¿ Introduction
Pelvic floor dysfunction often results from one or more disorders that affect the pelvic floor. These conditions are extremely common and affect up to 25% of the population. They cause significant comorbidity, affect quality of life and lead to psychological distress and work absenteeism. A working knowledge of these conditions and of the structure and function of the pelvic floor will enable a practicing gastroenterologist to better understand these disorders and hopefully better manage them.
The pelvic floor involves several muscles that are housed within the pelvic bones. It serves three primary functions, i.e., defecation, micturition and sexual function. Recent advances in the evaluation and management of disorders that affect the pelvic floor function will be discussed. These disorders include functional problems such as dyssynergic defecation and structural problems such as rectal prolapse, rectal mucosal intussusception, solitary rectal ulcer syndrome, rectocele, enterocele, or descending perineum syndrome (Table 1).
¿ Pelvic Floor Anatomy and Normal Physiology
The pelvic floor consists of superficial and deep muscle layers that interlace and envelope the rectum, the bladder and the uterus. Superficial muscle layers such as the internal and external anal sphincters, the perineal body and the transverse perinei muscles are relevant to bowel function.1 The deep pelvic muscles, also known as levator ani, consist of the pubococcygeus, the ileococcygeus and the puborectalis muscles.1 It is the traction of the puborectalis muscle that maintains the anorectal angulation and creates a mechanical barrier for stool flow from the rectum.2 Neurological innervations, primarily from the pudendal nerve, as well as from sympathetic and parasympathetic nerves, facilitate the normal function of the pelvic floor.
During normal defecation, the anal sphincters and the puborectalis muscle relax, which allows the anorectal angle to widen and the perineum to descend. Simultaneously, the voluntary effort of bearing down increases the intra-abdominal pressure, together with the contraction of the rectum and puborectalis muscle. These complex and mixed voluntary and involuntary movements facilitate the development of a stripping wave which moves the stool from the rectum and relaxes the pelvic floor muscles and the anus, resulting in stool evacuation.
¿ Structural Disorders of Defecation
Rectal prolapse, rectal mucosal intussusception, solitary rectal ulcer syndrome (SRUS), rectocele, enterocele and the descending perineum syndrome (DPS) constitute the most common structural pelvic floor disorders that affect defecation. Because these disorders predominantly affect women, a multidisciplinary team consisting of a gynecolo-gist or an urogynecologist, a gastroenterologist and a colorectal surgeon, will be most helpful in optimizing diagnosis and management.
¿ Rectal Prolapse
Definition, Pathogenesis, and Symptomatology. A rectal prolapse develops when all layers of the rectal wall protrude through the anus often associated with defecation. This should be differentiated from a rectal mucosal intussusception, where only the rectal mucosa protrudes within the confines of the rectum/anal canal. The incidence of rectal prolapse has been reported to be as high as 2.5/100 000 and has a high prevalence in elderly women.3
Constipation with years of straining weakens the pelvic floor muscles, damaging the pudendal nerve in the process. Besides constipation, aging and obstetric injury also contribute to pudendal neuropathy.3 The pudendal neuropathy leads to weakness of the anal sphincters. With the dilated anal sphincters offering no counter-acting forces, the rectal wall will easily protrude through the anus.
Most patients present with a rectal protrusion through the anus and/or passage of bloody stool or mucus. Fecal incontinence is seen in 20-100% of patients and constipation in 70%.3,4 On inspection, the rectum is edematous and sometimes friable with an ulcerated mucosa. Rectal prolapse is associated with other defecation disorders such as dyssynergic defecation and intussusceptions.3 Depending on the degree of protrusion, either up to the anal verge and/or inability to push back the prolapse, it is graded into four types, as follows: grade 1= up to the anal verge; grade 2= prolapse outside the anus but reduces spontaneously; grade 3=prolapses outside the anus but can be manually reduced; and grade 4= prolapse cannot be reduced manually.
Diagnosis
The diagnosis is made by a careful perineal examination while asking the patient to strain as if to defecate. If no abnormality is detected, it can be useful to have the patient strain in the lavatory. Although defecography will demonstrate the prolapse through the anus, this test is not essential if perineal examination has already exposed the abnormality. However, defecography may also reveal an obtuse anorectal angle and other anatomical defects such as rectocele, sigmoidocele, or enterocele.3 These findings suggest weakness of the pelvic floor and may help the surgeon to better plan a surgical intervention. Magnetic resonance (MR) defecography may provide additional details, especially of adjacent pelvic floor structures and co-existing problems.5
Management
Among the structural anorectal disorders, rectal prolapse has the clearest indication for surgery. There are currently two accepted approaches: trans-abdominal and perineal. The trans-abdominal approach involves mobilization of the rectum, with or without anterior resection of the rectum followed by fixation of the rectum to the promontory (rectopexy).3 Since patients with rectal prolapse usually have associated enteroceles and/or genital prolapse, the trans-abdominal approach offers the advantage of obliterating the pouch of Douglas.6
However, since patients with rectal prolapse are elderly and not suitable surgical candidates, perineal approaches have been considered in these cases. Among the perineal techniques, the Delorme's procedure or perineal proctosigmoidectomy (Altemeier) has been commonly recommended.3 The Delorme's procedure consists of stripping the mucosa of the prolapsed rectum, plicating the remaining muscular layer and anastomosing the remaining mucosa together.3 In contrast, proctosigmoidectomy involves resecting the prolapsed rectum from below, leaving at least 1 cm of rectum close to the dentate line and then performing a coloanal anastomosis.3 Lately, advancement in laparoscopy has allowed wider use of the abdominal approach in the elderly population. A recent study found that open and laparoscopic rectopexy have comparable outcomes in terms of recurrence, morbidity and length of hospital stay.7
¿ Rectal Mucosal Intussusception
Definition, Pathogenesis, and Symptomatology. Rectal mucosal intussusception is also known as "occult rectal prolapse" or "internal procidentia." Here, the rectal mucosa prolapses but the full thickness of the rectal wall does not protrude through the anus. Rectal intussusception itself is not pathologic since 50-60% of healthy subjects may demonstrate this phenomenon on defecography.3,8 In symptomatic patients, the rectal intussusception is larger when compared to asymptomatic counterparts.8 Furthermore, rectal intussusception rarely leads to a full-thickness rectal prolapse and is considered by many as a consequence of untreated dyssynergic defecation.3
Most patients with rectal intussusception complain of a feeling of incomplete evacuation. Patients usually have other structural abnormalities such as rectocele, SRUS, pudendal neuropathy or sphincter damage that may cause more symptoms such as soiling, bloody discharge, and/ or fecal incontinence.3,9
Diagnosis
Rectal examination detects only a third of patients with rectal intussusception, making defecography the standard test for establishing this diagnosis.3 However, rectal mucosal folds can sometimes be misread as rectal intussusception.3 Recently, dynamic three-dimensional computed tomography (CT), three-dimensional dynamic anorectal ultrasonography and dynamic magnetic resonance imaging (MRI) have shown promise as alternati-ve imaging options.10-12
Management
The role of surgery in rectal intussusception is controversial at best.
Patients are usually started on fiber-enriched diet and laxatives, followed by biofeedback therapy to correct the underlying dyssynergia. Several studies have shown favorable results with this approach.3 Some studies suggest avoiding surgery altogether because of the following: (1) rectopexy was found to correct incontinence but made constipation symptoms worse; (2) only a few patients improve after surgery; and (3) some patients have poor prognosis after surgery, especially those with preoperative diarrhea, fecal incontinence, DPS and proximal intussusception.13 Surgery should only be performed in patients with large intussusception with refractory symptoms and in whom all conservative and behavioral approaches have failed over several years.
Recently, a panel of experts recommended stapled transanal rectal resection (STARR) as a novel approach to restoring normal anatomy in patients with large symptomatic rectal intussusception and rectocele after failing conservative therapy.14 In the procedure, the rectovaginal septum is strengthened and the redundant tissues from the anterior and posterior rectal walls are resected.14 Complications from STARR include: bleeding, hematoma, urinary retention, severe pain, dehiscence, infection, fecal incontinence, recto-vaginal fistula, necrotizing fasciitis, peritonitis, and fecal urgency.14
¿ Rectocele
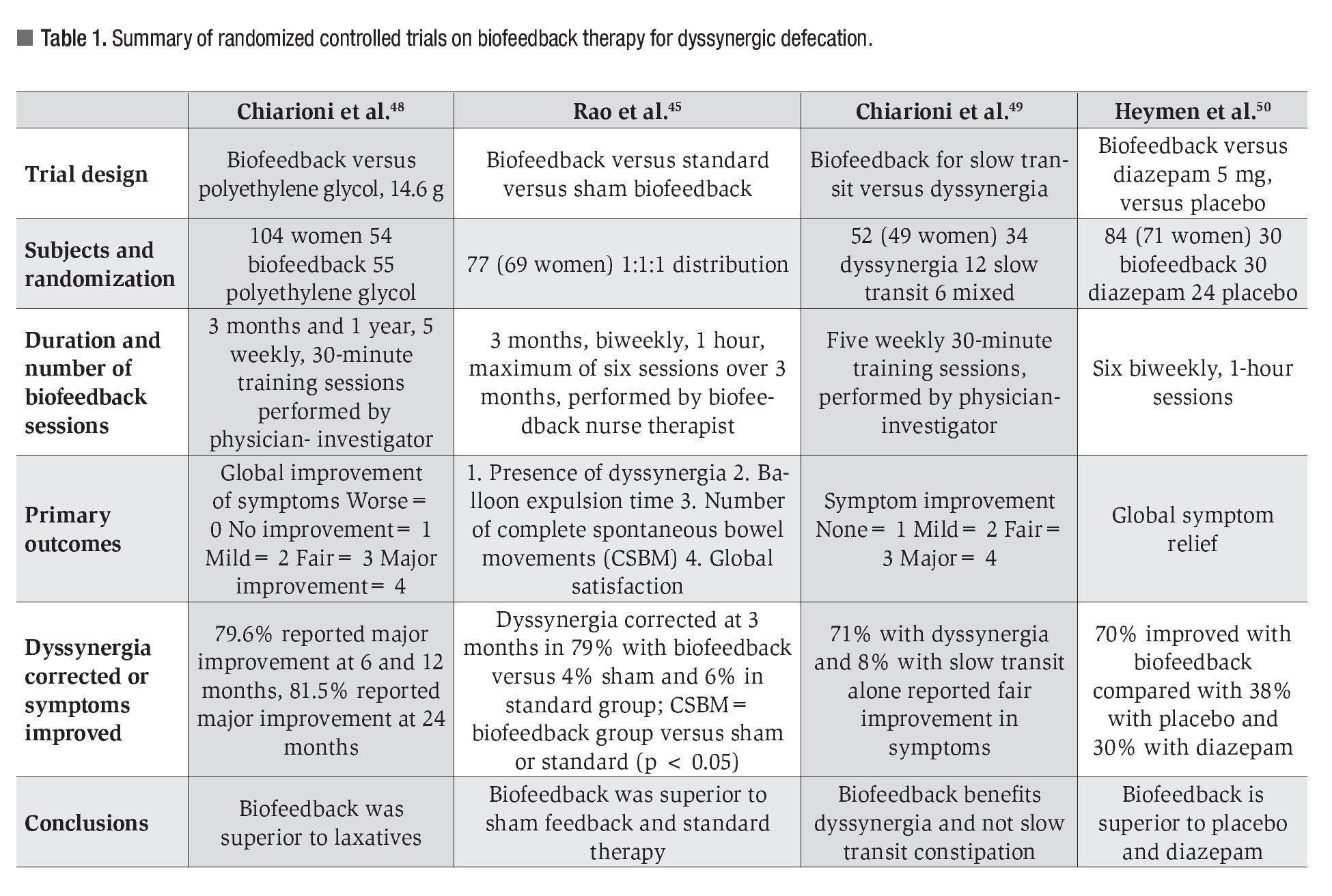
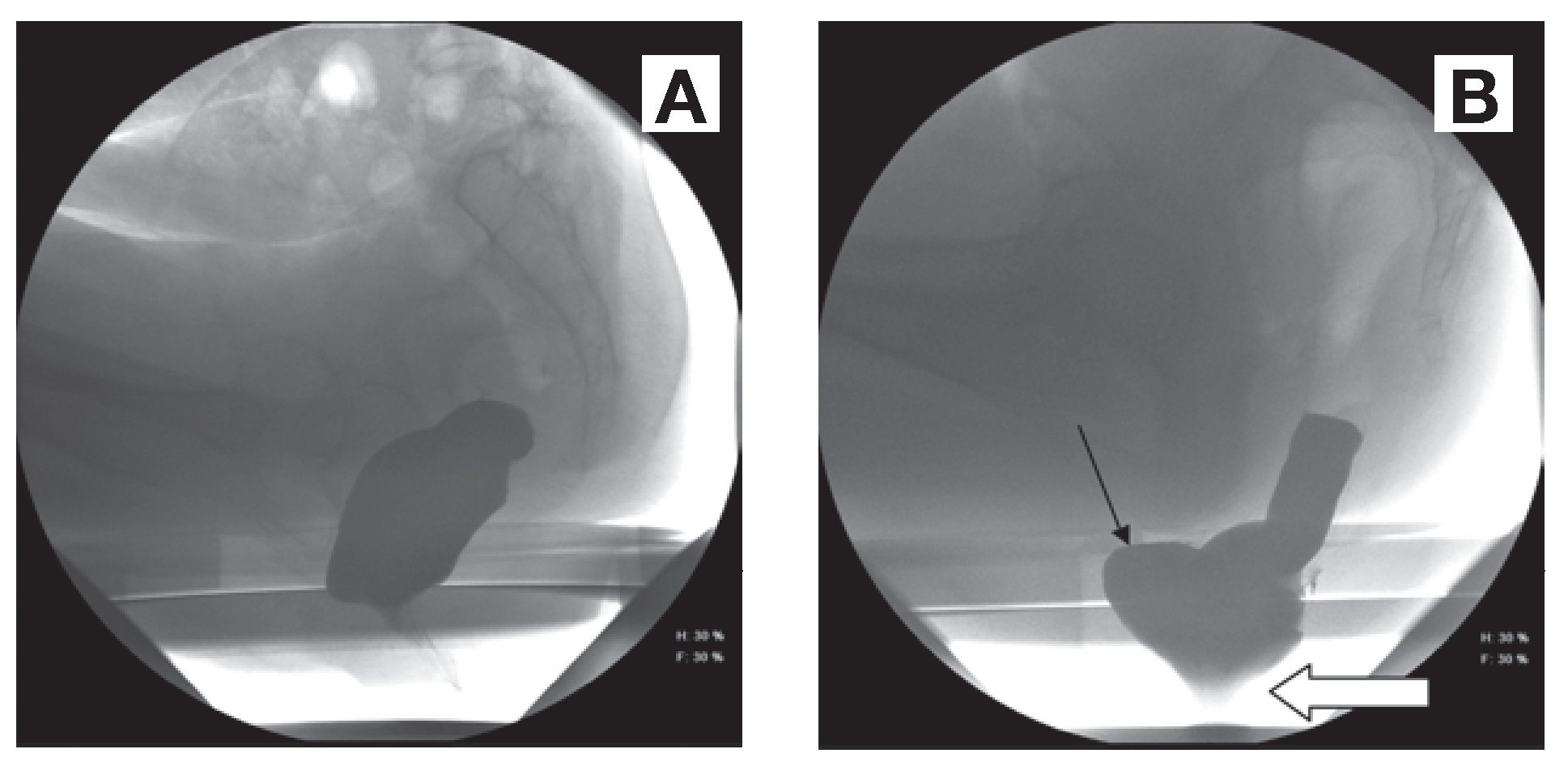
Definition, Pathogenesis, and Symptomatology. Rectocele is a protrusion of the rectal wall, usually anterior and towards the vagina (Figure 1). However, posterior rectocele may also occur. Using a subset the Pelvic Floor Distress Inventory questionnaire, the Pelvic Floor Disorders Network (PFDN) found the prevalence of pelvic floor disorders in the United States to be 23.7%.15
¿ Figure 1. Images on defecography at rest (A) and during straining (B); Figure A reveals an anorectal angle of 123 degrees and the anus at rest. Figure B shows a perineal descent of 5.2 cm from the pubococcygeal line (white arrow), showing descending perineum syndrome (DPS) together with an anterior rectocele (black arrow).
Rectocele is believed to be caused by weakness of the pelvic floor and the rectovaginal septum. Obstetric injury from multiple vaginal deliveries is a common cause.3,16 Rectoceles are also seen in postmenopausal women, especially if they are nulliparous.3,16 Childbirth and/or excessive and inappropriate straining weaken the vaginal septum and pelvic floor muscles, and damage the pudendal nerve. This process lowers the pelvic floor and makes the vaginal outlet bigger. Since the vagina cannot close on straining, this creates a pressure gradient between the rectum (high pelvic pressure) and the vagina (low atmospheric pressure).3 The high abdominal pressure generated during chronic straining pushes the rectal wall towards the vaginal, producing a rectocele. Hysterectomy, dyssynergic defecation and intussusception are often associated with rectoceles;3 whether they are a cause or an effect is unclear.
Aside from pelvic floor weakness and a rectocele, patients usually have non-specific symptoms. They can present with pelvic pain, feeling of prolapse, dragging, constant pressure, backache, constipation, fecal soiling, urologic complaints and dyspareunia.3 Vaginal splinting that consists of digital support can sometimes help with either initiating or completing defecation.
Diagnosis
Diagnosis is apparent on inspection and digital examination. Anterior bulging of the rectal wall is more evident on straining. Digital rectal palpation may also confirm the diagnosis. In the last decade, the Pelvic Organ Prolapse Quantification (POP-Q) has been used to grade pelvic organ pro-lapse.17 It takes 6 different measurements of the female genital area, grading genital prolapse into 4 standard stages.
Defecography can detect rectocele, document its size and identify concomitant retention;3 however, its usefulness in clinical work-up is limited. Eighty percent of asymptomatic controls may show small rectoceles.3,8 Hence, clinical correlation is of paramount importance and other etiologies should be searched for before labeling dyssynergia as due to rectocele. Only rectoceles larger than 3 cm or with significant contrast retention are considered clinically significant.3 MRI can also provide good visualization of the rectocele and pelvic floor movements.3 There are no physiologic findings that are specific for a rectocele.3 However, dyssynergic pattern of defecation on anorectal manometry was seen with increasing frequency in patients with rectocele (60%) compared to those without (24%).18
Management
First-line treatments are a fiber-rich diet, bulk-forming agents and laxatives to ensure easy defecation.
For both rectocele and rectal mucosal intussusception, surgical treatment leads to frequent recurrences.3 Recent studies have shown the importance of detecting "occult diseases" like psychological disorders (anxiety and depression), functional disorders (dyssynergic defecation, rectal hyposensation, pudendal neuropathy) and organic disorders (urogynecological disorders, solitary rectal ulcer).19 In these patients, conservative therapy in the form of psychological counseling and behavioral modification, including posture and education of the defecation process and biofeedback therapy are recommended before considering surgery.19
However, surgery is considered appropriate in patients with a large rectocele (> 4 cm) that causes a bulge in the vagina or a feeling of prolapse and after medical therapy has failed. The success rate of surgery is reportedly 75%, with the vaginal approach associated with a lower recurrence rate than the transanal approach.3 However, complications such as constipation and sexual dysfunction are very common.3 In patients with dyssynergic defecation associated with rectocele, the transanal approach is the ideal option.3 However, the transanal approach may impair anal sphincter pressures.3 The STARR procedure, described in the section about rectal intussusception, has also been performed with some success in patients with rectoceles.14 Although reducing a rectocele size may improve rectal emptying, it may not help relieve overall symptoms.
¿ Solitary Rectal Ulcer Syndrome
Definition, Pathogenesis, and Symptomatology.
The annual incidence of SRUS is 1 to 3.6 per 100 000; 80% of the patients are less than 50 years of age.3,20 The gender distribution is either equal or slightly higher in females.20 SRUS is often associated with rectal mucosal intussusception as revealed by defecography.3 Mucosal ulceration is believed to result from forceful straining against an immobile or a non-relaxing pelvic floor together with trauma from digital manipulations as well as from ischemic necrosis of the prolapsing rectal mucosa. Spontaneous resolution of SRUS during pregnancy suggests that hormones may play a role in its pathogenesis.21
Patients usually present with rectal bleeding, rectal pain, mucus discharge, straining and tenesmus, and a feeling of incomplete evacuation. About 55% of patients present with constipation, 20-40% with diarrhea, and 25% are asymptomatic.3 A quarter of the patients are misdiagnosed and treated as inflammatory bowel disease.3 Excessive rectal bleeding has been previously reported.3 Obsessive-compulsive disorder has been described in patients with SRUS.3
Diagnosis
Sigmoidoscopy with rectal biopsy is the gold standard for diagnosis. On endoscopic inspection, the ulcer in SRUS is usually a small, shallow lesion with a white slough or hyperemic mucosa on the anterior wall of the rectum.3 The lesions can be multiple (30%), ulcerated (57%), polypoid (25%), and show patches of hyperemic mucosa (18%).3 Histologically, the mucosa appears elongated with distorted glands at the base, with an edematous fibroblast-rich lamina propria and a thickened inner circular muscular layer.22 When the glands migrate down to the submucosa, bleeding may occur. Pathognomonic features in SRUS include: decussation of the two muscularis layers, nodular induration of the inner layer, and grouping of outer longitudinal layers into bundles.22 Biopsy is needed to differentiate SRUS from ulcers that are caused by non-steroidal anti-inflammatory agents, malignancy and sometimes rectal endometriosis.3
Defecography may show concomitant morphological abnormalities like intussusception in 45-80% of subjects.3 However, it is not essential for establishing a diagnosis. Barium enema may show nodularity of the rectal mucosa, thickening of the rectal folds, strictures, polypoid lesions or ulcerations.3
Management
Behavioral advice remains the mainstay of treatment. The most important step is to advice the patient not to strain and to stop digital rectal maneuvers. These recommendations may improve symptoms in 67% of patients.3,23 Variable response rates (19-70%) have been seen with a high-fiber diet, suggesting that although diet helps, it is often not enough.3,24 Local treatment with topical steroids and sulphasalazine has been generally ineffective.3 Although there is limited data, sucral-fate enemas and topical human fibrin sealant have been tried.3
To date, biofeedback therapy appears to be the most effective therapy; however, randomized clinical trials (RCTs) are lacking. Seventy-five percent (12/16) of patients with SRUS had symptomatic improvement after biofeedback therapy and 31% (5/16) had ulcer resolution on sigmoidoscopy.25 Mucosal flow improved in patients who felt subjectively better after biofeedback. Another study prospectively followed 11 patients with refractory SRUS treated with biofeedback;26 82% (9/11) had dyssynergic defecation. After biofeedback therapy, straining effort and stool frequency improved. Five patients discontinued digital maneuvers and bleeding ceased in 56%. Ulcer healing was reported in 10 patients: 4 had complete healing, 2 had > 50% healing and 4 had < 50% healing.
Constipation is a known complication of surgery and since it is the underlying mechanism for this problem, surgery should only be performed in highly selected cases.3 Surgical options include rectopexy, anterior rectopexy and Delorme's procedure.3
¿ Enterocele
Definition, Pathogenesis, and Symptomatology. In enterocele, a peritoneum-lined sac (usually the small bowel) herniates into the space between the vagina and rectum and produces symptoms of obstructed defecation. The epidemiology of enterocele is currently unknown.3 However, there is a high correlation between women who have undergone hysterectomy and developed enteroceles.27 Usually, patients with enteroceles have concomitant anorectal morphological abnormalities like rectocele, excessive perineal descent and rectal mucosal intussusception.3,27
Patients usually present with pelvic pain or heaviness (on standing), difficult defecation, and a feeling of incomplete evacuation. Upon lying down, both pain and heaviness disappear as the bowel repositions itself into the abdominal cavity.
Diagnosis
Enterocele is best diagnosed with defecography. Since enterocele usually involves herniated small bowel, it is crucial that the small bowel is opacified with oral contrast.3 Other imaging techniques like dynamic three-dimensional CT, anorectal ultrasound, and dynamic MRI may facilitate its diagnosis.10-12
Management
Improving defecation mechanics and biofeedback therapy are the initial treatments of choice for patients with enteroceles.3 However, some patients might benefit from surgery, especially those having persistent pelvic pain and constant urge to defecate. When surgery is indicated, the goal is to obliterate the pouch of Douglas.3 Abdominal approach (either open or laparoscopic) and transvaginal approach have been tried in the past.3
Post-surgical outcomes remain poor. In a study of 25 patients with enterocele, 5 underwent surgery; only 1 had complete resolution and 4 showed a partial relief of symptoms.27 Similarly, despite adequate surgical correction of the defect, symptoms of obstructed defecation persisted in 75% of the patients being followed for up to 85 months.28
Descending Perineum Syndrome. DPS (Figure 1), also known as "excessive perineal descent," is characterized by ballooning of the perineum several centimeters below the bony outlet of the pelvis during a straining effort.29 Clinically, patients present with painful defecation, impaired defecation, excessive straining, a sense of incomplete evacuation, or fecal incontinence.29,30 Hysterectomy is a known predisposing factor in the development of DPS.29 DPS is indicative of pelvic floor weakness and is usually associated with other anorectal disorders such as rectal prolapse, rectocele, SRUS or enterocele.30
According to Parks' and colleagues criteria, DPS can be diagnosed on physical examination.29 The patient is asked to lie on a left lateral position and the anal canal is examined for rapid descent of more than 3 cm during a straining effort.29 However, since this method is not physiologic (given the patient is on a lateral decubitus position), DPS is preferably diagnosed using defecography.31 Diagnostic criteria for DPS on defecography include: (1) over 4 cm perineal descent at rest; and (2) during a maximal push effort, the perineal descent exceeds 3 cm from the value measured at rest.31 When available, dynamic MR imaging is also a good diagnostic option.5,31
There is no surgical option for isolated DPS. Treatment consists mainly of correcting excessive straining, using an artificial device (a polycarbo-nate plate placed under the toilet seat to support the perineum during defecation), and biofeedback therapy.31 Although controlled trials are lacking, success with biofeedback therapy is around 50%.31
¿ Dyssynergic Defecation
Definition, Pathogenesis, and Symptomatology. Dyssynergic defecation has been described in the literature with a plethora of other terms such as anismus, pelvic floor dyssynergia, obstructive defecation, paradoxical puborectalis contraction, pelvic outlet obstruction, and spastic pelvic floor syndrome.32 However, the term dyssynergic defecation is now recommended by most experts.33 In tertiary care setting, the prevalence of dyssynergic defecation among patients with chronic constipation is about 40-50%.34
It was suggested that paradoxical anal contraction or involuntary anal spasm during defecation was the main cause of this problem. However, simple myectomy or chemo-paralysis of the anal sphincter with botulinum toxin led to a minimal improvement in patients with dyssynergia, suggesting that other mechanisms may play a role.32 Later studies have found that a failure of recto-anal coordination, manifesting as either impaired rectal contraction, paradoxical anal contraction, or inadequate anal relaxation were primarily responsible for this condition.35
In a large series of well characterized patients with dyssynergic defecation, the most common symptoms were excessive straining (84%), feeling of incomplete evacuation (76%), abdominal bloating (74%), passage of hard stools (65%), and less than 3 bowel movements per week (62%).34 Although these patients would not readily admit to their doctors, digital maneuvers such as disimpaction or vaginal splinting to relieve symptoms were frequently encountered (approximately in 50% of the cases).32
Diagnosis of Dyssynergic Defecation
Medical History and Physical Examination
Diagnosis of dyssynergic defecation starts with a good history, physical examination and a digital rectal exam. The rectal exam should include a careful inspection of the perianal skin for excoriation, fissures, skin tags or hemorrhoids. Perianal sensation and anocutaneous reflex can be examined by stroking the perianal skin with a cotton bud. When asked to bear down on digital exam, patients with dyssynergia may demonstrate paradoxical contraction of the external sphincter and puborectalis muscle, ineffective perineal descent, impaired push effort or a combination.
Laboratory Tests, Imaging and Physiologic Tests
Underlying metabolic and pathologic disorders should first be excluded by using routine laboratory blood tests, colonoscopy and/or barium enema. Physiologic tests are essential for the diagnosis of dyssynergic defecation, especially anorectal manometry (ARM) and balloon expulsion test.32 ARM provides information on rectal and anal pressures at rest and during simulated defecation.32 It also tests for rectal compliance, rectal sensation, and recto-anal reflexes.32 For example, it can exclude Hirschsprung's disease. However, caution should be exercised when diagnosing dyssynergic defecation based on ARM alone. An ARM performed with the patient lying in the left lateral position can sometimes show a dyssynergic pattern, but when the patient is seated on a commode and particularly when provided with a sensation of stooling, most normal subjects exhibit a normal defecation pattern.36-38 Similarly, many fail to expel the artificial stool in the lying position but can expel the balloon easily in a sitting position.36-38 Thus, dyssynergia is best diagnosed by asking the subject to evacuate in a sitting position after evoking a sensation of stooling.36-38 Four patterns of dyssynergia have been described with ARM.32 In addition, the defecation index (DI), which is calculated from ARM-generated pressures, can serve as a simple and useful quantitative measure of recto-anal coordination during defecation.32,37
Rome III Criteria for Diagnosis of Dyssynergic Defecation
Dyssynergic defecation is diagnosed when a patient fulfills Rome III criteria for functional constipation and exhibits a dyssynergic pattern of defecation on ARM or electromyogram (EMG) and demonstrates another quantifiable measure of abnormal defecation such as an abnormal balloon expulsion test, a prolonged delay in colonic transit or an incomplete evacuation during defecography.33
The balloon expulsion test provides information on the patient's ability to expel an artificial stool. A normal individual can pass within 1 minute a 50-ml water-filled balloon placed in the rectum. Although this test has an 80-90% specificity for dyssynergia,32,39 its sensitivity is only 50%.32
Since slow transit constipation may coexist with dyssynergic defecation, a colonic transit study may prove useful. A colonic transit is considered abnormal if more than five markers (20% markers retained) are present on plain abdominal film taken 5 days (120 hours) after ingestion of a Sitzmarker® capsule containing 24 radio-opaque markers.
Other Imaging Modalities for Dyssynergic Defecation
Several imaging modalities may identify dyssynergic defecation. Defecography is performed by placing barium paste into the patient's rectum. A video fluoroscopic imaging system records the anatomic and functional changes as the patient tries to evacuate the barium. Patients with dyssynergic defecation show poor activation of the levator muscles, prolonged retention of the contrast material, inability to expel the barium or absence of a stripping wave in the rectum.32 Defecography is only considered an adjunct to clinical and manometric assessment of anorectal function. Endoanal and dynamic pelvic MRI (MRI defecography) is the only imaging study that can evaluate pelvic floor anatomy in dynamic motion.32 Recently, 3-dimensional dynamic anorectal ultrasonography (echodefecography) has been tested against standard defecography showing good agreement for detecting rectocele and dyssynergia.12
Management of Dyssynergic Defecation
Standard Treatment and other Medical Options
Standard treatment consists of avoiding constipating medications (i.e. narcotics), increasing fiber and fluid intake, exercise activity, and timed toilet training. A fiber intake of 20 grams per day is recommended. Currently, there is insufficient data to recommend synthetic fiber supplements. Only psyllium, a natural fiber supplement, was given a grade B recommendation both by the American College of Gastroenterology (ACG) Task Force40 and a systematic review.41 Medications that promote bowel movement such as stool softeners (sodium and calcium docusate compounds), stimulant laxatives (senna, bisacodyl, and castor oil), osmotic laxatives (salts of magnesium, phosphate and sulfate, lactulose, sorbitol, mannitol, polyethylene glycol) can all be useful.41 A recent study reported relief of constipation in 52% of patients treated with polyethylene glycol compared to 11% of those receiving placebo.42 Lubiprostone, a chloride channel-2 activator, given at 24 µg twice daily was more effective than placebo in decreasing straining, improving stool frequency, and relieving symptoms of chronic constipation43,44 but whether these medications help dyssynergic defecation is not known.
Biofeedback Therapy
Biofeedback therapy is proven to be effective in patients with dyssynergic defecation.45 The main purpose of biofeedback is to restore the normal pattern of defecation using an instrument-based learning process that is based on "operant conditioning" techniques.46,47 There are two primary goals: (1) correcting the underlying dyssynergia involving the abdominal, rectal and anal muscles; and (2) improving rectal sensory perception in patients with impaired rectal sensation.
Biofeedback consists of improving abdominal push effort and manometry-guided pelvic floor relaxation, followed by simulated defecation training. This procedure involves inserting in the patient's rectum a manometric probe that would capture rectal and anal pressure readings on a monitor, providing the patient instant feedback regarding his performance and helping him to learn quickly. Patients are then taught to coordinate their abdominal push effort showing a rise in intrarectal pressure and synchronized relaxation of the anal sphincter depicted by decreased anal pressure on the monitor. About 10-15 maneuvers are usually attempted in a single session. Next, a balloon in the rectum is distended with 60 cc of air to provide the patient a sensation of rectal fullness or the desire to defecate. Patients are asked to attempt defecation while observing the pressure changes in the monitor. These maneuvers are then repeated 5-10 times. Lastly, an artificial stool (a 50 mL water-filled balloon) is placed in the rectum. The patient is asked to sit on a commode and attempt defecation. The patient is taught to relax the pelvic floor muscles, to correct his posture, and breathing techniques. Gradually, the patient learns to coordinate the defecation maneuver and is able to successfully expel the balloon.
Since about half of patients with dyssynergic defecation have impaired rectal sensation, rectal sensory training is also beneficial. The goal of this training is enhancing sensory perception in order to improve awareness for stooling.47 This is accomplished through repeated inflations and deflations of the rectal balloon, establishing newer thresholds for rectal perceptions in the process.
Depending on patient's needs, the number of biofeedback sessions and the length of each session should be customized. Typically, each biofeedback training session takes an hour. Patients are asked to visit the clinic every 2 weeks and attend an average of 4-6 training sessions. Aside from manometry, biofeedback therapy has been tried using different instruments. EMG biofeedback, balloon defecation training, and home training devices have been described.32
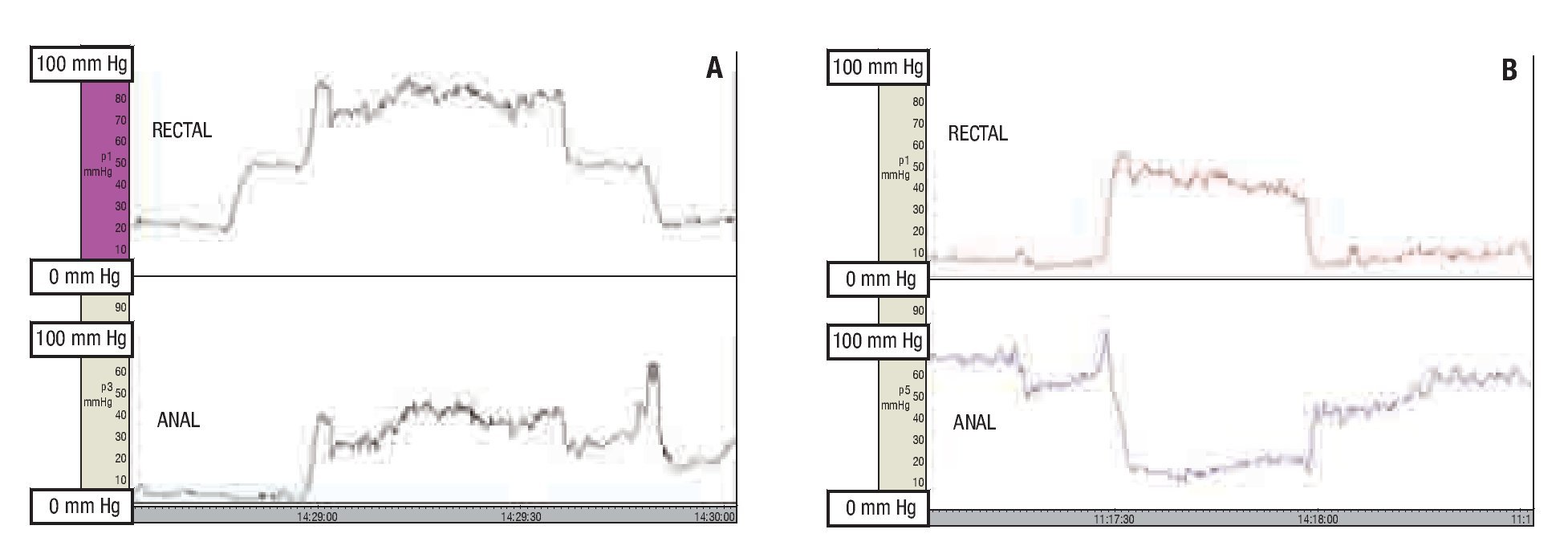
Four randomized clinical trials have evaluated the use of biofeedback in patients with dyssynergic defecation (Table 1).45,48-50 All of these studies showed that biofeedback is superior to laxatives, sham feedback, standard therapy, placebo, and diazepam. Figure 2 shows manometry tracings of a patient with dyssynergic defecation before and after biofeedback therapy. It is notable that the rectal and anal pressures normalized after biofeedback.
¿ Figure 2. Rectal and anal pressure changes during anorectal manometry in a subject with dyssynergic defecation before (A) and after (B) biofeedback therapy.
Despite its proven effectiveness, biofeedback is only offered in a few motility centers in the US. To treat dyssynergic patients in the community, a home-based biofeedback program maybe important. Although there are studies that have demonstrated the feasibility of home training, no controlled trial has compared home training with the standard office biofeedback.32
¿ Conclusions
Pelvic floor disorders that include dyssynergic defecation and anatomical abnormalities of the anorectum are very common, particularly in females. The role of biofeedback is increasingly recognized not only in patients with dyssynergic defecation but also in others with rectal mucosal intussusception, rectocele, SRUS, and enterocele. This underscores the importance of performing physiologic tests such as an anorectal manometry and a balloon expulsion test to properly diagnose dyssynergic defecation. Correcting the underlying pathophysiological dysfunction offers patients better control of symptoms when compared to performing surgical correction of the anatomic defects. Further research is required to define the phenotype of these patients, to examine predisposing factors, and to examine the correlation of symptoms with diagnostic tests and longitudinal follow-up. Most importantly, randomized controlled trials are urgently needed to test current and evolving therapies for these disorders.
Correspondence: Satish S.C. Rao, MD, PhD, FRCP (LON).
The University of Iowa Hospitals & Clinics. Internal Medicine, GI Division. 200 Hawkins
Drive, 4612 JCP. Iowa City, IA 52242.
Telephone: 319 3536 602 Fax: 319 353 6399
E mail:satish-rao@uiowa.edu