Acute colonic pseudo-obstruction (ACPO) is an obstructive phenomenon of functional origin characterized by pain and acute colonic dilatation in the absence of a mechanical cause. It primarily affects patients with several comorbidities, postoperative patients, and/or critically ill patients. The most widely accepted pathophysiologic theory is enteric autonomic dysfunction. Diagnosis must be confirmed through abdominal tomography, ruling out causes of mechanical obstruction and revealing complications, such as ischemia and/or bowel perforation. Treatment is stepped, taking symptom severity, cecal diameter, hemodynamic stability, and/or the presence of complications into account. It is started with conservative measures, and in cases with partial response or refractoriness, there are pharmacologic, endoscopic, and surgical therapeutic options. The aim of this article is to describe four cases of patients diagnosed with ACPO treated at a tertiary care hospital.
La pseudo-obstrucción colónica aguda (ACPO) es un fenómeno obstructivo de origen funcional caracterizado por dolor y dilatación colónica aguda en ausencia de causa mecánica. Afecta principalmente a pacientes con varias comorbilidades, en estado postoperatorio y/o críticamente enfermos. La teoría fisiopatológica más aceptada es la disfunción autonómica entérica. El diagnóstico se debe confirmar con tomografía abdominal que descarte causas de obstrucción mecánicas y revele complicaciones como isquemia y/o perforación intestinal. El tratamiento es escalonado considerando gravedad de síntomas, diámetro cecal, estabilidad hemodinámica y/o presencia de complicaciones, iniciando con medidas conservadoras y para casos con respuesta parcial y/o refractarios, existen opciones terapéuticas farmacológicas, endoscópicas y quirúrgicas. El objetivo de este artículo es describir 4 casos de pacientes atendidos en un hospital de tercer nivel de atención con diagnóstico de ACPO.
Acute colonic pseudo-obstruction (ACPO) is a functional disorder characterized by dysmotility and dilatation of the colon, in the absence of an identifiable mechanical obstruction.1 Its incidence is unknown, and its etiology is not fully defined, but it is recognized as a multifactorial entity. An imbalance in autonomic nervous system regulation that affects the function of the interstitial cells of Cajal, contributing to altered colonic motility, has been proposed.2 The most common risk factors associated with ACPO include prolonged hospitalization, age above 60 years, male sex, the use of medications that have an effect on intestinal motility (opioids and anticholinergics), and hydroelectrolyte disorders (hypokalemia and hypocalcemia), infections, postoperative states, and decompensated chronic diseases, such as hypothyroidism, cardiovascular diseases, and neurodegenerative disorders.3 Clinically, patients may present with acute abdominal pain and distension, nausea, vomiting, constipation, or diarrhea (in up to 40% of cases). Computed tomography (CT) is the diagnostic method of choice, because it enables documenting colonic dilatation –usually of the cecum and ascending colon–, confirming the absence of mechanical obstruction, and ruling out the differential diagnoses.3 Three clinical variants of ACPO have been described: the classic variant; the variant with secretory diarrhea (SD), intestinal loss of potassium, and refractory hypokalemia;4 and a variant associated with hypothyroidism5 (Table 1). Treatment is divided into the conservative approach, with measures of metabolic status support and correction (effective in 77–96% of patients with the classic variant), and pharmacologic (neostigmine) and/or endoscopic decompression (effective in 60–94% of patients with the classic variant). The SD variant tends to have a lower response to conventional strategies and warrants the chronic use of spironolactone. Ischemia and bowel perforation are among the most relevant complications, reported in 3–15% of cases, and are associated with fever, abdominal hyperalgesia, cecal dilatation greater than 12 cm, and clinical symptom persistence for more than six days. The mortality rate is lower than 1% in uncomplicated cases but can reach up to 50% when there are complications.2 The treatment of ACPO has three main goals: to decompress the colon, prevent complications, and reduce the risk of recurrence. In patients with a cecal diameter < 12 cm and no signs of complications, the initial approach is based on correcting the hydroelectrolyte status, suspending drugs that affect motility, managing comorbidities, encouraging walking, and decompressing the colon with a transanal decompression tube (TDT). This conservative strategy has a reported success rate of close to 70%.1 Clinical response is the evacuation of large volumes of gas and stools; radiologic response is a decrease in colonic diameter to <12 cm, seen on imaging studies (thus, radiologic studies every 48 h are part of the follow-up for evaluating disease progression); and sustained response is the resolution of symptoms and dilatation for at least 72 h.4,5 Pharmacologic (neostigmine) or endoscopic decompression is recommended in patients with an initial cecal diameter > 12 cm or with no clinical response after 48−72 h, who continue with no complications,6 due to the increased risk of perforation. Endoscopic decompression is recommended for patients in whom neostigmine is contraindicated or who have no pharmacologic response. There are currently no randomized clinical trials that compare the efficacy of neostigmine versus endoscopic decompression, or that support the use of the latter, but available evidence suggests there are similar safety and success rates comparable to pharmacologic treatment (36–88%).7,8 An additional advantage of endoscopic decompression is that it enables direct evaluation of the colonic mucosa, when looking for ischemic changes.9
Characteristics that distinguish the clinical variants of acute colonic pseudo-obstruction.
| Clinical subtype | Clinical data | Treatment response |
|---|---|---|
| Classic (80−90%) | Acute abdominal pain and distension, nausea, vomiting, constipation (60%). | Effectiveness of conservative treatment (74%), pharmacologic treatment (89%), and endoscopic colonic decompression (82%). Lower mortality rate (1−8%) than the other subtypes. |
| Secretory diarrhea (<5%) | Associated with the presence of pre-existing nephropathy (80%), hypokalemia, metabolic acidosis, and diarrhea, with imaging studies consistent with ACPO. | Effectiveness of conservative treatment (36%), pharmacologic treatment (7%), and endoscopic colonic decompression (50%); Has a higher mortality rate (21%). |
| Requires chronic use of spironolactone. | ||
| Atrophic visceral myopathy (<1%) | Associated with late-onset hypothyroidism; signs of atrophic visceral myopathy (thin wall, atrophic muscularis propria layer with no inflammation or fibrosis) are identified, along with unaffected ganglion cells and myenteric plexus. | Refractory to conventional treatment, shows good response to thyroid hormone replacement. |
ACPO: acute chronic pseudo-obstruction.
The classic neostigmine application route is an intravenous bolus (2 mg in 5 minutes), under cardiac monitoring because of the risk of bradycardia or arrhythmias. Relative contraindications include recent myocardial infarction, bradycardia, kidney failure, peptic ulcer, reactive respiratory disease, and acidosis. Absolute contraindications are bowel obstruction and urinary retention. The reported success rate with this strategy is up to 90%. The mechanism of action of neostigmine is based on increased circulating acetylcholine that potentiates parasympathetic tone and colonic smooth muscle contractility. In cases of partial response (reduced pain, moderate decrease in the cecal diameter with respect to initial size, partial tolerance of oral diet), or in the absence of clinical improvement, the bolus can be repeated in 24 h.3 Retrospective studies have suggested that continuous neostigmine infusion (0.4−0.8 mg/h) can induce an effective clinical response. Compared with bolus, continuous infusion showed a greater effect on reducing the intestinal diameter (73.7% vs 40.5%), albeit with a longer time to initial response (3.5 h vs 1.4 h).10–12 Lastly, more recent evidence has proposed the use of subcutaneous neostigmine administration. In a study that included 182 patients with ACPO, ileus, or refractory constipation, clinical response in a median of 29 h, with no increase of adverse effects, was reported.13,14 Regarding endoscopic decompression, the available international guidelines on the management of ACPO suggest avoiding the use of pre-decompression colonic preparation, but recommend the use of decompression tubes with intermittent suction, flushed with saline solution every 4−6 h, as well as the post-procedure administration of polyethylene glycol (29.5 g dissolved in 500 ml of water, divided into two doses), due to the association with a lower recurrence rate after the decompression.15
Endoscopic percutaneous cecostomy (EPC) is a therapeutic option in patients with refractoriness to the abovementioned strategies, especially in high-risk surgical patients. It can be performed endoscopically or under radiologic guidance. The most frequent complications are localized pain, bleeding, the formation of granulomas, or perforation. Reported technical success rates are above 80%. Even though the studies on its effectiveness are limited and retrospective, international guidelines recognize EPC as part of the therapeutic management of ACPO.6 In the presence of ischemia or bowel perforation, surgical management with subtotal or total colectomy with a protective stoma are indicated. Finally, the use of prokinetics, such as pyridostigmine or prucalopride, and osmotic laxatives, such as polyethylene glycol, may contribute to recurrence prevention.
This case series describes the experience with the diagnosis and treatment of ACPO at a tertiary care hospital, emphasizing the clinical characteristics, risk factors, complications, and therapeutic progression of the patients evaluated. The current literature was also reviewed to contextualize the clinical findings and support the management decisions.
Material and methodsA retrospective review of the clinical records of patients diagnosed with ACPO, within the time frame of March and September 2024, at the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”, was carried out. Clinical, laboratory, imaging, and therapeutic data, as well as clinical outcomes, were documented.
ResultsFour patients diagnosed with ACPO were included in the study. They were all older adults (age range: 77–89 years), with multiple comorbidities. Three cases had clinical resolution with conservative or pharmacologic treatment, and one case required invasive management with EPC.
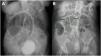
Case 1A 77-year-old woman, with a past medical history of high blood pressure and rheumatoid arthritis in treatment with an immunosuppressant, was admitted to the hospital due to community-acquired pneumonia and pleural effusion. Antimicrobial treatment was started, along with potassium replacement, given that moderate hypokalemia (2.9 mEq/l) was reported in the laboratory work-up. During hospitalization, she developed abdominal distension and constipation. CT showed generalized colonic dilatation (max. 10.1 cm), with no signs of mechanical obstruction (Fig. 1A). Conservative treatment with a TDT was given. Forty-eight hours later, the patient presented with improvement in abdominal distension, she passed gas, and her stools had no signs of inflammation, all of which was congruent with the decrease in the cecal dilatation shown in the control abdominal x-ray, compared with the initial diameter (Fig. 1B). Oral diet was restarted and treatment with polyethylene glycol, 17 g/day every 24 h, was begun to prevent recurrence. However, the patient presented with adult respiratory distress syndrome, causing her death.
Case 2An 89-year-old woman, with a past medical history of high blood pressure, heart failure, diabetes mellitus, and vascular dementia, had presented with two episodes of secretory-type ACPO in the past six months. She sought medical attention for bloating, nausea, pain, and constipation alternating with diarrhea. Moderate hypokalemia (2.5 mEq/l) with no identifiable extraintestinal cause stood out in the laboratory work-up. CT showed colonic dilatation (max. 10 cm) with no obstruction. Conservative treatment was initially indicated. Given the lack of response, an initial dose of 2 mg of neostigmine was administered as a bolus, after which the patient passed gas. A repeat dose was attempted at 24 h, but there was no passage of stools and no significant decrease in the cecal diameter. Due to the recurrent ACPO events, EPC was performed, with favorable clinical progression. The patient was discharged, with pharmacologic management (prucalopride 1 mg every 24 h, polyethylene glycol 17 g every 24 h, and spironolactone 25 mg every 24 h). During follow-up she presented with localized pain as the only complication and continues in follow-up as an outpatient.
Case 3An 86-year-old man, with a past medical history of Parkinson’s disease, high blood pressure, and ischemic heart disease, sought medical attention for nausea, abdominal pain, and three days of acute diarrhea and bloating. Physical examination revealed a painful distended abdomen, with reduced peristaltic sounds. Laboratory work-up showed mild hyponatremia, hypokalemia, and hypomagnesemia. CT identified dilatation of the right colon (max. 8.6 cm), with no signs of obstruction. Conservative treatment with a TDT was given, achieving clinical resolution with no complications. Management was started with prucalopride 0.5 mg every 24 h and polyethylene glycol 17 g every 24 h, maintaining the passage of gas, the absence of abdominal distension, and one bowel movement per day during the 48 h of in-hospital observation. Oropharyngeal dysphagia secondary to Parkinson’s was identified, along with inadequate food intake and hydration. The patient was referred to speech therapy for swallowing rehabilitation. Instructed to adhere to pharmacologic treatment for his comorbidities and maintain prucalopride and polyethylene glycol use, the patient was discharged for outpatient follow-up.
Case 4An 88-year-old man had a past medical history of ischemic heart disease, non-specified arrhythmia, vascular dementia, and a previous episode of ACPO. He sought medical attention for nausea, vomiting, bloating, and acute constipation. Physical examination revealed the patient was stable, with a tympanic abdomen and decreased peristalsis. Laboratory work-up showed moderate hypokalemia (2.7 mEq/l). CT identified generalized colonic dilatation, predominantly in the sigmoid colon (max. 9 cm). Conservative treatment with a TDT was started. At 48 h the distension persisted, with secondary hypokalemia, which in the approach, was consistent with gastrointestinal losses, suggesting the secretory variant of ACPO. Control imaging identified colonic diameter progression (max. 10 cm). Due to his history of arrythmia, the patient was not a candidate for neostigmine use, and management with endoscopic colonic decompression was proposed, but refused by his relatives. Given the absence of ischemia, perforation, and abdominal sepsis, medical treatment was started with prucalopride 1 mg every 24 h, spironolactone 25 mg every 24 h, and oral erythromycin in suspension 250 mg every 8 h for 5 days, resulting in clinical improvement and voluntary discharge.
DiscussionACPO is an underdiagnosed entity that primarily affects older adults with multiple comorbidities. In our series, conservative treatment was effective in three of the four cases, reinforcing its role as first-line treatment in the absence of complications. In the patient with recurrence and the clinical secretory variant – identified by refractory hypokalemia with no apparent secondary cause – it was necessary to implement stepped drug treatment with neostigmine and later perform EPC, with a favorable outcome. Said case strengthens support for the performance of EPC as a useful alternative in patients with refractoriness and those who are high-risk surgical patients. Timely suspicion of the clinical subtypes, such as the secretory variant, enables treatment personalization (for example, chronic use of spironolactone). The experience presented herein reinforces the stepped approach proposed in the literature and provides practical information on the implementation of strategies, according to clinical response and patient context.
ConclusionOur four cases showed that general measures continue to be effective as first-line management in most patients with ACPO. Awareness of the different clinical subtypes enables a more precise therapeutic approach to be carried out. Nevertheless, in patients with recurrence and limited response to conservative and pharmacologic treatment, EPC is a useful therapeutic alternative in selected settings. Its successful implementation depends on local knowledge of the procedure, adequate case selection, the experience of the treating team, and the available multidisciplinary support.
FundingNo funding of any kind was received to carry out this article.
The authors declare that they have no conflict of interest