Bacterial resistance may hamper the antimicrobial management of acute gastroenteritis. Bacterial susceptibility to rifaximin, an antibiotic that achieves high fecal concentrations (up to 8,000μg/g), has not been evaluated in Mexico.
ObjectiveTo determine the susceptibility to rifaximin and other antimicrobial agents of enteropathogenic bacteria isolated from patients with acute gastroenteritis in Mexico.
Material and methodsBacterial strains were analyzed in stool samples from 1,000 patients with diagnosis of acute gastroenteritis. The susceptibility to rifaximin (RIF) was tested by microdilution (<100, <200, <400 and <800μg/ml) and susceptibility to chloramphenicol (CHL), trimethoprim-sulfamethoxazole (T-S), neomycin (NEO), furazolidone (FUR), fosfomycin (FOS), ampicillin (AMP) and ciprofloxacin (CIP) was tested by agar diffusion at the concentrations recommended by the Clinical & Laboratory Standards Institute and the American Society for Microbiology.
ResultsIsolated bacteria were: enteropathogenic Escherichia coli (E. coli) (EPEC) 531, Shigella 120, non-Typhi Salmonella 117, Aeromonas spp. 80, enterotoxigenic E. coli (ETEC) 54, Yersinia enterocolitica 20, Campylobacter jejuni 20, Vibrio spp. 20, Plesiomonas shigelloides 20, and enterohemorrhagic E. coli (EHEC 0:157) 18. The overall cumulative susceptibility to RIF at <100, <200, <400, and <800μg/ml was 70.6, 90.8, 99.3, and 100%, respectively. The overall susceptibility to each antibiotic was: AMP 32.2%, T-S 53.6%, NEO 54.1%, FUR 64.7%, CIP 67.3%, CLO 73%, and FOS 81.3%. The susceptibility to RIF <400 and RIF <800μg/ml was significantly greater than with the other antibiotics (p<0.001).
ConclusionsResistance of enteropathogenic bacteria to various antibiotics used in gastrointestinal infections is high. Rifaximin was active against 99-100% of these enteropathogens at reachable concentrations in the intestine with the recommended dose.
La resistencia bacteriana puede dificultar el tratamiento antimicrobiano de las gastroenteritis agudas. La susceptibilidad bacteriana de los enteropatógenos a la rifaximina, un antibiótico que alcanza altas concentraciones fecales (hasta 8,000μg/g) no se ha evaluado en México.
ObjetivosDeterminar la susceptibilidad a rifaximina y a otros antimicrobianos de bacterias enteropatógenas aisladas de pacientes con gastroenteritis aguda en México.
Material y métodosSe analizaron las cepas bacterianas en las heces de 1,000 pacientes con diagnóstico de gastroenteritis aguda. Se probó la susceptibilidad a la rifaximina (RIF) con microdilución (< 100, < 200, < 400 y < 800μg/ml), la susceptibilidad a cloranfenicol (CLO), trimetoprim-sulfametoxazol (T-S), neomicina (NEO), furazolidona (FUR), fosfomicina (FOS), ampicilina (AMP) y ciprofloxacino (CIP) se probó por difusión-agar a las concentraciones recomendadas por CLSI y ASM.
ResultadosLas bacterias aisladas fueron: Escherichia coli (E. coli) enteropatógena (EPEC) 531, Shigella 120, Salmonella no-typhi 117, Aeromonas spp. 80, E. coli enterotoxigénica 54, Yersinia enterocolitica 20, Campylobacter jejuni 20, Vibrio spp. 20, Pleisiomonas shigelloides 20 y E. coli enterohemorrágica (EHEC 0:157) 18. La susceptibilidad global acumulada a RIF < 100, < 200, < 400, < 800μg/ml fue del 70.6, el 90.8, el 99.3 y el 100%, respectivamente. La susceptibilidad global a cada antibiótico fue: AMP 32.2%, T-S 53.6%, NEO 54.1%, FUR 64.7%, CIP 67.3%, CLO 73%, FOS 81.3%. La susceptibilidad a RIF < 400 y < 800μg/ml fue significativamente mayor que con los otros antimicrobianos (p<0.001).
ConclusionesLa resistencia de las bacterias enteropatógenas a antimicrobianos utilizados en gastroenteritis es alta. La rifaximina fue activa contra el 99-100% de las bacterias en concentraciones alcanzables en el contenido intestinal con las dosis recomendadas.
Acute diarrhea is an important health problem, mainly in the developing countries. Despite the decrease in mortality rates from this disease in the last decade in various countries, including Mexico1–3, acute gastroenteritis continues to be a health problem due to its high morbidity. In Mexico, it is the second most common infectious disease, only preceded by respiratory diseases, with more than 5 million new cases per year4.
Despite the presence of certain clinical signs, it is difficult to determine the causal agent of acute diarrhea in a patient based solely on clinical findings. Acute gastroenteritis is often due to a viral infection, especially in children under 5 years of age1, whereas bacterial infection is more habitual in older children and adults. The most frequently detected enteropathogenic bacteria in patients with endemic gastroenteritis are Escherichia coli (E. coli) (EPEC, EIEC, EHEC, ETEC), Campylobacter jejuni (C. jejuni), Shigella spp, Salmonella spp, Yersinia enterocolitica (Y. enterocolitica)5–8, and less frequently, Aeromonas spp, Vibrios spp9–12, and Pleisomonas shigeloides (P. shigeloides)13–15. The number of cases vary in relation to geographic region, patient age, and the season of the year in which the study was conducted.
The aims of therapeutic management of patients affected with gastroenteritis are to preserve life, relieve symptoms, prevent complications, cut the disease short, and prevent the spread of the pathogenic agents to the population. Oral rehydration is the standard treatment in acute gastroenteritis and antimicrobial agents are indicated in severe or prolonged cases, when shigellosis or cholera are suspected, or when the pathogen is known, in order to prevent contagion16. However, in daily clinical practice, when the result of the stool culture is reported, the patient is already recovering or the treatment is delayed. Knowing the susceptibility of the bacteria causing a syndrome to the antimicrobial agents is important in 2 aspects: one is the early therapeutic approach, out of empirical necessity, and the other is the epidemiologic surveillance of bacterial resistance that is useful for taking measures to prevent it.
The empirical use of antimicrobials can be inefficient due to the emergence of bacterial resistance. Therefore the possible therapeutic regimens should be frequently updated, taking the pattern of regional resistance into account17–20. Some clinical studies indicate that quinolones are superior to other antibiotics or to placebo in the empirical treatment of adult patients with diarrhea17,20–22. Other antimicrobial drugs, such as ampicillin (AMP), trimethoprim-sulfamethoxazole (T-S), chloramphenicol (CHL), furazolidone (FUR), and non-absorbable antibiotics, such as neomycin and recently rifaximin (RIF), have been used in children, in whom quinolones are not indicated, and also in adults17,23–25. Nevertheless, recent data on the bacterial susceptibility to these antimicrobial agents is not available in Mexico. RIF is a semisynthetic antibiotic derived from broad spectrum rifamycin S that inhibits the synthesis of bacterial RNA, is not absorbable when taken orally, and reaches a very high concentration in the intestinal lumen (∼8,000μg/g of feces). It has excellent bactericide activity on enteropathogenic microorganisms and does not cause important alterations in the gut microbiota26.
The aim of this study was to investigate the susceptibility of the acute gastroenteritis-causing bacteria to rifaximin and the most widely used antibiotics in the treatment of gastrointestinal infections in Mexico.
MethodsBacterial isolationsBacterial strains obtained from the stool samples of 1,000 patients diagnosed with gastroenteritis or acute diarrhea were analyzed in 10 hospital laboratories in Mexico City that attend to hospitalized patients and outpatients. The strains were conserved and frozen before carrying out the biochemical identification, serology, and the antimicrobial susceptibility test in milk broth and soy broth with glycerol at −70°C;27 the initial primary isolations from the stool samples were carried out at each particular laboratory, and the following culture media were used: MacConkey Agar, Sorbitol-MacConkey Agar, Salmonella Shigella Agar, XLD Agar, Campylobacter Agar, Yersinia Agar, TCBS Agar, Brilliant Green Agar, and Tetrathionate Broth28.
The isolates were biochemically identified in each laboratory using AutoScan 4, Walkaway (MicroScan), or Vitek 2 (Biomeriux) manual and automated processes and systems29–31 with an acceptance probability >95% in the identification; the typing and serologic identification in determined bacterial species, such as E. coli, Salmonella, and Shigella, were then performed using the Bio-Rad32, Phadebact33, Oxoid34, Sanofi-Pasteur35, “O” Beli36, and Probac37 specific antisera and agglutination or coagglutination reagents. The detected serogroups and serotypes are shown in Table 1.
Bacteria isolated from 1,000 patients diagnosed with acute gastroenteritis and their serotypes.
| Bacteria | Number | Serotypes involved |
|---|---|---|
| Enteropathogenic E. coli group A,B, and C | 531 | O127:B8, O111:B4, O55:B5, O26:B6 and other serotypes |
| O119:B4, O128:B12, O124:B17, O86:B7, O126:b16 and other serotypes | ||
| O142:B86, O119:K90, O124:B17, O86:B7, O126:B16 and other serotypes that should correspond to: O128:K73, O44:K74, O18:K77, O20:K61, and O20:K84 | ||
| Enterotoxigenic (LT) E. coli | 54 | Only thermolabile (TL) toxin-producing serotypes |
| Enterohemorrhagic E. coli | 18 | Only O:157 serotype |
| Shigella dysenteriae | 54 | All the serotypes that are agglutinable with the specific antiserum |
| Shigella flexneri | 24 | All the serotypes agglutinable with the specific antiserum |
| Shigella boydii | 27 | All the serotypes that are agglutinable with the specific antiserum |
| Shigella sonnei | 15 | All the serotypes that are agglutinable with the specific antiserum |
| Salmonella Group A | 24 | Most likely species: S.paratyphi |
| Salmonella Group B | 21 | Most likely species: S.typhimurium |
| Salmonella Group C1 | 24 | Most likely species: S.choleraesuis |
| Salmonella Group C2 | 24 | Most likely species: S.newport |
| Salmonella Group D | 24 | Most likely species: S.enteritidis |
| Vibrio spp. | 20 | – |
| Yersinia enterocolitica | 20 | – |
| Campylobacter jejuni | 20 | – |
| Pleisomonas shigeloides | 20 | – |
| Aeromonas | 80 | – |
Antimicrobial susceptibility testing (AST) was done through the agar diffusion method (CHL, T-S, neomycin [NEO], FUR, fosfomycin [FOS], AMP, and ciprofloxacin [CIP]), and by microdilution (RIF) following the recommendations of the Clinical & Laboratory Standards Institute38 and the American Society for Microbiology39. The concentrations of the antibiotics assayed and AST conditions were: AMP 10μg/ml, T-S 1.25/23.75μg/ml, CHL 30μg/ml, CIP 5μg/ml, FUR 100μg/ml, NEO 30μg/ml, FOS 50μg/ml. RIF was tested at 100μg/ml and the strains that were not susceptible at this concentration were successively exposed to concentrations of 200μg/ml, 400μg/ml, and 800μg/ml. Susceptibility to RIF was considered at a minimal inhibitory concentration (MIC) of MIC100, whereas it was MIC90 for the other antibiotics.
Statistical analysisBacterial susceptibility to RIF was compared with the susceptibility to the other antimicrobial agents with the Z test and statistical significance was set at a p<0.05. The Statistica 8.0 and Stata 11 statistical software were employed.
ResultsThe stool samples from 511 men and 489 women were analyzed. Sixty-five percent of the participants were above 20 years of age. The isolated bacteria were E. coli 603 (EPEC 531, ETEC 54, EHEC 18), Shigella 120 (dysenteriae, flexneri, boydii, sonnei), Salmonella 117 (paratyphi, typhimurium, choleraesuis, newport, enteritidis), Vibrio spp. 20, Y. enterocolitica 20, C. jejuni 20, P. shigeloides 20, and Aeromonas spp. 80.
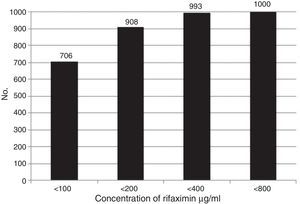
Overall susceptibility of the bacteria to the different concentrations of RIF (MIC100) are shown in Figure 1. The results of accumulated susceptibility patterns in all the strains assayed were at <100μg/ml: 70.6%; at <200μg/ml: 90.8%; at <400μg/ml: 99.3%; and at <800μg/ml: 100.0%. Table 2 shows the susceptibility of each group of bacteria to the different RIF concentrations. More than 99% of the strains of Shigella, Salmonella, Yersinia, Campylobacter, Vibrio, Pleisiomonas, and Aeromonas were susceptible to concentrations <100 or <200μg/ml, whereas 11-15% of the E. coli required <400 or even <800μg/ml.
Overall accumulated susceptibility of the isolated bacteria to rifaximin. In the 1,000 strains, RIF was tested at a concentration of 100μg/ml. The bacteria that were not susceptible at that concentration were successively exposed to concentrations of 200μg/ml, 400μg/ml, and 800μg/ml. The accumulated susceptible strains were 706 (<100), 908 (<200), 993 (<400), and 1,000 (<800).
Susceptibility of the bacteria to different concentrations of rifaximin.
| Bacteria | Number | <100μg/ml | <200μg/ml | <400μg/ml | <800μg/ml |
|---|---|---|---|---|---|
| EPEC | 531 | 58.95 | 25.61 | 14.69 | 0.75 |
| ETEC | 54 | 79.63 | 7.41 | 7.41 | 5.56 |
| EHEC | 18 | 72.22 | 16.67 | 11.11 | – |
| Shigella | 120 | 90.00 | 10.00 | – | – |
| Salmonella | 117 | 67.52 | 31.62 | 0.85 | – |
| Yersinia | 20 | 80.00 | 20.00 | – | – |
| Campylobacter | 20 | 70.00 | 30.00 | – | – |
| Vibrio | 20 | 100.00 | – | – | – |
| Plesiomonas | 20 | 100.00 | – | – | – |
| Aeromonas | 80 | 100.00 | – | – | – |
| All | 1000 | 70.6 | 20.2 | 8.5 | 0.7 |
Results expressed in % of susceptible strains.
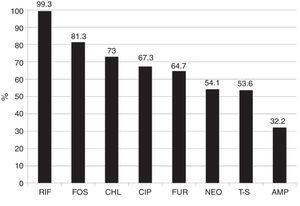
Overall susceptibility of all the bacterial species to each antibiotic was: RIF (< 400μg/ml) 99.3% and (< 800μg/ml) 100%, FOS 81.3%, CHL 73.0%, CIP 67.3%, FUR 64.7%, NEO 54.1%, T-S 53.6%, and AMP 32.2%. Overall susceptibility to RIF with <400 and <800μg/ml was superior (p <0.001) to the overall susceptibility to each of the 8 drugs studied (fig. 2). This was also true in the majority of the cases in each bacterial group alone.
Overall susceptibility of the 1,000 isolated bacterial strains to the different antibiotics.
Rifaximin vs the other antimicrobials p <0.0001.
AMP: ampicillin; CHL: chloramphenicol; CIP: ciprofloxacin; FOS: fosfomycin; FUR: furazolidone; NEO: neomycin; RIF: rifaximin (<400μg/ml); T-S: trimethoprim-sulfamethoxazole.
Table 3 shows the susceptibility of each bacterial group to the antibiotics tested.
Susceptibility of each group of bacteria to the antimicrobials tested.
| Group of bacteria | FOS | CHL | CIP | FUR | NEO | T-S | AMP |
|---|---|---|---|---|---|---|---|
| EPEC | 7.1 | 66.6 | 51.9 | 66.4 | 48.5 | 49.1 | 27.6 |
| ETEC | 75.9 | 88.8 | 50.0 | 77.7 | 53.7 | 50.0 | 29.6 |
| EHEC | 55.5 | 83.3 | 100 | 50.0 | 61.1 | 16.6 | 16.6 |
| Shigella | 68.3 | 82.5 | 97.5 | 47.5 | 46.6 | 27.5 | 48.3 |
| Salmonella | 77.8 | 64.1 | 89.7 | 64.1 | 52.1 | 74.4 | 50.4 |
| Yersinia | 90.0 | 80.0 | 90.0 | 80.0 | 30.0 | 2.5 | 100 |
| Campylobacter | 80.0 | 95.0 | 30.0 | 100 | 100 | 30.0 | 45.0 |
| Vibrio | 90.0 | 80.0 | 100 | 90.0 | 70.0 | 90.0 | 55.0 |
| Plesiomonas | 100 | 95.0 | 80.0 | 85.0 | 90.0 | 90.0 | 20.0 |
| Aeromonas | 67.5 | 86.0 | 87.5 | 50.0 | 80.0 | 78.7 | 20.0 |
| All | 81.3 | 73.0 | 67.3 | 64–7 | 54.1 | 53.6 | 32.2 |
Results expressed in % of susceptible strains.
As can be seen, a large number of strains show a very high proportion of bacterial resistance to NEO, T-S, and AMP, but Yersinia continues to be very susceptible to AMP (100%), Vibrio to T-S (90%), and Campylobacter to NEO.
DiscussionThe prevalence and incidence of the bacteria that cause gastroenteritis worldwide vary according to the type of population studied, the geographic location of the study, the season of the year in which the microbiologic diagnosis is made, and other clinical and sociodemographic characteristics of the patients studied6,40–42. Even though ours was not a prevalence study, the strains were collected throughout the entire year of 2013 and therefore the seasonal variations were not influential. The frequency of the strains received in the clinical analysis laboratories was reflected and the dominating ones were E. coli, Shigella, Salmonella, and Aeromonas. These data coincide with other reports in Mexico23,43,44.
Standards for defining susceptibility or bacterial resistance have been published for the majority of antibiotics38. Nevertheless, there are no clinical cut-off points for rifamycins against enteropathogens, although a value of ≤ 32μg/ml of rifaximin was applied by some researchers in traveler's diarrhea45–47. Bacterial susceptibility to an antibiotic implies that the isolations are inhibited by the usually achievable concentration of the antimicrobial agent at the infection site when the recommended dose is used. Fecal concentrations of rifaximin have been reported of up to 8,000μg/g (mean 7,961μg/g) after 3 days of treatment at the recommended dose of 800mg/day, and there were still mean fecal concentrations of 3,266μg/g 3 days after the end of the therapy48. In our study, we evaluated the susceptibility to rifaximin at 4 concentrations: <100, <200, <400, and <800μg/ml. The general susceptibility observed at these concentrations implies a certain bacterial resistance to rifaximin at concentrations of <100μg/ml that was overcome at higher concentrations, but always much below the achievable values in the intestinal lumen. The cut-off points for considering bacteria to be resistant to rifaximin have been established at 32, 128, or even 256μg/ml46–49, but, as observed in our study, the bacteria resistant to lower concentrations of rifaximin were not resistant if the concentration of the antibiotic was increased in vitro. Finally, 99.3% were susceptible at concentrations <400μg/ml and 100% at concentrations <800μg/ml, which are 10-20 times lower than the mean concentration achieved in vivo with the recommended treatment.
The concentrations in the intestinal lumen, or fecal concentrations, of the other antimicrobial agents tested in the present study are not known, so the internationally recommended concentrations were used for the susceptibility analysis. In general, the bacteria were more susceptible to rifaximin than to the other antimicrobial agents tested in our analysis. Recent studies with enteropathogen isolates from travelers coming from Latin America and Asia also found a greater proportion of bacteria susceptible to rifaximin than to other antimicrobial agents. It was striking that the C. jejuni strains from Asia had a variable resistance to rifaximin, whereas those from Mexico and Guatemala were 100% susceptible46,47. In our study, the C. jejuni strains were 100% susceptible at concentrations of <100-200μg/ml. Several strains of E. coli required rifaximin concentrations greater than 100μg/ml, concurring with other observations on bacteria coming from Mexico50.
Rifaximin has a broad spectrum of susceptibility that includes anaerobic bacteria26,49. Bacterial resistance is very low and is not easily induced51. In addition, Ouyang–Latimer et al. compared the susceptibility of enteropathogenic bacteria obtained in 2006-2008 with those obtained 10 years earlier and found no increase in the MIC90 levels for rifaximin in any of the organisms analyzed46. In fact, the normal intestinal bacteria that became resistant to 100μg/ml of rifaximin after 5 days of treatment spontaneously disappeared from the stools within a few weeks52.
The low proportion of bacteria that are susceptible to AMP, T-S, neomycin, FUR, and CIP, from 32.2 to 67.3% in that order, coincides with studies conducted in other regions of the world53–56 and can be explained by the induction of bacterial resistance due to the widespread use or abuse of those antimicrobial agents. In this respect, it is striking that CHL, which conserves an acceptable general susceptibility of 73%, has been restricted, after its widespread use in Mexico, to treating typhoid fever for the last 30 years. And comparatively, in our study we introduced the test of susceptibility to FOS. This antibiotic is used very little in Mexico, and mainly in urinary infections. The general susceptibility of FOS was greater than that of the other antimicrobial agents, just below rifaximin.
Neomycin is a poorly absorbed antibiotic that is widely used in Mexico and does not require a prescription. According to our data, it is clear that AMP, T-S, neomycin, and FUR would not be good therapeutic options. RIF has been successfully used for treating acute infectious diarrhea in children and adults, as well as for traveler's diarrhea, with excellent tolerance57–60. RIF would appear to be the best option, given that it has a high therapeutic index, combining high efficaciousness with a low frequency of adverse effects26. Adverse effects are frequently attributed to other antibiotics, especially to beta-lactams and T-S mainly due to allergies, and the sulfonamides and CIP are known to be neurotoxic61.
Treatment with antimicrobial agents is a valuable tool in the control of several gastrointestinal infections, given that the length of time and intensity of the disease is reduced, potentially severe complications are prevented, and disease transmission is decreased. Unfortunately, for several decades, enteropathogenic bacteria strains have been selected that are resistant to the commonly used antimicrobial agents and to those that once were considered first choice62. The selection and dissemination of antimicrobial resistance among the different bacterial species that cause gastroenteritis is a growing health problem that complicates the therapeutic management of the severe cases. Studies conducted in many parts of the world have revealed an important increase in antimicrobial-resistant bacteria in many infectious diseases, including gastroenteritis63,64. To reduce the appearance of antimicrobial-resistant bacterial strains, it would seem prudent to avoid the indiscriminate use of antibiotics in cattle, periodically determine local bacterial resistance patterns, reduce the easy access to antimicrobial drugs (for example: prevent self-medication with antimicrobials), and strengthen the medical education on this topic.
Study limitations. The stool cultures were obtained from 10 laboratories that sent positive stool cultures with enteropathogen bacteria from patients diagnosed with acute gastroenteritis or acute diarrhea. Therefore, it was not possible to obtain the clinical data, such as the length of time of symptom progression, stool characteristics, the symptoms accompanying the diarrhea, and the intensity or severity of the clinical symptoms that would enable their correlation with the genus and species of the bacterial isolates. The stool cultures were from Mexico City and do not necessarily reflect those from the rest of the country.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for this study.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureThis study was financially supported by Alfa Wassermann S.A. de C.V.
Conflict of interestAlberto Frati is presently the medical director of Alfa Wassermann S.A. de C.V., which produces rifaximin. He participated in the planning of the project and manuscript revision, but did not intervene in any phase of the study, the enumeration of the results, or the statistical analysis. The other authors declare that there is no conflict of interest.
Please cite this article as: Novoa-Farías O, Frati-Munari AC, Peredo MA, Flores-Juárez S, Novoa-García O, Galicia-Tapia J, et al. Susceptibilidad de las bacterias aisladas de infecciones gastrointestinales agudas a la rifaximina y otros agentes antimicrobianos en México. Revista de Gastroenterología de México. 2016;81:3–10.
See related content at doi: http://dx.doi.org/10.1016/j.rgmx.2016.01.001, Remes Troche JM. Reflexiones sobre la resistencia a antibióticos y qué hacer al respecto. Rev Gastroenterol Méx. 2016;81(1):1–2.