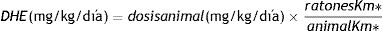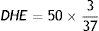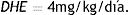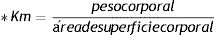Cirrhosis is the common outcome of liver diseases. It can be decompensated and lead to the development of complications, such as encephalopathy. Hyperammonemia that develops due to liver dysfunction is etiopathologically related to hepatic encephalopathy. Caffeine increases the activity of the urea cycle in the liver, augmenting ammonia degradation. By antagonizing adenosine receptors, it also has a hepatoprotective effect, impeding the formation of fibrosis, as well as having a stimulating effect on the central nervous system. The present study analyzed the effects of caffeine on the progression of cholestatic liver fibrosis and hepatic encephalopathy.
Materials and methodsAn experimental model of cholestatic liver fibrosis, through common bile duct ligature, and of hepatic encephalopathy, through the administration of a high-protein diet, was constructed. Male Wistar rats (n=32) were equally divided into 4 groups. The experiment lasted 28 days, with the administration of 50mg/kg/day of caffeine. Laboratory tests, histologic analyses of the liver and encephalon, open field tests (OFTs), and daily behavioral analyses were carried out.
ResultsThe ligated animals treated with caffeine had lower mean transaminase levels and improved histologic aspects of the liver and encephalon. The untreated ligated animals were clearly lethargic and apathetic at the last week of the experiment, confirmed by reduced exploratory activity during the OFT.
ConclusionCaffeine improved the microarchitecture of the liver and encephalon of the cirrhotic animals and prevented the decrease in exploratory behavior of the animals during the OFT.
La cirrosis es el desenlace común de las enfermedades hepáticas. Puede ser descompensada y llevar al desarrollo de complicaciones como la encefalopatía. La hiperamonemia que se desarrolla debido a la disfunción hepática está etiopatológicamente relacionada con la encefalopatía hepática. La cafeína (CAF) aumenta la actividad del ciclo de la urea en el hígado, aumentando la degradación del amonio. Además, al antagonizar receptores de adenosina, ejerce un efecto hepatoprotector, impidiendo la formación de fibrosis y tiene un efecto estimulante en el sistema nervioso central. El objetivo del presente estudio fue el análisis de los efectos de la cafeína sobre la evolución de la fibrosis hepática colestásica y de la encefalopatía hepática.
Material y métodosSe construyó un modelo experimental de fibrosis hepática colestásica, por medio de la ligadura del conducto biliar común, y de encefalopatía hepática, con la administración de una dieta hiperprotéica. Treinta y dos ratas Wistar machos fueron divididas igualmente en 4 grupos. El experimento duró 28 días, con administración de CAF 50mg/kg/día. Se realizaron pruebas de laboratorio, análisis histológicos de hígado y encéfalo, pruebas de campo abierto (PCA) y análisis conductuales diarios.
ResultadosLos animales ligados tratados con CAF presentaron menores medias de transaminasas y mejorías histológicas en hígado y encéfalo. El grupo ligado sin tratamiento se presentó claramente letárgico y apático en la última semana de experimento, lo cual fue comprobado por la disminución de la actividad exploratoria durante la PCA.
ConclusiónLa CAF mejoró la microarquitectura hepática y encefálica de los animales cirróticos. Además, impidió la disminución de la actividad exploratoria de los animales durante la PCA.
Liver diseases are of great importance for public health due to their socioeconomic impact, given that they are associated with high treatment costs, long periods of outpatient follow-up, and hospital admissions.1 Those diseases cause high morbidity and mortality, as a result of the progression to cirrhosis, a common final stage of chronic liver diseases.2 In the United States, cirrhosis caused approximately 49,500 deaths and was the eighth leading cause of death in 2010.3 According to the World Health Organization (WHO), liver cirrhosis is the 11th leading cause of death worldwide.4
There are numerous causes of liver disease, due to liver inflammation or cholestasis. It can end in cirrhosis, which is defined as a diffuse process of nodular formation and fibrosis after liver cell necrosis, the common outcome of chronic liver lesions.5
Regardless of the etiologic agent, cirrhosis can be asymptomatic in its compensated form. However, when decompensated, one or more liver failure complications appear, such as encephalopathy, which is one of the most prevalent.6,7
In patients with liver cirrhosis, hepatic encephalopathy is present in about 30 to 45% of cases and minimal hepatic encephalopathy is present in 80% of those individuals.8–10 It is a potentially reversible metabolic disorder that consists of the functional disorder of the central nervous system, associated with hepatocellular insufficiency. Hepatic encephalopathy results from acute or chronic liver diseases and the presence of portosystemic shunts, which can be spontaneous or surgical (portosystemic intrahepatic transjugular shunting). Ammonia is the main element related to its development, in addition to the interference of several neurotoxins and various factors, such as cerebral edema, GABAergic tone, and the microelements, zinc and manganese.11–13 Because a large part of ammonia is metabolized to urea inside the liver through the urea cycle, the serum ammonia level increases in cases of liver dysfunction, causing it to cross the blood-brain barrier and become toxic to the central nervous system.13,14 It is therefore understood that substances that decrease said concentration can be used in the management of said complication, and caffeine (CAF) is one of them.15
CAF is a pharmacologically active alkaloid that belongs to the group of methylxanthines.16 It is present in several types of food, such as chocolates, soft drinks, teas, energy drinks, and coffee, which is its main source.16,17 Coffee intake has recently been linked to a reduced risk of presenting with several chronic diseases, such as type 2 diabetes mellitus, Parkinson’s disease, inflammatory diseases, and liver diseases.18,19 Specifically regarding liver diseases, CAF consumption has been linked to a decrease in liver enzyme levels and a lower risk of fibrosis.20,21
CAF also increases the activity level of several enzymes that affect the urea cycle, including carbamoyl-phosphate synthetase 1, ornithine transcarbamylase, argininosuccinate synthetase, and argininosuccinate lyase.22,23 All of those enzymes work in favor of the cycle, i.e., they catalyze the urea formation reaction, then increase its synthesis and decrease serum ammonia concentration.24 In addition, one of the main effects of CAF is the stimulation of the central nervous system, by antagonizing adenosine receptors.25
Based on the above, the aim of the present study was to analyze the effects of CAF on the progression of cholestatic liver fibrosis induced by ligation of the common bile duct (LCBD) and correlate its use with the development of encephalopathy caused by hyperammonemia, resulting from liver disease.
Materials and methodsExperimental DesignThirty-two male Wistar rats (200-350g), obtained from the Federal University of Santa Catarina (Florianópolis, Brazil), were equally and randomly divided into four groups (n=8 per group): Sham-operated (SO) + CAF; SO + H2O; LCBD+CAF; and LCBD + H2O. Throughout the experiment, the animals were kept in the animal facility laboratory of the Medical Research Institute (Curitiba, Brazil) in 47×34×18cm plastic boxes, at a 12h light/dark cycle, with relative humidity of 45-55%, and temperature between 24±1°C. The rats had access to filtered water and food ad libitum. All animal protocols in our study were approved by the Animal Use Ethics Committee of the Mackenzie Evangelical College of Paraná (Curitiba, Brazil), and the procedures were performed according to the criteria of the “Guide for the Care and Use of Laboratory Animals”, recommended by the National Academy of Sciences and published by the National Institutes of Health.26
Experimental model of cholestatic liver fibrosisThe experiment lasted 28 days, which was sufficient time for the development of cholestatic liver fibrosis. The procedures were performed according to Tag et al., 2015.27 The animals were anesthetized with ketamine 90mg/kg and xylazine 10mg/kg. Trichotomy and disinfection of the abdominal region were performed with 2% chlorhexidine, followed by a xiphopubic laparotomy. After opening the abdominal cavity, the liver was elevated and the intestinal loops were lowered, exposing the common bile duct. Two ligatures were placed, one proximal and one distal, with polyglactin 7.0 thread. Afterwards, the peritoneum, muscle layer, and skin were sutured. The entire procedure was performed aseptically. In the SO animals, only the abdominal cavity was opened, with no ligation.
Experimental model of hepatic encephalopathyIn addition to the LCBD, the administration of high-protein animal feed was required. So, from day 21 to day 28, the animals were offered a diet containing 76% soy protein. A mixture was made of the usual crushed feed, soy protein, water, and wheat flour, resulting in a soft dough. The dough was divided into small cylinders and baked at 200°C for 45min, to acquire a consistency more similar to that of standard animal feed. The soy protein and wheat flour were obtained from PopHouse Alimentos (Curitiba, Brazil). This model, with some adaptations, was described by Jover et al., in 2006.28 Daily records of the behavior of the animals were kept, using the West Haven criteria (Table 1) as a guide,29 to document the gradual progression to clinical hepatic encephalopathy.
West Haven criteria for the classification of hepatic encephalopathy.47.
| Grade | Consciousness | Intellect/behavior | Neurologic findings |
|---|---|---|---|
| 0 | Normal | Normal | Normal exam |
| 1 | Mild lack of awareness | Shortened attention span, impaired addition/subtraction | Mild asterixis or tremor |
| 2 | Lethargic and apathetic | Disoriented; inappropriate behavior | Obvious asterixis; slurred speech |
| 3 | Somnolent | Gross disorientation; bizarre behavior | Muscular rigidity, clonus, hyperreflexia |
| 4 | Coma | Coma | Decerebrate posturing |
The CAF, obtained from the Quallitá Farmácias (Curitiba, Brazil), was dissolved in 2ml of warm water (45°C) and administered intragastrically at a dose of 50mg/kg/day.30 The treatment was given from the first to the last day of the experiment, for a total of 28 days. The 50mg/kg administered dose was calculated, based on the allometric formula of the human equivalent dose (HED),31 considering an average daily human consumption of 4mg/kg:32
The dose of 4mg/kg/day, in a 70kg adult, corresponds to the consumption of 280mg/day, which is the equivalent of two to three cups of coffee per day.33 In the control groups, 2ml of H2O were administered in the same manner.
Open Field Test (OFT)OFTs reproduce the natural animal behavior of exploring a new environment. Thus, exploratory and locomotor activity can be assessed by placing the animals in an open area larger than what they are used to.34 We conducted the test in an open square wooden MDF box (120cmx120cmx40cm) with a checkered floor, made at Rudegon Marcenaria (Curitiba, Brazil). On the last day of the experiment, each animal was placed in the center of the box and individually exposed for 5minutes, while being filmed with a Nikon D3400 camera.35 The videos were analyzed by counting the quadrants each animal passed through and by the acts of standing only on the hind legs (rearing) and of self-cleaning (grooming). The test was applied by the same person in a closed room, isolated from external sounds, to prevent any changes in the behavioral habits of the animals.36
Method of euthanasia and sample collectionOn day 28 of the experiment, the animals were anesthetized with ketamine 90mg/kg and xylazine 10mg/kg. Three milliliters of arterial blood were collected by cardiac puncture, after which the liver and encephalon were removed.
Laboratory analysisBlood samples were centrifuged at 3,000rpm for 20min and the resulting sera were stored at –20°C. The samples were then sent to the Bionostic clinical analysis laboratory (Curitiba, Paraná). The liver integrity tests were performed using the UV kinetic method for ALT and AST, and the colorimetric method for bilirubin and fractions.
Histologic evaluationThe collected organs were stored in buffered formaldehyde for 48h. Fragments were then selected for processing, according to the conventional histologic technique. Fragments of liver and encephalon were embedded in Paraplast® and cut as cross sections of the organs. The liver sections were stained with hematoxylin-eosin and Masson’s trichrome and the encephalon was stained with hematoxylin-eosin. The histologic examination was performed by a pathologist that had no information about the experimental groups.
Statistical analysisValues were expressed as mean and standard deviation (SD) and the analysis was performed using GraphPad Prism, version 7.0, for Windows 10. To evaluate differences between the groups, the data were analyzed by one-way analyses of variance (ANOVA), followed by the post-hoc Tukey-Kramer test. A p<0.05 was considered statistically significant.
Ethical considerationsAll the animal protocols in this study were approved by the Animal Use Ethics Committee of the Mackenzie Evangelical College of Paraná (protocol number 1566/2017). The procedures were performed according to the guidelines of the National Council for the Control of Animal Experimentation (CONCEA) and the criteria of the “Guide for the Care and Use of Laboratory Animals”, recommended by the National Academy of Sciences and published by the National Institutes of Health.26
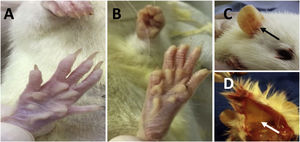
ResultsPhenotypic changesThere were no significant differences in the body weight of the animals throughout the entire experiment. Around day 7, the LCBD+CAF and LCBD + H2O groups started to show signs of jaundice (Fig. 1), visible in the region of the legs, ears, and back. The coloring intensified as the days passed and was more evident at the end of the experiment.
Jaundice in the animals that underwent LCBD. A) anicteric skin; B) jaundice of the skin and fur on day 28 of the experiment; C) jaundice of the ear region (black arrow) on day 28 of the experiment; D) jaundice of the hypodermis (white arrow) on day 28 of the experiment.
LCBD: ligation of the common bile duct.
The animals also presented with choluria, a characteristic sign of hyperbilirubinemia.
Throughout the experiment, the LCBD + H2O group showed evident signs of apathy, torpor, disorientation, muscle rigidity, and tremors, particularly visible during the last week of the experiment, characterized as grade II or grade III hepatic encephalopathy, according to the West Haven criteria (Table 1).29 In the LCBD+CAF group, only tremors were occasionally seen, characterized as grade 0 or I.29 No behavioral changes were observed in the control groups.
Open Field Test evaluationThe number of rearings and quadrants covered by the animals of the LCBD+CAF group was significantly higher, when compared with the LCBD + H2O group (Table 2). There was no difference between the mean number of rearings and quadrants covered between the LCBD+CAF group and its controls but the LCBD + H2O group showed a reduction in those means, compared with its corresponding control group.
Parameters obtained in the open field behavioral test.
| SO+CAF | SO + H2O | LCBD+CAF | LCBD + H2O | |
|---|---|---|---|---|
| Squares covered | 120±25.5 | 106.8±12.4 | 108.4±7.9b,* | 56.8±30.5a,* |
| Rearing | 25.13±7.4 | 25.6±4.16 | 24.6±5.17b,* | 13±4.24a,* |
| Grooming | 4.37±2.6 | 2.8±0.84 | 4.4±2.4 | 2.6±1.61 |
Mean (SD).
CAF: caffeine; LCBD: ligation of the common bile duct; SD: standard deviation; SO: sham-operated.
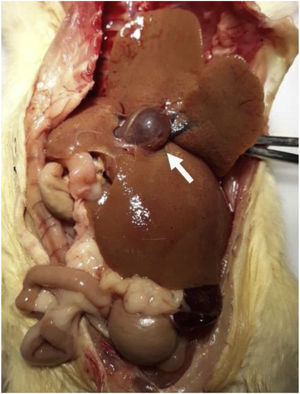
The livers of the animals from the control groups were macroscopically normal (Fig. 2), with a reddish-brown color, triangular shape, well-defined edges, smooth and shiny surface, and soft consistency. However, the livers of the animals with LCBD were yellowish-brown, and in some cases, greenish, as well as oval-shaped, with well-defined edges, easily friable, and had an irregular surface with a micronodular aspect, fibroelastic consistency, and important hepatomegaly (Fig. 2). Some animals also presented dilation of the portal vein (Fig. 2), possibly as a result of portal hypertension.
Regarding the encephalon, there were no macroscopic differences. The organ was pinkish-white, soft, and friable.
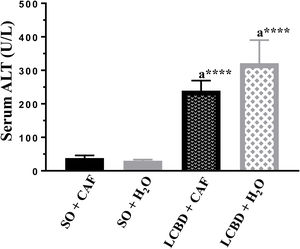
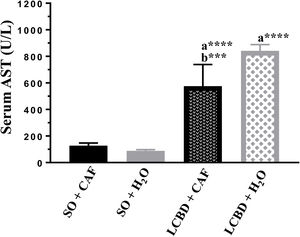
Laboratory test evaluationA significant increase in the values of the ALT and AST transaminases (Figs. 3 and 4) and of direct bilirubin and indirect bilirubin (Table 3) was observed in all the animals with LCBD, compared with their respective controls, but the LCBD+CAF group had lower mean values than the LCBD + H2O group.
AST serum levels in each group. Mean (SD).
a: Significant difference between the LCBD groups and their respective controls.
b: Significant difference between the LCBD+CAF and LCBD + H2O groups.
***p<0.001; ****p<0.0001.
CAF: caffeine; LCBD: ligation of the common bile duct; SD: standard deviation.
Bilirubin serum levels expressed in mg/dl.
| SO+CAF | SO + H2O | LCBD+CAF | LCBD + H2O | |
|---|---|---|---|---|
| Direct bilirubin | 0.16±0.05 | 0.1 | 4.16±0.6a,**** | 5.22±1.6a,**** |
| Indirect bilirubin | 0.1 | 0.14±0.05 | 1.04±0.18a,*** | 1.18±0.17a,**** |
| Total bilirubin | 0.26±0.05 | 0.24±0.05 | 5.2±0.67a,**** | 6.4±1.61a,**** |
Mean (SD).
CAF: caffeine; LCBD: ligation of the common bile duct; SD: standard deviation; SO: sham-operated.
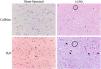
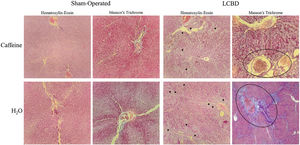
With respect to the architectural distortion of the parenchyma and widening of the portal spaces of the liver tissue, the LCBD + H2O group showed the worst result, with 100% of the animals presenting with a moderate/accentuated degree of distortion. The LCBD+CAF group showed better results, with a mild degree of distortion in 40% and moderate/accentuated distortion in 60% of the animals (Fig. 5).
Photomicrographs of liver sections stained in hematoxylin-eosin and Masson’s trichrome (magnification, x100). Architectural distortion of the parenchyma (better visualized in the hematoxylin-eosin staining), proliferation of bile ducts (black arrows), widening of the portal spaces (inside the black circles), and fibrosis (blue in the Masson’s trichrome staining) can be observed in a moderate/accentuated degree in the LCBD + H2O group. The LCBD+CAF group showed significant improvement in those characteristics. The control groups had normal histology.
CAF: caffeine; LCBD: ligation of the common bile duct.
Regarding bile duct proliferation, 100% of the LCBD + H2O group was classified as having moderate/accentuated proliferation. Eighty percent of the animals in the LCBD+CAF group presented with moderate/accentuated proliferation and 20% had slight proliferation (Fig. 5).
The ISHAK scale was used to determine the degree of liver fibrosis, with grade 0 indicating the absence of fibrosis and grade 6 indicating cirrhosis.37 The LCBD+CAF group showed better results, compared with the LCBD + H2O group. A total of 62.5% of the animals had grade 3 (portal fibrosis with occasional portal-to-portal septa), 25% had grade 4/5 (portal fibrosis with portal-to-portal septa and occasional nodules), and 12.5% had grade 6. In the LCBD + H2O group, 62.5% of the animals presented with grade 4/5 fibrosis and 37.5% with grade 6 (Fig. 5).
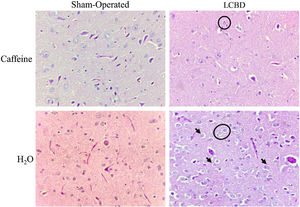
The LCBD+CAF group showed significant improvement in the encephalon tissue, regarding the presence of Alzheimer type II astrocytes, edema, and laminar necrosis. Their presentation was mild in 80% of those animals and moderate/accentuated in only 20%. The LCBD + H2O group presented with moderate/accentuated necrosis in 80% of the animals and Alzheimer type II astrocytes and edema in 100% of them. The spongiform aspect was mild in 100% of the animals in the LCBD+CAF group, whereas it was moderate/accentuated in 80% of the animals in the LCBD + H2O group and mild in 20% of them (Fig. 6).
Photomicrographs of encephalon sections stained in hematoxylin-eosin. (magnification, x400). Alzheimer type II astrocytes (under the black stars), necrosis (black arrows), edema (white halo around the cells), and spongiform aspect can be observed in a moderate/accentuated degree in the LCBD + H2O group. The LCBD+CAF group showed significant improvement in those characteristics. The control groups had normal histology.
CAF: caffeine; LCBD: ligation of the common bile duct.
The liver enzymes, ALT and AST, are markers of toxicity and severity of liver damage, and their release into the bloodstream demonstrates the presence of damage to the liver parenchyma.38 In our study, the LCBD-induced liver damage, which was evidenced by the significant increase of those enzymes in the blood, a fact already corroborated by other studies using the same model.27,39 As reported in similar studies, CAF treatment decreased the release of those enzymes into the bloodstream, showing a hepatoprotective effect and possible improvement in the liver architecture.30,40
With the LCBD, direct bilirubin from the liver is unable to complete its flow through the bile ducts to the intestine, regurgitating and accumulating in the liver, and is captured by the hepatic sinusoids, increasing its serum concentration.41 With the chronicity of biliary obstruction, the bile acids accumulated in the liver begin to damage the cells responsible for conjugating indirect bilirubin, derived from hemolysis, into direct bilirubin, which causes the serum level of indirect bilirubin to also increase.5,41 In the present study, both the direct bilirubin and indirect bilirubin levels in the ligated animals were increased. The ligated group that was untreated had the highest mean total bilirubin of the groups. On the other hand, the caffeine-treated group showed a decrease in indirect bilirubin, compared with the untreated ligated group, demonstrating that CAF administration resulted in less damage to the cells responsible for the conjugation of indirect bilirubin into direct bilirubin. According to the literature, total bilirubin levels greater than 2 or 3mg/dl are sufficient for the pigment to leak into the skin and mucous membranes and lead to the development of jaundice.5,41 In both LCBD groups, the amount of bilirubin was greater than 2mg/dl, which explains the intense yellowish color visible in the animals after day 7 of the experiment.
Hepatomegaly was also macroscopically evident. That condition is often associated with hepatobiliary diseases and is caused by the retention of bile content in the liver tissue and by the diffuse damage resulting from its accumulation.42,43 In the histopathologic assessment of the liver in the groups that underwent LCBD, both areas had only slight architectural distortion of the parenchyma and areas with intense fibrosis, similar to results from other studies using the same model.27,39,42 The parameters of architectural distortion, portal enlargement, ductal proliferation, and hepatic fibrosis were considered moderate/accentuated in all the untreated ligated animals.
Hepatic myofibroblasts, derived from stellate cells and portal fibroblasts, are the main cells involved in the formation of hepatic fibrosis.44 Those cells express adenosine receptors, which when activated (usually by liver injury), stimulate the formation of fibrosis through myofibroblasts. CAF is an antagonist of adenosine receptors, i.e., it prevents the activation of those receptors and consequently decreases the formation of fibrotic tissue.45 TGF-β is the main regulatory cytokine of the liver and one of its functions is to activate liver stellate cells, stimulating the formation of fibrosis.46 According to the literature, TGF-β levels are increased in experimental models of liver disease, but when CAF is administered, TGF-β levels are lower, meaning there is less activation of the stellate cells due to downregulation of that cytokine.47,48
In vitro studies have shown that CAF also prevents the activation of liver stellate cells by inhibiting the Snail-1 transcription factor, which is essential in the activation of those cells. CAF also activates the enzyme, Nrf2, which regulates antioxidant protein expression. Consequently, it acts by reducing oxidative stress, which is one of the causes of liver fibrosis.30 Through all those mechanisms, CAF exerts its hepatoprotective effect, resulting in less liver damage, thus releasing fewer transaminases into the bloodstream.
In the present study, the liver tissue of the ligated animals treated with CAF generally had a histologic pattern that was more similar to the physiologic one. The induced liver damage could be viewed under microscopy, but there was less damage than that seen in the untreated LCBD group.
As a result, a dose of 50mg/kg/day of CAF, in the presence of cholestatic liver fibrosis, exerted a hepatoprotective effect, especially with respect to the formation of fibrotic tissue, corroborating its effects on injured liver tissue, described in recent studies.30,45,47,48
In hepatic encephalopathy, the main microscopic changes in the encephalon are edema and Alzheimer type II astrocytes,49,50 which are modified cells that show swelling, enlarged nuclei, and nuclear inclusions.41 Laminar necrosis and a spongiform appearance can also be found in the deep layers of the cerebral cortex, subcortical white matter, basal nuclei, and the cerebellum.51
In the present study, the untreated ligated animals intensely presented with the characteristic parameters of encephalopathy, mainly edema and Alzheimer type II astrocytes. Those features were classified as accentuated in 100% of the animals, corroborating the findings of other studies in the literature.28,52
The result of the histologic analysis of the encephalon slides of the LCBD group treated with CAF was satisfactory, with the administration of CAF producing a significant decrease in edema, Alzheimer type II astrocytes, the spongiform aspect, and laminar necrosis. Edema and Alzheimer type II astrocytes, the main histologic changes observed in encephalopathy,49,50 were classified as accentuated in only 20% of the animals, whereas, in the LCBD + H2O group, they were accentuated in 100% of the animals.
In the last days of the experiment, the animals in the untreated LCBD group were clearly more lethargic, confused, disoriented, and apathetic than the animals in the treated group. During gavage, the animals did not struggle against the administration of H2O, unlike the other groups or at other times of the experiment. That reduced consciousness was demonstrated and confirmed by the open field test (OFT), during which the animals showed a decrease in two of the three parameters used to assess exploratory and locomotor activity.29 That state of consciousness is characterized as grade II or grade III of hepatic encephalopathy, according to the West Haven criteria (Table 1), which was expected for the encephalopathy model chosen.28,53,54 Those findings corroborate the results of other studies that used a similar methodology to induce hepatic encephalopathy.28,52,55,56
In the LCBD group supplemented with CAF, no signs of lethargy or prostration were observed. The mean values of the three parameters assessed in the OFT were similar to those in the control groups, showing that the administration of CAF prevented the animals from having the clinical signs characteristic of grade II and grade III of the West Haven criteria.29,53
CAF is known to have a modulatory effect on the state of consciousness due to its stimulating effect on the central nervous system, by antagonizing adenosine receptors.25,57 CAF also decreases the serum concentration of ammonia,57 by increasing the activity of several enzymes in the urea cycle, especially carbamoyl phosphate synthetase 1.22,23 It catalyzes the urea formation reaction, increasing the synthesis of the compound, using ammonia as a substrate, which, in excess, can lead to the development of hepatic encephalopathy.24,53 Through those mechanisms, CAF, at a dose of 50mg/kg/day, prevented the hepatic encephalopathy from progressing to the most severe degrees. In the human body, that dose is equivalent to 280mg/day for a 70kg adult, corresponding to two or three cups of coffee per day.33 That amount of CAF is extremely safe. Side effects, such as anxiety, bipolar disorders, insomnia, psychomotor agitation, and tachycardia, are caused only with larger doses (>400mg/day).58 Thus, CAF would be a viable alternative in the management of patients with liver disease and/or hepatic encephalopathy.
Based on the above, and with the results of the present study, we conclude that the effect of caffeine as a stimulant of the central nervous system, associated with its catalytic action in the urea cycle and its hepatoprotective effect of attenuating liver injury, prevented induced hepatic encephalopathy from progressing to the most severe degrees. It impeded the appearance of clinical signs and the decrease in exploratory and locomotor activity, characteristic of West Haven criteria grade II and grade III,29 and attenuated the appearance of histologic changes in the encephalon. Future studies that measure cerebral ammonia and determine the urea cycle enzymes are strongly recommended for a better understanding of the pathophysiology involved in the prevention of hepatic encephalopathy severity, with the use of caffeine.
Financial disclosureThe present work was supported by the Mackenzie Evangelical College of Paraná (grant number 03/2017,082017/072018). The funding source had no involvement in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Guth I, Matos-Pardal CF, Ferreira-Lima R, Loureiro-Rebouças R, Sobral AC, Moraes-Marques CA, et al. La cafeína atenúa da˜no hepático y mejora signos neurológicos en un modelo de encefalopatía hepática con ratas, Rev Gastroenterol Méx. 2022;87:159–169.