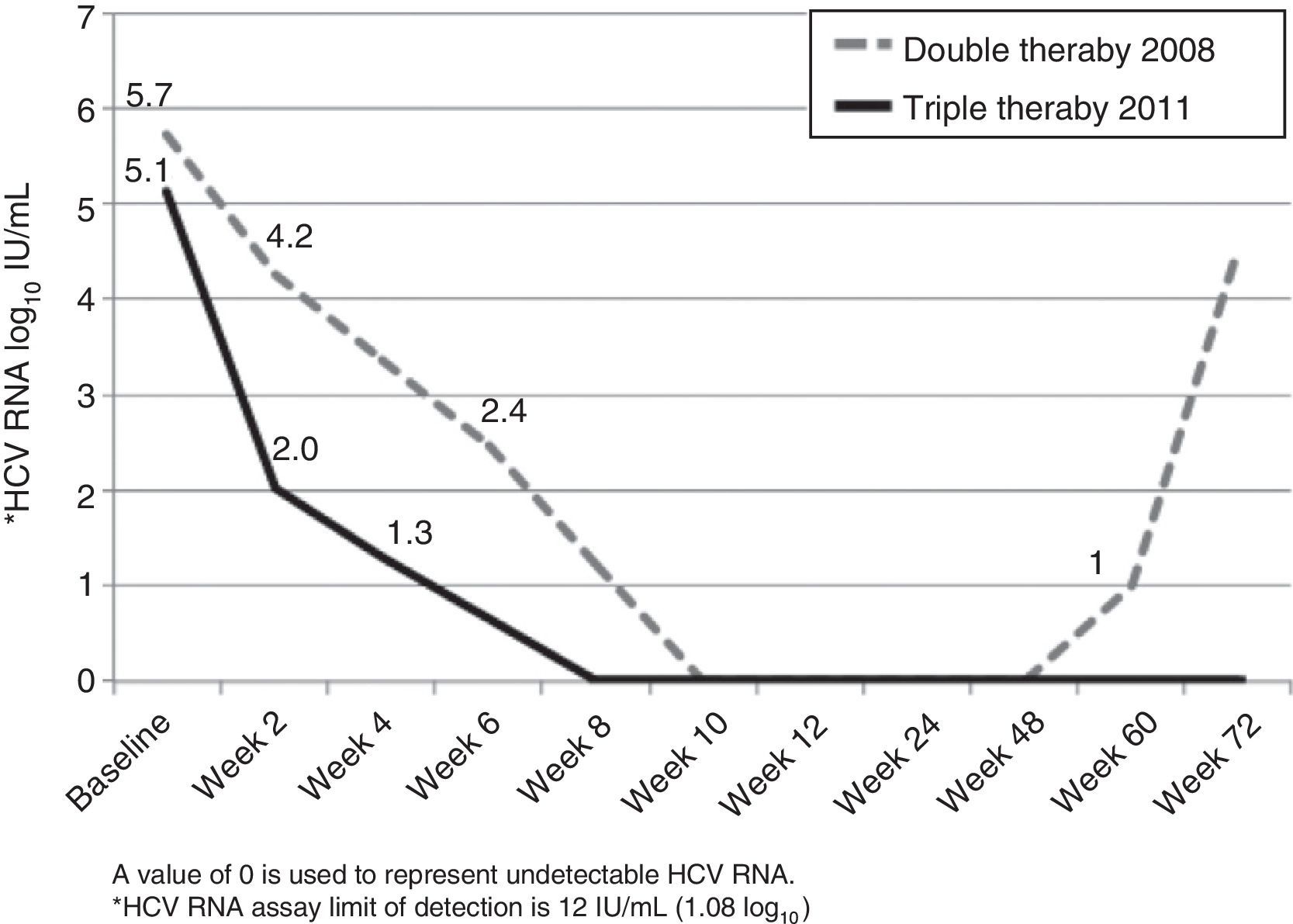
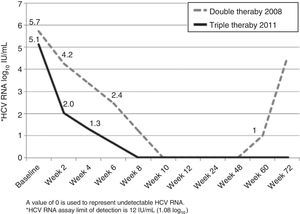
A 48-year-old Caucasian woman with chronic hepatitis C virus (HCV) infection, genotype 4, presented to our clinic in the summer of 2011 to be evaluated for antiviral treatment. She had evidence of portal hypertension (splenomegaly, low platelets, small esophageal varices), cirrhosis stigmata, and a liver biopsy from 2006 had shown bridging fibrosis. IL28B genotypes were subsequently found to be TT at both rs12979860 and rs8099917 loci. The patient had been treated in 2006 with peginterferon (peg-IFN) alpha-2a with 180 mcg SQ weekly and ribavirin (RBV) 1,200mg/day for 48 weeks. Treatment was unsuccessful with a complete early virological response (EVR) followed by detectable HCV RNA at end-of-treatment visit, and she was then referred to our center for further evaluation. In 2008, she was treated with identical doses of peg-IFN/RBV, achieving a complete EVR (fig. 1). Due to dose-reductions in RBV resulting from anemia (800mg/d on average), and in light of her prior breakthrough, it was planned for her to continue treatment for a total of 72 weeks. Unfortunately, HCV RNA was positive around week 60 (viral breakthrough) and treatment was stopped.
The patient was particularly interested in trying the direct-acting antivirals (DAA), newly approved in 2011. After full disclosure of the lack of evidence on the effect of DAA in genoype 4, we agreed to try telaprevir-based regimen (TPV). In July 2011, she was initiated on her third course of peg-IFN/RBV (same doses), now with TPV (750mg every 8h) for the first 12 weeks. Virological response is shown in Figure 1. There was a 3 log10-drop after 2 weeks of treatment (compared to the 1.5 log10-drop with the previous regimen), followed by complete EVR (undetectable HCV RNA at week 8). Treatment was complicated by pancytopenia, requiring multiple adjustments in peg-IFN (down to 135 mcg SQ weekly) and RBV (600mg/day on average) doses, erythropoietin, and red blood cell transfusions (8 packed red blood cells vs 12 on previous course). The patient completed 48 weeks of treatment and achieved a sustained virological response (SVR) 24 weeks following end-of-treatment.
FDA approval of TPV and boceprevir (BOC) in May 2011 has revitalized treatment of patients with HCV, genotype 1. However, for those with genotype 4 infection, treatment options remain limited. Although genotype 4 is uncommon in Europe and rare in North America, it is the most common in the Middle East and Africa.1 Nonetheless, genotype 4 has SVR rates similar to genotype 1 (40-50% in Europeans), when using peg-IFN/RBV. The improved SVR rates observed in genotype 1 after addition of TPV or BOC (≈60-70%), now makes genotype 4 the genotype most difficult to treat.2 Currently approved DAA have been developed and primarily studied for treatment of genotype 1 infection, but there is some suggestion that their effectiveness may extend to other genotypes as well. One study has shown that TPV and BOC are effective inhibitors of NS3/4A isolated from genotype 4.3 We report a case of successful treatment using a TPV-containing regimen on a patient with genotype 4 infection, who had previously failed treatment with peg-IFN/RBV. A phase II randomized clinical trial showed effectiveness of 2 weeks of TPV monotherapy in reducing HCV RNA in treatment-naive patients with genotype 4. This effect was much greater when TPV was combined with peg-IFN/RBV, and the effect of triple therapy was greater than the effect of peg-IFN/RBV dual therapy (HCV RNA reduction of -0.77, -4.32, and -1.58 log10 IU/mL, for TPV, TPV+peg-IFN/RBV, and peg-IFN/RBV, respectively). The number of patients included in this study was, however, too small (n=8 per group) to draw any conclusions on triple therapy antiviral efficacy.4 Remarkably, there is no published experience on treatment with TPV for longer than two weeks in patients with genotype 4. To the best of our knowledge this is the first reported case of a treatment-experienced patient with genotype 4 infection to achieve SVR after a full-length TPV-containing regimen (12 weeks). We believe this isolated clinical experience with a difficult-to-treat patient contributes to the limited knowledge on the effectiveness of a TPV-based antiviral regimen on genotype 4. Without a doubt, new DAA (such as the new NS5B polymerase inhibitor) will open the door for new antiviral regimens with a specific activity against genotype 4.5,6 In the meantime, defining the role of the already approved DAA (particularly TPV) for the treatment of HCV genotype 4, based on well-designed studies, should be encouraged.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Deneke M, Dranoff Ja, Duarte-Rojo A. Erradicación exitosa del virus de la hepatitis C, genotipo 4, con terapia triple antiviral estándar más telaprevir. Revista de Gastroenterología de México. 2014;79:64–66.