, 11p, and 12a. For total radical gastrectomy, the extent of lymphadenectomy included lymph nodes 1, 2, 3, 4sa, 4sb, 4d, 5, 6, 7, 8a, 9, and 10 (only in cases needing splenectomy).15 Bursectomy was performed in cases where tumors penetrated the serosa of the posterior gastric wall.4 Omentectomy was performed routinely. Multivisceral resections were done in cases of direct invasion, and splenectomy was carried out only in patients with greater curvature tumors or positive lymph nodes at the splenic hilum. Roux-en-Y reconstruction was performed after total gastrectomy (TG), and Billroth II or Roux-en-Y reconstructions were carried out in cases of subtotal gastrectomy.4 Esophagojejunal anastomosis was performed according to surgeon preference. A circular stapler was utilized in the majority of those procedures and duodenal sections were performed manually in 28 patients (4.1%), with a linear stapler (n = 663) (95.9%).
Postoperative assessmentPostoperative management was carried out by a multidisciplinary team consisting of psychologists, nutritionists, physiotherapists, nurses, and surgical oncologists. At postoperative day 1, all patients received physical and respiratory therapy and the nasogastric tube was withdrawn. Oral diet was routinely initiated on postoperative day 3. Patients were discharged when they were able to tolerate a soft diet for more than 24 h and prophylactic abdominal drains were removed at the same time. Postoperative morbidity included all medical and surgical complications that occurred within the first 90 days. Postoperative complications were recorded from a prospective database and medical records were then analyzed by a medical audit team.16 Postoperative mortality included all patients that died due to surgical or medical complications within the first 90 days.
Statistical analysisA descriptive analysis of the information was carried out through frequencies, percentages, and summary measures (mean and standard deviation).
The association of qualitative variables with morbidity and mortality, respectively, was evaluated with the chi-square test, applying the corresponding Yates correction, when appropriate. Regarding the quantitative variables, differences between patient groups, with and without complications, and between groups of living and deceased patients, were evaluated by the Student’s t test for independent samples or by its corresponding non-parametric test, after testing for normality.
The qualitative variables significantly associated with morbidity and mortality were age, ASA grade, BMI, albumin level, hemoglobin level, tumor location, type of gastrectomy, multivisceral resection, and pancreatosplenectomy. Their influence on morbidity and mortality was evaluated through a binary logistic regression analysis.
A p value <0.05 was considered significant for an association, difference, or influence.
The SPSS 22 statistical program was used in the analyses.
Ethical considerationsThe present study complies with current regulations on bioethical research and was evaluated and approved by the Ethics Committee of the Instituto Nacional de Enfermedades Neoplásicas (INEN) in Lima, Peru.
The authors declare that this article contains no personal information that could identify the study patients.
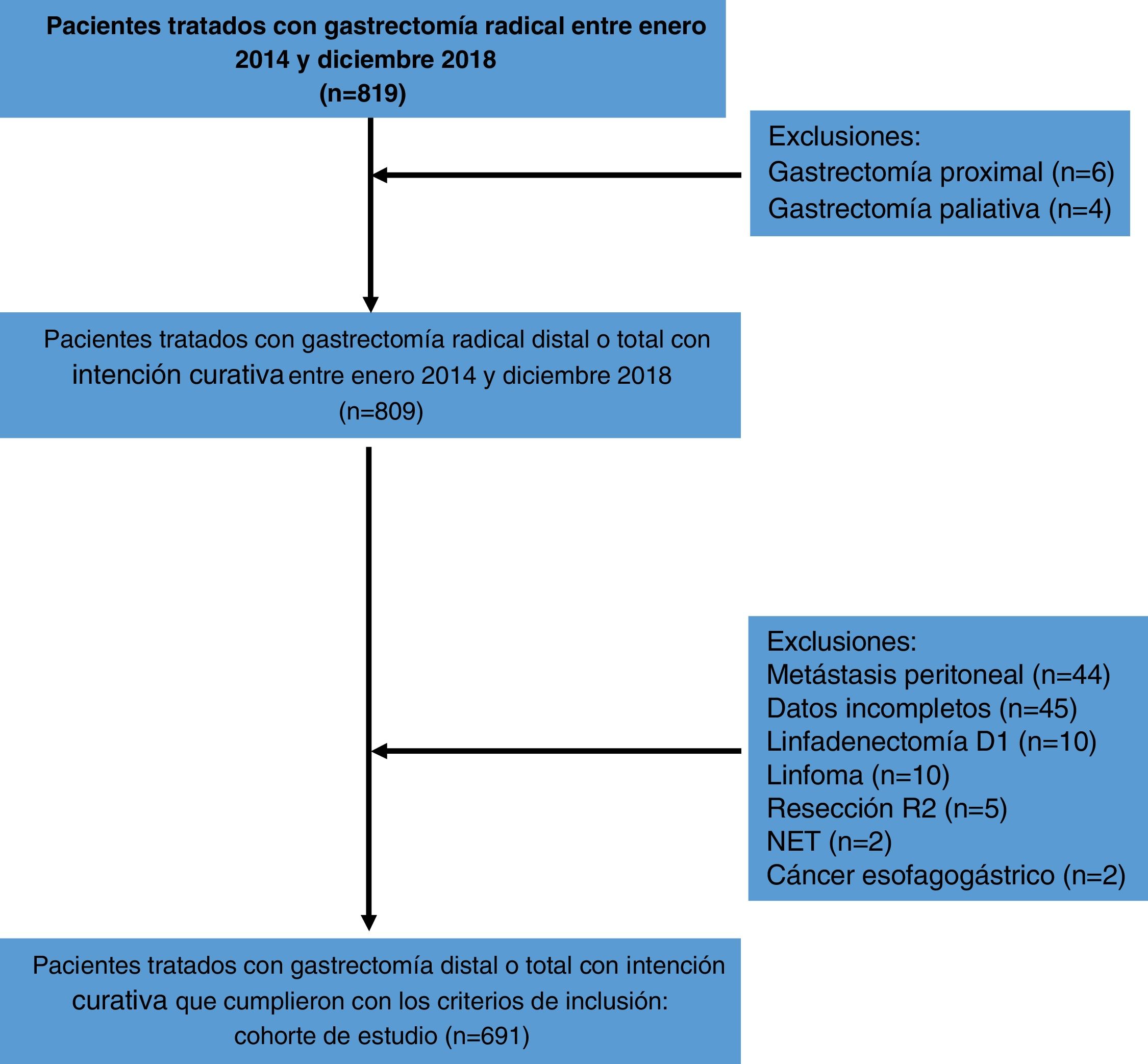
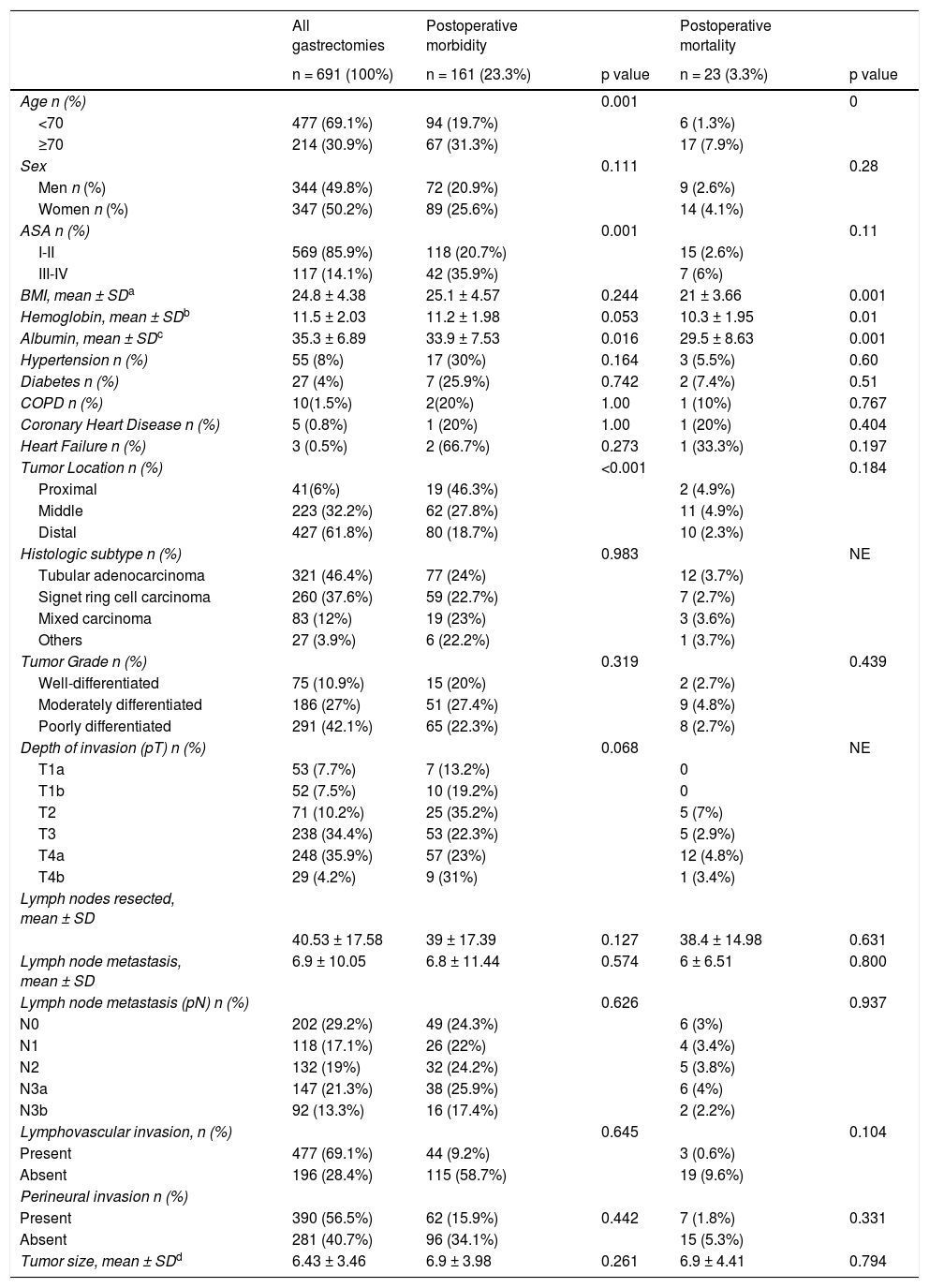
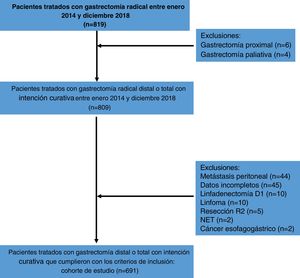
ResultsFrom January 2014 to December 2018, a total of 819 patients were treated with D2 radical gastrectomy, and 691 patients met the inclusion criteria. The causes of exclusion are shown in Fig. 1. Four hundred seventy-seven patients (69.1%) were under 70 years of age and 214 patients (30.9%) were ≥70 years of age. Three hundred and forty-seven patients (50.2%) were women and 344 (49.8%) were men. Mean hemoglobin was 11.5 g/dl and mean albumin was 35.3 g/l. Most patients (n = 569, 85.9%) were classified as ASA I-II and a minority (n = 117, 14.1%) were classified as ASA III-IV. Mean BMI was 24.8 kg/m2. Fifty-five patients (8%) were diagnosed with hypertension, 27 (4%) with diabetes mellitus, 10 (1.5%) with chronic obstructive pulmonary disease, 5 (0.8%) with coronary heart disease, and 3 patients (0.5%) with heart failure, in the preoperative assessment. Distal tumors were the most frequent (n = 427, 61.8%) and tubular adenocarcinoma was the most common histologic subtype (n = 321, 46.4%). The mean number of harvested lymph nodes was 40.53 and mean lymph node metastasis was 6.9. The clinical-pathologic characteristics of the patients are summarized in Table 1.
Clinical/pathologic characteristics of patients that underwent distal and total D2 gastrectomy.
| All gastrectomies | Postoperative morbidity | Postoperative mortality | |||
|---|---|---|---|---|---|
| n = 691 (100%) | n = 161 (23.3%) | p value | n = 23 (3.3%) | p value | |
| Age n (%) | 0.001 | 0 | |||
| <70 | 477 (69.1%) | 94 (19.7%) | 6 (1.3%) | ||
| ≥70 | 214 (30.9%) | 67 (31.3%) | 17 (7.9%) | ||
| Sex | 0.111 | 0.28 | |||
| Men n (%) | 344 (49.8%) | 72 (20.9%) | 9 (2.6%) | ||
| Women n (%) | 347 (50.2%) | 89 (25.6%) | 14 (4.1%) | ||
| ASA n (%) | 0.001 | 0.11 | |||
| I-II | 569 (85.9%) | 118 (20.7%) | 15 (2.6%) | ||
| III-IV | 117 (14.1%) | 42 (35.9%) | 7 (6%) | ||
| BMI, mean ± SDa | 24.8 ± 4.38 | 25.1 ± 4.57 | 0.244 | 21 ± 3.66 | 0.001 |
| Hemoglobin, mean ± SDb | 11.5 ± 2.03 | 11.2 ± 1.98 | 0.053 | 10.3 ± 1.95 | 0.01 |
| Albumin, mean ± SDc | 35.3 ± 6.89 | 33.9 ± 7.53 | 0.016 | 29.5 ± 8.63 | 0.001 |
| Hypertension n (%) | 55 (8%) | 17 (30%) | 0.164 | 3 (5.5%) | 0.60 |
| Diabetes n (%) | 27 (4%) | 7 (25.9%) | 0.742 | 2 (7.4%) | 0.51 |
| COPD n (%) | 10(1.5%) | 2(20%) | 1.00 | 1 (10%) | 0.767 |
| Coronary Heart Disease n (%) | 5 (0.8%) | 1 (20%) | 1.00 | 1 (20%) | 0.404 |
| Heart Failure n (%) | 3 (0.5%) | 2 (66.7%) | 0.273 | 1 (33.3%) | 0.197 |
| Tumor Location n (%) | <0.001 | 0.184 | |||
| Proximal | 41(6%) | 19 (46.3%) | 2 (4.9%) | ||
| Middle | 223 (32.2%) | 62 (27.8%) | 11 (4.9%) | ||
| Distal | 427 (61.8%) | 80 (18.7%) | 10 (2.3%) | ||
| Histologic subtype n (%) | 0.983 | NE | |||
| Tubular adenocarcinoma | 321 (46.4%) | 77 (24%) | 12 (3.7%) | ||
| Signet ring cell carcinoma | 260 (37.6%) | 59 (22.7%) | 7 (2.7%) | ||
| Mixed carcinoma | 83 (12%) | 19 (23%) | 3 (3.6%) | ||
| Others | 27 (3.9%) | 6 (22.2%) | 1 (3.7%) | ||
| Tumor Grade n (%) | 0.319 | 0.439 | |||
| Well-differentiated | 75 (10.9%) | 15 (20%) | 2 (2.7%) | ||
| Moderately differentiated | 186 (27%) | 51 (27.4%) | 9 (4.8%) | ||
| Poorly differentiated | 291 (42.1%) | 65 (22.3%) | 8 (2.7%) | ||
| Depth of invasion (pT) n (%) | 0.068 | NE | |||
| T1a | 53 (7.7%) | 7 (13.2%) | 0 | ||
| T1b | 52 (7.5%) | 10 (19.2%) | 0 | ||
| T2 | 71 (10.2%) | 25 (35.2%) | 5 (7%) | ||
| T3 | 238 (34.4%) | 53 (22.3%) | 5 (2.9%) | ||
| T4a | 248 (35.9%) | 57 (23%) | 12 (4.8%) | ||
| T4b | 29 (4.2%) | 9 (31%) | 1 (3.4%) | ||
| Lymph nodes resected, mean ± SD | |||||
| 40.53 ± 17.58 | 39 ± 17.39 | 0.127 | 38.4 ± 14.98 | 0.631 | |
| Lymph node metastasis, mean ± SD | 6.9 ± 10.05 | 6.8 ± 11.44 | 0.574 | 6 ± 6.51 | 0.800 |
| Lymph node metastasis (pN) n (%) | 0.626 | 0.937 | |||
| N0 | 202 (29.2%) | 49 (24.3%) | 6 (3%) | ||
| N1 | 118 (17.1%) | 26 (22%) | 4 (3.4%) | ||
| N2 | 132 (19%) | 32 (24.2%) | 5 (3.8%) | ||
| N3a | 147 (21.3%) | 38 (25.9%) | 6 (4%) | ||
| N3b | 92 (13.3%) | 16 (17.4%) | 2 (2.2%) | ||
| Lymphovascular invasion, n (%) | 0.645 | 0.104 | |||
| Present | 477 (69.1%) | 44 (9.2%) | 3 (0.6%) | ||
| Absent | 196 (28.4%) | 115 (58.7%) | 19 (9.6%) | ||
| Perineural invasion n (%) | |||||
| Present | 390 (56.5%) | 62 (15.9%) | 0.442 | 7 (1.8%) | 0.331 |
| Absent | 281 (40.7%) | 96 (34.1%) | 15 (5.3%) | ||
| Tumor size, mean ± SDd | 6.43 ± 3.46 | 6.9 ± 3.98 | 0.261 | 6.9 ± 4.41 | 0.794 |
ASA: American Society of Anesthesiologists; BMI: body mass index; COPD: chronic obstructive pulmonary disease; n: number of patients.
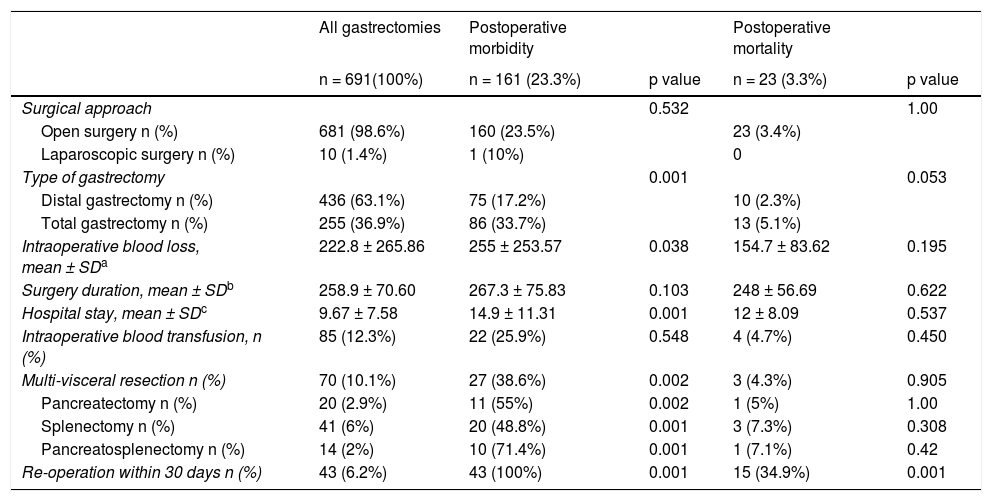
Open gastrectomy was performed on 681 patients (98.6%) and laparoscopic surgery on 10 patients (1.4%). Distal gastrectomy (DG) was carried out on 436 patients (63.1%) and TG on 255 patients (36.9%). Mean intraoperative blood loss was 222.8 ml and mean surgery duration was 258.9 min. Eighty-five patients (12.3%) underwent intraoperative blood transfusion. Mean hospital stay was 9.67 days. Seventy patients (10.1%) had multivisceral resection, whereas pancreatectomy was performed on 20 patients (2.9%) and splenectomy on 41 (6%). Fourteen patients (2%) underwent pancreatosplenectomy. The surgical characteristics are summarized in Table 2.
Surgical characteristics of patients that underwent distal and total D2 gastrectomy.
| All gastrectomies | Postoperative morbidity | Postoperative mortality | |||
|---|---|---|---|---|---|
| n = 691(100%) | n = 161 (23.3%) | p value | n = 23 (3.3%) | p value | |
| Surgical approach | 0.532 | 1.00 | |||
| Open surgery n (%) | 681 (98.6%) | 160 (23.5%) | 23 (3.4%) | ||
| Laparoscopic surgery n (%) | 10 (1.4%) | 1 (10%) | 0 | ||
| Type of gastrectomy | 0.001 | 0.053 | |||
| Distal gastrectomy n (%) | 436 (63.1%) | 75 (17.2%) | 10 (2.3%) | ||
| Total gastrectomy n (%) | 255 (36.9%) | 86 (33.7%) | 13 (5.1%) | ||
| Intraoperative blood loss, mean ± SDa | 222.8 ± 265.86 | 255 ± 253.57 | 0.038 | 154.7 ± 83.62 | 0.195 |
| Surgery duration, mean ± SDb | 258.9 ± 70.60 | 267.3 ± 75.83 | 0.103 | 248 ± 56.69 | 0.622 |
| Hospital stay, mean ± SDc | 9.67 ± 7.58 | 14.9 ± 11.31 | 0.001 | 12 ± 8.09 | 0.537 |
| Intraoperative blood transfusion, n (%) | 85 (12.3%) | 22 (25.9%) | 0.548 | 4 (4.7%) | 0.450 |
| Multi-visceral resection n (%) | 70 (10.1%) | 27 (38.6%) | 0.002 | 3 (4.3%) | 0.905 |
| Pancreatectomy n (%) | 20 (2.9%) | 11 (55%) | 0.002 | 1 (5%) | 1.00 |
| Splenectomy n (%) | 41 (6%) | 20 (48.8%) | 0.001 | 3 (7.3%) | 0.308 |
| Pancreatosplenectomy n (%) | 14 (2%) | 10 (71.4%) | 0.001 | 1 (7.1%) | 0.42 |
| Re-operation within 30 days n (%) | 43 (6.2%) | 43 (100%) | 0.001 | 15 (34.9%) | 0.001 |
n: number of patients.
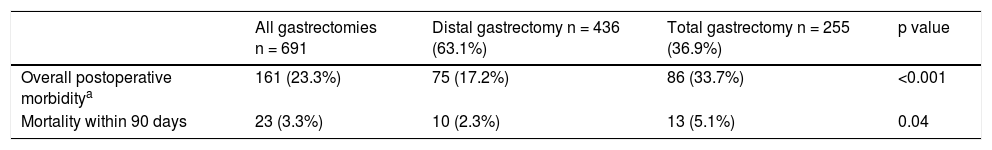
Overall postoperative morbidity was 23.3%. Seventy-five patients (17.2%) that underwent DG and 86 (33.7%) that underwent TG had postoperative complications within the first 90 days (p < 0.001) (Table 3).
Overall morbidity and mortality in distal and total gastrectomy.
| All gastrectomies n = 691 | Distal gastrectomy n = 436 (63.1%) | Total gastrectomy n = 255 (36.9%) | p value | |
|---|---|---|---|---|
| Overall postoperative morbiditya | 161 (23.3%) | 75 (17.2%) | 86 (33.7%) | <0.001 |
| Mortality within 90 days | 23 (3.3%) | 10 (2.3%) | 13 (5.1%) | 0.04 |
The most frequent complication was hospital-acquired pneumonia in both groups, with 29 cases (6.6%) in the DG group and 33 (12.9%) in the TG group. The most frequent postoperative surgical complications in the DG group were pancreatic fistula (2.3%), abdominal hemorrhage (1.6%), and bowel obstruction (1.3%). Gastrojejunal anastomosis leak was reported in 2 patients (0.5%). In the TG group, the most common postoperative surgical complications were pancreatic fistula (10.2%), abdominal hemorrhage (4%), and esophagojejunal anastomosis leak (3.5%).
The overall 90-day mortality rate was 3.3%, with 10 patients (2.3%) in the DG group and 13 patients (5.1%) in the TG group (p = 0.04). In the DG group, postoperative 90-day mortality was associated with mesenteric venous thrombosis (3 patients), peritonitis (2 patients), duodenal leak, abdominal hemorrhage, gastric perforation, hospital-acquired pneumonia, and pulmonary embolism (one patient each).
The most frequent cause of postoperative mortality in the TG group was esophagojejunal anastomosis leak (5 patients), followed by mesenteric venous thrombosis (2 patients), abdominal hemorrhage (2 patients), hospital-acquired pneumonia (2 patients), intestinal perforation (one patient), and pulmonary embolism (one patient).
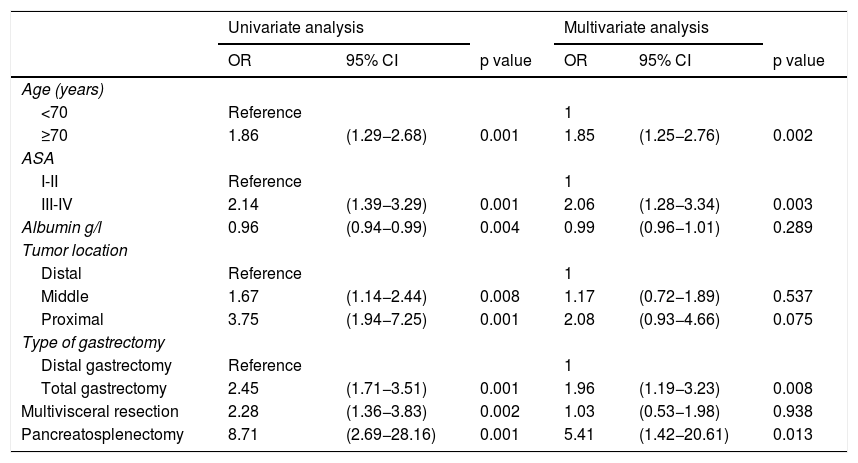
In the univariate analysis, age ≥70 years (p = 0.001), ASA classification III-IV (p = 0.001), hypoalbuminemia (p = 0.004), middle tumors (p = 0.008), proximal tumors (p = 0.001), total gastrectomy (p = 0.001), multivisceral resection (p = 0.002), and pancreatosplenectomy (p = 0.001) were significantly associated with the presence of complications in distal and total D2 gastrectomy (Table 4).
Univariate and multivariate analyses of risk factors associated with morbidity in distal and total gastrectomy.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age (years) | ||||||
| <70 | Reference | 1 | ||||
| ≥70 | 1.86 | (1.29−2.68) | 0.001 | 1.85 | (1.25−2.76) | 0.002 |
| ASA | ||||||
| I-II | Reference | 1 | ||||
| III-IV | 2.14 | (1.39−3.29) | 0.001 | 2.06 | (1.28−3.34) | 0.003 |
| Albumin g/l | 0.96 | (0.94−0.99) | 0.004 | 0.99 | (0.96−1.01) | 0.289 |
| Tumor location | ||||||
| Distal | Reference | 1 | ||||
| Middle | 1.67 | (1.14−2.44) | 0.008 | 1.17 | (0.72−1.89) | 0.537 |
| Proximal | 3.75 | (1.94−7.25) | 0.001 | 2.08 | (0.93−4.66) | 0.075 |
| Type of gastrectomy | ||||||
| Distal gastrectomy | Reference | 1 | ||||
| Total gastrectomy | 2.45 | (1.71−3.51) | 0.001 | 1.96 | (1.19−3.23) | 0.008 |
| Multivisceral resection | 2.28 | (1.36−3.83) | 0.002 | 1.03 | (0.53−1.98) | 0.938 |
| Pancreatosplenectomy | 8.71 | (2.69−28.16) | 0.001 | 5.41 | (1.42−20.61) | 0.013 |
CI: confidence interval; OR: odds ratio.
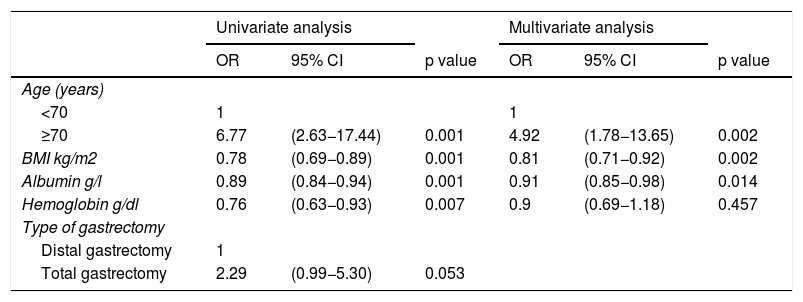
In the univariate analysis, age ≥70 years (p = 0.001), lower BMI (p = 0.001), hypoalbuminemia (p = 0.001), and hemoglobin (p = 0.007) were significantly associated with mortality in distal and total D2 gastrectomy (Table 5).
Univariate and multivariate analyses of risk factors associated with mortality in distal and total gastrectomy.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age (years) | ||||||
| <70 | 1 | 1 | ||||
| ≥70 | 6.77 | (2.63−17.44) | 0.001 | 4.92 | (1.78−13.65) | 0.002 |
| BMI kg/m2 | 0.78 | (0.69−0.89) | 0.001 | 0.81 | (0.71−0.92) | 0.002 |
| Albumin g/l | 0.89 | (0.84−0.94) | 0.001 | 0.91 | (0.85−0.98) | 0.014 |
| Hemoglobin g/dl | 0.76 | (0.63−0.93) | 0.007 | 0.9 | (0.69−1.18) | 0.457 |
| Type of gastrectomy | ||||||
| Distal gastrectomy | 1 | |||||
| Total gastrectomy | 2.29 | (0.99−5.30) | 0.053 | |||
CI: confidence interval; OR: odds ratio.
In the multivariate analysis, age ≥70 years (OR = 1.85, 95% CI: 1.25−2.76), ASA class III-IV (OR = 2.06, 95% CI: 1.28−3.34), total gastrectomy (OR = 1.96, 95% CI: 1.19−3.23), and pancreatosplenectomy (OR = 5.41, 95% CI: 1.42−20.61) were identified as predictors for postoperative morbidity in D2 radical gastrectomy (Table 4).
In the multivariate analysis, a greater risk of postoperative mortality was observed in patients ≥70 years of age (OR = 4.92, 95% CI:1.78−13.65), compared with patients <70 years of age.
In addition, there was a reduced risk of postoperative mortality in patients with a higher BMI (OR = 0.81, 95% CI: 0.71−0.92) and an increased albumin level (OR = 0.91, 95% CI: 0.85−0.98) (Table 5).
DiscussionPrevious publications report variable postoperative morbidity and mortality in gastrectomies for gastric cancer in different regions of the world. In Asia, reported morbidity was between 15.2% and 16.4%,5,7 and mortality was lower than 1%.17 In Western countries, morbidity was shown to be significantly higher and varied from 23.6% to 43%.9,18 Postoperative mortality was also higher in Western countries and ranged between 6% and 8%.19,20
Generally, the decision to perform DG or TG is based on tumor location and the possibility to ensure free resection margins.15,21 In the present study, most patients had tumors that infiltrated the subserosa (T3) and serosa (T4a) (70.3%), with a high presence of lymph node metastasis (pN3). Those results are comparable to findings in other South American studies.11 At our medical center, most patients are diagnosed with locally advanced stages, consisting of large tumors that require more extensive surgery to ensure free margins, and in selected cases, multivisceral resection, which possibly explains our results.2
Previous studies from South America have reported postoperative morbidity between 31.1% and 42.9%.11,22 Similarly, in a Peruvian study that included 899 patients, the authors described postoperative complications in 109 patients, with an overall morbidity of 12.1%, from 1990 to 2000.2 Our finding of overall morbidity of 23.3% is comparable to those previously reported results.
In the present study, the TG group presented with more overall morbidity and greater mortality, compared with the DG group. That result is concordant with those of previous studies.20,23 In the multivariate analysis, TG was significantly associated with increased morbidity (OR = 1.96, 95% CI: 1.19−3.23). In the univariate analysis, TG was not significantly associated with an increased risk of mortality.
Recent systematic reviews and meta-analyses report higher postoperative morbidity in TG versus DG (21.9% vs 15.1%).24 However, in a meta-analysis by Kong et al., no significant difference in terms of overall morbidity was found between the two procedures. Importantly, patients in poor condition were excluded from randomization in that meta-analysis.25
According to our results, the most frequent complication was hospital-acquired pneumonia. Several studies have reported similar results, with an incidence as high as 16.2%.18,26,27 In our study, the primary postoperative surgical complication in both groups was pancreatic fistula, (2.3% in the DG group and 10.2% in the TG group). It is worth noting that grade B was the most frequent pancreatic fistula in DG and only required medical treatment. In the TG group, biochemical leaks were the most frequent type of pancreatic fistula. However, recent publications no longer consider a biochemical leak a true complication.28 Even without taking the biochemical leaks into account, pancreatic fistula was still the most common complication in both groups. Abdominal hemorrhage was the second most frequent surgical complication in both groups, resulting in the death of one patient in the DG group and 2 patients in the TG group.
Several retrospective cohort studies have reported that esophagojejunal anastomosis leak is the most common surgical complication in TG.18 Systematic reviews and meta-analyses have also reported higher rates of anastomotic leak with TG.24,25 In the present study, esophagojejunal anastomosis leak was the third most frequent postoperative complication in TG (3.5%) and caused the death of more than 50% of those patients.
The overall postoperative 90-day mortality in our study was 3.3%. Retrospective studies from South America have described postoperative mortality ranging from 2.1% to 4.7%, in distal and total gastrectomies.11,22 Ruiz et al. reported that the postoperative mortality rate decreased from 4.4% to 2.2% in Peru, between 1950 and 2000.2
Overall mortality in the present study was higher in the TG group vs the DG group (p = 0.04). A similar result was also reported in a meta-analysis comparing DG vs TG.24 The main cause of mortality in the DG group was mesenteric venous thrombosis (MVT). MVT as a postoperative complication after gastrectomy had previously been reported with an incidence of less than 1%.29
Esophagojejunal anastomosis leak was one of the main causes of mortality in the TG group in our analysis, and Gertsen et al. reported that anastomotic leak had the greatest overall impact on postoperative mortality (PAF 29.2%).26
Age ≥70 years, ASA III-IV, total gastrectomy, and pancreatosplenectomy were identified in the multivariate analysis of our study as factors associated with an increase in postoperative complications in DG and TG. Several studies reported increased morbidity in older patients, compared with younger patients, but different cutoff values for age were utilized.30 Robb et al. reported that age ≥60 years was a predictive factor of postoperative complications, in the multivariate analysis (p = 0.001)31 and Nelen et al. found that patients above 70 years of age had increased morbidity.32
ASA classification was reported in multiple studies as a predictive factor of postoperative complications. In a multivariate analysis, Martin et al. found that ASA IV and V were related to an increase in postoperative complications.13
Other studies suggested that ASA classification III could also be a risk factor for complications, by multivariate analyses.31,32 Several studies found that multivisceral resection in gastric cancer is associated with increased postoperative morbidity.12,17 In a retrospective study, Norero et al. reported that a greater number of resected organs was a predictive factor of postoperative mortality.11
In the present study, multivisceral resection was significantly associated with increased morbidity, in the univariate analysis (OR = 2.28, 95% CI: 1.36−3.83), but no association was found in the multivariate analysis. Pancreatosplenectomy was also significantly associated with an increased risk of postoperative morbidity, in the univariate and multivariate analyses (OR = 5.41, 95% CI: 1.42−20.61).
Likewise, age ≥70 years, lower BMI, and hypoalbuminemia were associated with an increase in mortality, in the multivariate analysis. Papenfuss et al. reported that age and weight loss were factors associated with mortality, by multivariate analysis.9 Martin et al. described similar results to ours, but in their study, blood transfusion was also a risk factor for mortality.13
In our region, Norero et al. reported that ASA III and multivisceral resection were factors associated with mortality.11 In addition, Ruiz et al. reported that albumin of <3.5 g/l, blood transfusion, and reoperation rate were factors related to increased postoperative mortality in distal and total gastrectomies.2
We presented herein one of the largest case series in South America that describes radical D2 gastrectomies with curative intent for gastric cancer. As stated before, D2 lymphadenectomy was introduced at our institution by surgeons trained in Japan in the 1990s and subsequently maintained as standard treatment. Our 90-day morbidity and mortality analysis provided more accurate data regarding complications and postoperative death, compared with the 30-day rate.33,34 In contrast to other studies worldwide, our morbidity and mortality rates were adequate and even lower.
The risk factors associated with a higher rate of postoperative complications and mortality were analyzed separately, enabling us to more accurately identify the factors that could be related to a greater possibility of postoperative death.
Among the limitations of our study was its retrospective design, despite the prospective use of a complications database. In addition, the ASA scores were determined by different anesthesiologists, and thus were subjective. We chose that classification because it has been widely used in previous studies, and even with said limitation, ASA III and IV were associated with increased postoperative morbidity, in the multivariate analysis.
In conclusion, D2 radical gastrectomy for gastric cancer was a safe procedure, with low morbidity and mortality. Age ≥70 years, ASA III-IV, total gastrectomy, and pancreatosplenectomy were factors associated with an increased rate of complications. Age ≥70 years, lower BMI, and hypoalbuminemia were predictors of mortality in distal and total radical gastrectomies.
Conflict of interestThe authors declare there are no conflicts of interest.
The authors wish to thank all the members of the Department of Abdominal Surgery at the Instituto Nacional de Enfermedades Neoplásicas (INEN) in Lima, Peru.
Please cite this article as: Paredes-Torres OR, García-Ruiz L, Luna-Abanto J, Meza-García K, Chávez- Passiuri I, Berrospi-Espinoza F, et al., Factores de riesgo asociados con morbilidad y mortalidad postoperatoria en gastrectomía radical D2 por cáncer gástrico, Rev Gastroenterol México. 2022;87:149–158.