Introduction
Gastrointestinal fistulas are infrequent, and can develop as a complication of any gastrointestinal surgery, trauma, malignant disease, radiotherapy or by iatrogenic mechanisms.1-3 Gastrointestinal leaks or fistulas represent a feared surgical complication, since they are related to high morbidity rates4 and seldom improve with medical treatment,5,6 thus, most of the times, patients need to undergo surgery in order to fix the anatomic defect.5,6
Endoscopic interventions, such as the application of tissue glue4,7-10 or using temporary metallic stents2,11,12 have been reported as a feasible and effective method to treat gastrointestinal leaks, and avoid surgical treatment. 1,2,11,13
The largest available series reports on the use of partially covered nytinol self-expanding metal stents (SEMS) as an effective treatment in 21 cases that developed upper gastrointestinal fistulas following laparoscopic bariatric surgery. The use of SEMS appears to be effective in sealing fistulas because they bypass the leak and act as barrier to fluids; they also develop tissue hyperplasia at both ends of the stent, preventing its migration and increasing its water proofing, however, removal of SEMS after a long period of being in place becomes difficult and requires different techniques to successfully retrieve them.11
Self-expanding polyflex stents (SEPS) have been successfully used to treat different esophageal conditions including malignancies, perforations and leaks.14,15
In this paper, we report the results of a case series of upper gastrointestinal fistulae following gastrointestinal surgery that were managed with SEPS.
Material and methods
Medical records of 5 patients (2 female and 3 male, ages ranging from 29 to 65 years old) referred to our endoscopy unit to treat a gastrointestinal fistula that resulted from gastrointestinal surgery were retrospectively reviewed. Cases are summarized in Table 1.
All patients underwent esophagogastroduodenoscopy (EGD) and SEPS placement. Fistula closure was assessed by direct endoscopic vision and radiologic examination.
Case presentation
Case 1: A 29 y/o female presented 5 years later after esophageal surgical myotomy for achalasia, with dysphagia secondary to distal esophageal stenosis. Hydrostatic dilation (CRE widewired balloon dilator; Boston Scientific, Natick MA) was attempted but was unsuccessful. The patient underwent another surgical myotomy that was complicated with a distal esophageal fistula that was recognized one week after the procedure. An EGD was performed and a polyflex stent was placed at the fistula site; the patient was able to resume oral feedings the day after the stent placement. Nine months later, the patient was asymptomatic and the fistula had closed; an abdominal X-ray showed that the stent migrated into the stomach. The stent was retrieved uneven-tfully using standard biopsy forceps. The patient was discharged and remains asymptomatic.
Case 2: A 65 y/o male presented with 2 months of progressive dysphagia due to upper esophageal stenosis and an esophageal leak, five years after the subsequent resection of a laryngeal cancer and radiotherapy. A PEG tube was placed for nutritional support, hydrostatic dilation (CRE widewired balloon dilator; Boston Scientific, Natick MA) of the stenotic portion of the esophagus was performed uneventfully and a polyflex stent was placed at the site of the fistula. Four months after the procedure, the patient had food contents oozing throughout the fistula. An EGD was performed showing distal stent migration to the stomach. The stent was removed with polipectomy loop and a new stent was placed. Four months later the stent remained in place and was removed easily using a foreign body forceps. The patient remains asymptomatic
Case 3: A 60 y/o female presented to the endos-copy unit with a gastrocutaneous fistula 3 months after undergoing gastric bypass for morbid obesity. The patient underwent an EGD that showed a fistulous tract at the anastomotic site. A self-expandable plastic stent was placed at the anastomotic site covering the fistulous orifice completely. One month after the procedure, the cutaneous site of the fistulae had closed. An EGD and x-ray showed no evidence of the fistulous tract. The plastic stent remained in place and was retired easily using a foreign body forceps. The patient remains asymptomatic.
Case 4: A 42 y/o male with nausea, postpran-dial vomit and a cutaneous fistulous tract was referred to the endoscopy unit 2 months after undergoing gastric bypass surgery. An EGD was performed showing a stenosis at the gastrojejunal anastomosis and a fistulous tract proximal to the anastomotic site. A hydrostatic balloon dilation (CRE widewired balloon dilator; Boston Scientific, Natick MA) was performed at the anastomotic site and a plastic stent was placed covering the fistulous orifice. The patient was discharged, but one week later, the fistula output reappeared. An X ray showed stent migration to the stomach. The stent was removed with standard biopsy forceps and a new SEPS was successfully placed. Prior to stent replacement, cyanoacrilate and fibrin were applied in the fistula. Two days later, a control X ray showed that the stent migrated again into the stomach. The EGD showed that the fistulous tract persisted. The stent was removed with a polipectomy loop and it was replaced with a new stent; easily with a standard biopsy forceps. One month later, an EGD showed that the stent migrated to the efferent loop, so it was removed with a polipectomy loop and another stent was replaced; prior to the placement, fibrin was applied in the fistulous tract. One week later, there was no evidence of the cutaneous fistula orifice. An EGD was performed showing that the stent had migrated again to the stomach but there was no evidence of the fistulous tract. The stent was withdrawn easily with standard biopsy forceps and the patient was discharged. The patient is currently asymptomatic.
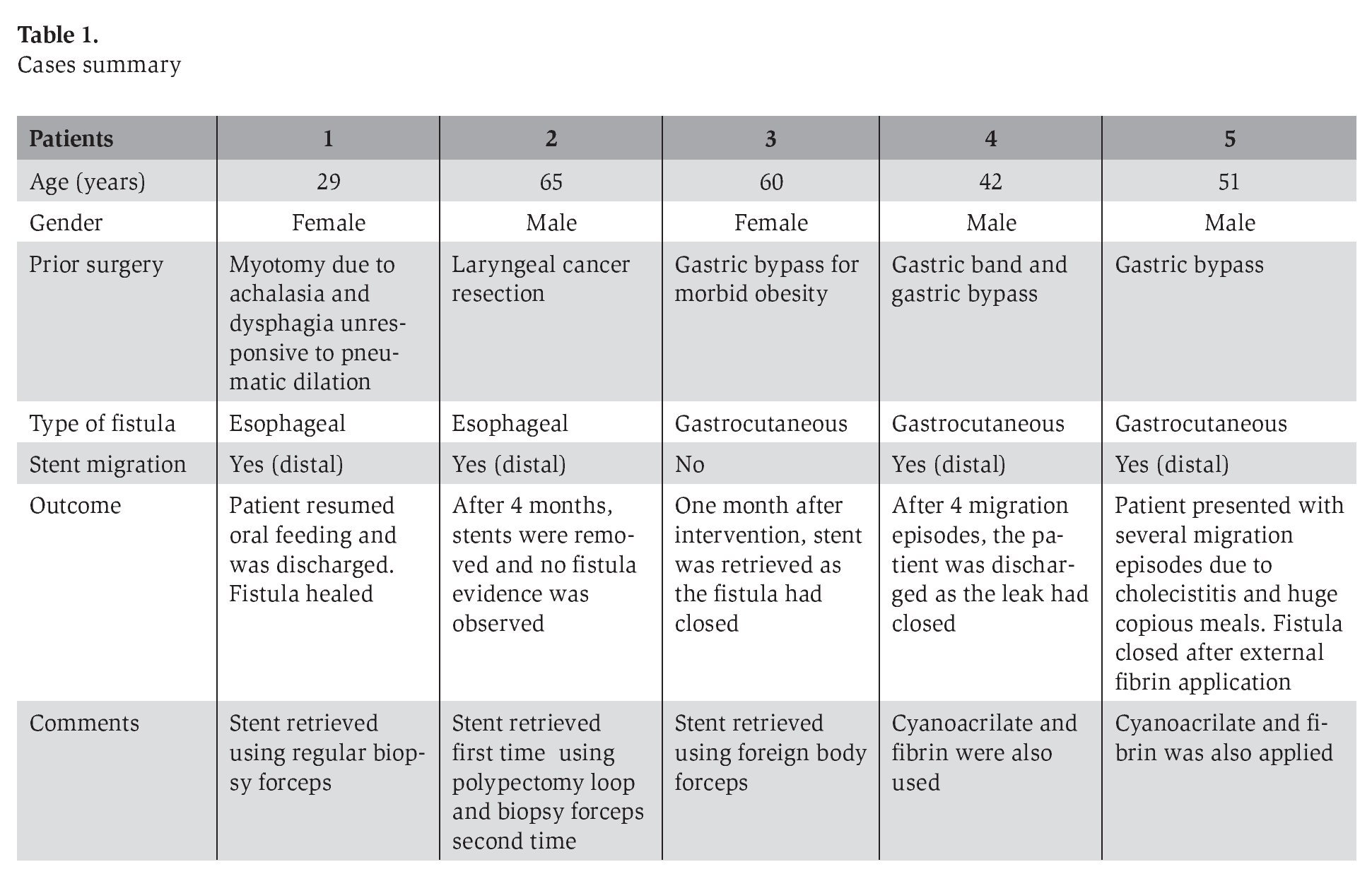
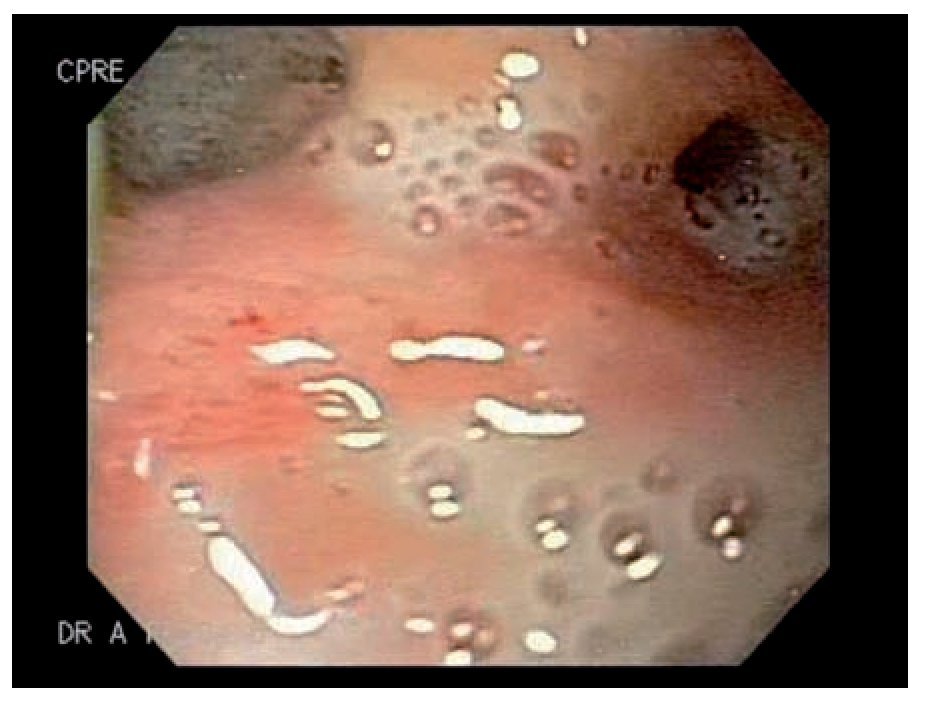
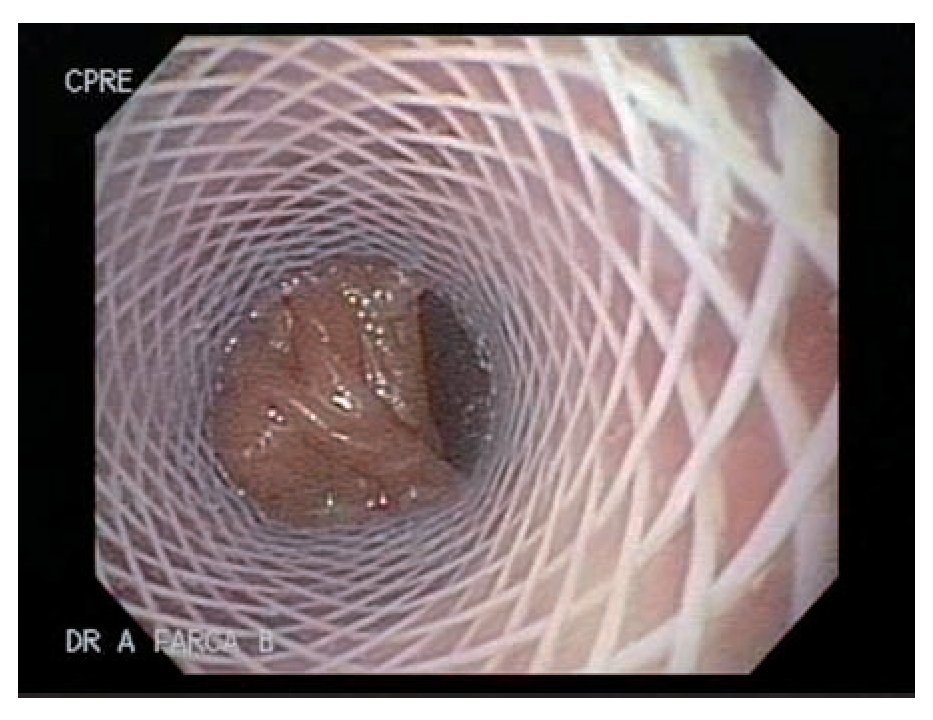
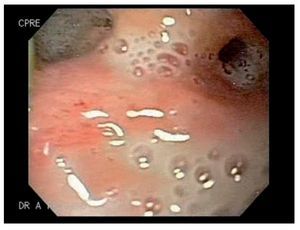
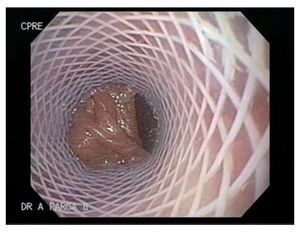
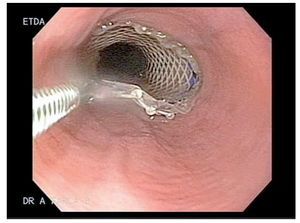
Case 5: A 51 y/o male developed a gastrocutaneous leak 3 months after gastric bypass. An EGD showed narrowing at the gastrojejunal anastomotic site. An EGD with hydrostatic balloon dilation (CRE widewired balloon dilator; Boston Scientific, Natick MA) was performed and a 4 mm. fistulous orifice was identified at the anastomotic site (Figure 1). One week later a 9 cm polyflex stent was placed due to an increased fistula output (Figure 2). Two weeks later, the patient developed cholecystitis and after an episode of emesis the stent migrated into the stomach. The stent was easily removed with foreign body forceps and a new stent was successfully placed (Figure 3). One week later, the patient had persistent nausea. An EGD showed that the stent migrated into the stomach, so it was removed by using a standard biospy forceps and replaced. Prior to placement, cyanoacrilate was applied. The stent migrated 2 more times to the efferent loop and into the stomach (1 week and a month later, respectively) after copious meal intake and emesis. Each time it was retrieved and replaced successfully. After 1 week, the patient underwent surgery as the fistulous output persisted. Four months after surgery the fistula re-opened. Fibrin was applied and the leak closed.
Figure 1. Fistulous opening at the anastomotic site
Figure 2. SEPS placed and covering the fistulous opening
Figure 3. SEPS being replaced above the fistulous opening, using standard biopsy forceps
Overall, SEPS were left in place for a median of 90 days (range: 30-279 days). The SEPS migrated in 80% of the cases, but were successfully and easily replaced. No complications related to SEPS placement, replacement or withdrawal was recorded. Fistulas closed in 60% of the cases when SEPS were the only treatment used, in 2 cases, SEPS failed to achieve fistula closure as a single therapy; thus, in one case cyanoacrliate was applied, leading to later resolution of the fistula and in other case, application of fibrin was the only therapy that closed the fistula.
Discussion
Treatment options for upper digestive tract fistulas, mainly for larger or chronic leakages have been divided into repair techniques that use clips, sealants or suturing devices and diversion techniques that use stents.4,9,16 The rationale for using either SEMS or SEPS in gastrointestinal fistulas is that most of the patients with this condition are weak, have numerous comorbidities and comprise a high group with surgical risk.
In this retrospective series we demonstrated that endotherapy using SEPS is a feasible, safe and effective therapeutic option, with an overall success rate of 60%, in sealing upper gastrointestinal tract fistulas, when used as single therapy; however the success rate might be overrated considering the limited number of cases in our series although similar outcomes have been previously reported.14,17
A downside in our series, is that in 4 (80%) cases the SEPS migrated and needed to be replaced, something that seldom occurs with SEMS; however, the latter ones are more difficult to remove and most of the use of SEPS is needed to induce pressure necrosis of the underlying SEMS hyperplasia. It has also been reported that nearly 50% of fistulas treated with SEMS, needed to be replaced 2, to 3 times during follow-up.17
When comparing SEMS with SEPS, the latter present some practical advantages for their use: they are easily removed regardless the time they are left in place, less expensive, and although they have a higher chance to migrate, they are easily replaced. On the other hand SEMS are more expensive, and if they are left in place more than 6 weeks (something common in the treatment of gastrointestinal fistulas) they are more difficult to extract and usually need the use of SEPS to aid in their withdrawal.11,15,17,18
If either SEMS or SEPS are the most effective and cost-effective ones sealing gastrointestinal fistulas, is yet to be clarified. It is our belief that the use of either SEMS or SEPS in the management of gastrointestinal fistulas has proved to be effective and reliable. According to the available reports, SEPS seem to be better suited when fistulas are associated to strictures (since this might prevent the SEPS to migrate) meanwhile SEMS are more indicated in malignant and benign non stricture related fistulas, however we lack of clear indications for their use.
As shown in case 4 and 5, the concomitant use of synthetic seals as cyanoacrilate or fibrin with plastic stent placement could be a useful tool and surgical attempt to close gastrointestinal fistulas and which clinical scenario (i.e. location, size, output, associated stricture) including complication rates and cost effectiveness. Clear clinical indications other than personal preferences for either SEMS or SEPS use, are needed.
Although our series is small and therefore lacks of statistical power to advise change in management, the results resemble those previously reported in larger series. Comparative and larger randomized controlled studies should evaluate if endotherapy could be performed prior to any surgical attempt to close gastrointestinal fistulas and which clinical scenarios (i.e. location, size, output, stricture association of fistulas) will benefit the most from the use of SEMS or SEPS including complication rate and cost effectiveness. Clear clinical indications other than personal preferences for either SEMS or SEPS use, are needed.
Correspondence author: Mario Peláez-Luna, MD. Dr. Balmis 148. CP 06726, Mexico City, Mexico. Phone: (52) 55 5623-2673. Fax (52) 55 5623-2669.
E-mail: mariopl@prodigy.net.mx
Received: April 30th, 2008 Accepted on: April 16th, 2009