Acute liver failure is a rare but serious syndrome, with an incidence of approximately 2,000 to 3,000 cases per year in North America. Its pathophysiology and clinical course vary, depending on the cause of the primary liver injury, and can lead to high morbidity and mortality or the need for liver transplantation, despite available therapies. This syndrome involves excessive activation of the immune system, with damage in other organs, contributing to its high mortality rate. The most accepted definition includes liver injury with hepatic encephalopathy and coagulopathy within the past 26 weeks in a patient with no previous liver disease. The main causes are paracetamol poisoning, viral hepatitis, and drug-induced liver injury, among others. Identifying the cause is crucial, given that it influences prognosis and treatment. Survival has improved with supportive measures, intensive therapy, complication prevention, and the use of medications, such as N-acetylcysteine. Liver transplantation is a curative option for nonresponders to medical treatment, but adequate evaluation of transplantation timing is vital for improving results. Factors such as patient age, underlying cause, and severity of organ failure influence the post-transplant outcomes and survival.
La falla hepática aguda es un síndrome poco común pero grave, con una incidencia de aproximadamente 2000 a 3000 casos por año en América del Norte. Su fisiopatología y curso clínico varían según la causa del daño hepático primario, y puede llevar a una alta morbimortalidad o necesidad de trasplante hepático, a pesar de las terapias disponibles. Este síndrome involucra una activación excesiva del sistema inmunológico con daño en otros órganos, lo que contribuye a su alta tasa de mortalidad. La definición más aceptada incluye daño hepático con EH y coagulopatía en las últimas 26 semanas en un paciente sin enfermedad hepática previa. Las principales causas son intoxicación por paracetamol, hepatitis viral, lesión hepática inducida por drogas, entre otras. Es crucial identificar la causa, ya que influye en el pronóstico y tratamiento. La supervivencia ha mejorado con medidas de soporte, terapia intensiva, prevención de complicaciones y el uso de medicamentos como la N-acetilcisteína. El trasplante hepático es una opción curativa para casos no respondedores al tratamiento médico, pero la evaluación adecuada del momento para el trasplante es crucial para mejorar los resultados. Factores como la edad del paciente, la causa subyacente y la gravedad de las fallas orgánicas influyen en los resultados y la supervivencia post-trasplante.
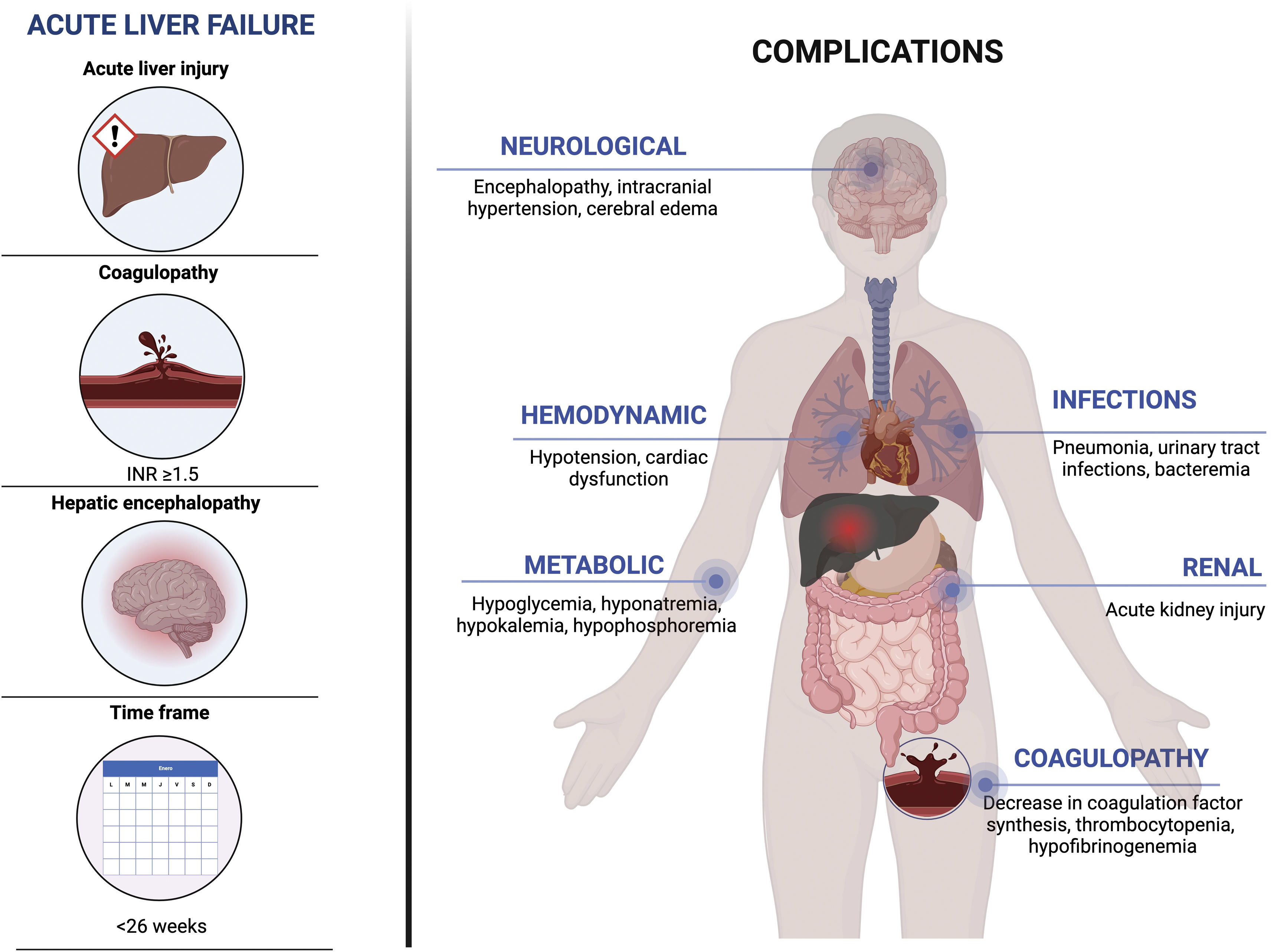
Acute liver failure (ALF) is a complex, multisystemic, unpredictable, and rapidly progressing syndrome caused by numerous etiologies (viral infections, herbal medicine and drugs, autoimmune diseases, genetic and/or environmental factors). Although rare, it has a high death rate. This entity is potentially reversible and is characterized by rapid liver injury (<26 weeks), with the development of hepatic encephalopathy (HE) and coagulopathy (INR>1.5) in an individual with no pre-existing liver disease.1 The annual reported incidence is from 2,000 to 3,000 cases in the United States.2
The probability of spontaneous recovery or transplant-free survival (TFS) is related to etiology and HE grade. At present, the number of patients with TFS has increased, thanks to supportive medical management options, such as the use of N-acetylcysteine (NAC), continuous renal replacement therapy (CRRT), and less need for invasive mechanical ventilation.3–5 Nevertheless, the reported mortality rate ranges from 25 to 30% of patients that get on a liver transplantation waitlist. Nationally, transplant is considered an emergency procedure in patients that meet the criteria, and currently, one and 5-year post-transplant survival is 90 and 80%, respectively, with results becoming comparable to the population undergoing transplant for a chronic liver disease.6,7
A sudden decline in liver function, known as acute-on-chronic liver failure, is often misinterpreted in patients with chronic liver disease. Differentiating other conditions that are not part of the ALF spectrum, such as alcoholic hepatitis, sepsis, extensive liver resection, and liver trauma, is also vital for guaranteeing optimum and timely management.8
Differences between acute liver failure and acute-on-chronic liver failureDistinguishing between ALF and acute decompensation of cirrhosis, or acute-on-chronic liver failure, can be difficult. Cirrhosis of the liver is highly prevalent worldwide and the development of decompensations (HE, variceal bleeding, and ascites) tends to appear at some point in the course of the disease, but determining the history of the chronic liver disease is easy the majority of the time. On the other hand, acute-on-chronic liver failure is characterized by intense systemic inflammation, a close temporal relation to a precipitating event, and the presence of one or multiple organ failures. The presentation of acute-on-chronic liver failure is sudden in a patient with decompensated cirrhosis of the liver and has a high short-term mortality rate (>20% at 28 days). It usually develops in the context of an added insult that causes a systemic inflammatory reaction. The causes can be intrahepatic (e.g., viral, drug-induced, alcohol-induced, etc.) or extrahepatic (e.g., hypoperfusion due to cardiac dysfunction, Budd-Chiari syndrome, etc.).9 It is vitally important to identify the differences between these entities because management changes radically. For example, ALF has a 1A status on the transplant waitlist, whereas acute-on-chronic liver failure is presently given no priority and the patient is placed on the list according to the MELD score.10
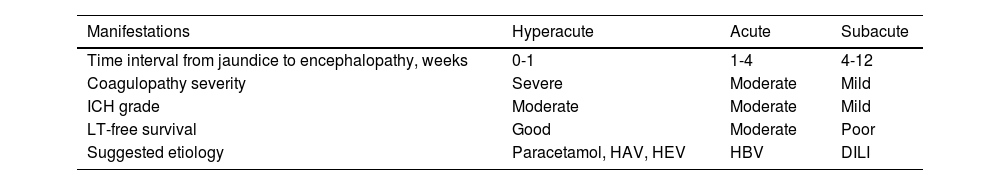
EpidemiologyALF is considered a relatively rare disease, with an annual incidence of 2,000 to 3,000 cases per year.1 It accounts for 4 to 5% of all liver transplants. Causes are diverse and vary according to region and socioeconomic level. In developed countries, paracetamol poisoning predominates in 45 to 55% of cases, with an incidence of 5.5 to 6.2 cases per million per year11 (Table 1). However, in developing countries, paracetamol overdose is less frequent and viral hepatitis (hepatitis A and E) and herbal medicines and other drugs play a fundamental role in the development of ALF.3,12,13 The etiology of the disease cannot be identified in 11 to 15% of adults and 50% of pediatric patients, with a possible 34% increase in developing countries,14 often due to lack of access to adequate laboratory testing.4,15,16 There is not much information on the epidemiology of ALF in Mexico. An increase in incidence and mortality attributable to viral causes of ALF have been seen in the country, but those observations are based on retrospective studies utilizing national registers, making them susceptible to information biases and variations in detection, depending on access to healthcare services.17
Incidence of liver failure and causes
| Country | Incidence | Cause |
|---|---|---|
| Global18 | Viral ALF epidemiology | HAV: (2-20%) |
| HBV: (20-22%) | ||
| HCV: (9%) | ||
| HEV: (32%) | ||
| Countries with high admission ratesa,3 | 5.5-6.2 cases/million inhabitants/year | Main cause: PP |
| Countries with medium-low admission ratesa,3 | Scant information due to lack of tertiary care referral centers | Main causes: indeterminate, viral hepatitis (HEV), herbal medicine, DILI |
| The United States1 | 1case/million inhabitants/year | PP (45.7%), DILI (10%), HBV: (8%) |
| Other causes: associated with pregnancy, Budd-Chiari syndrome, non-hepatotropic viral infections, malignancy (15%) | ||
| Germany19 | 11.3 cases/one million inhabitants/year | DILI (32%), PP: (32%) |
| Indeterminate (24%) | ||
| Viral (21%) | ||
| Kenya3 | 15.7 cases/one million inhabitants/year | Non-specified |
| Tailandia3 | 62.9 cases/one million inhabitants/year | Indeterminate (69.4%) |
| Non-paracetamol DILI (26.2%) |
ALF: acute liver failure; DILI: Drug-induced liver injury; HAV: hepatitis A virus; HBV: hepatitis B virus; HCV: hepatitis C virus; HEV: hepatitis E virus; PP: paracetamol poisoning.
The course of the disease is variable and unpredictable. The mortality rate is 25 to 30% and the main causes of death are sepsis and cerebral edema.20 Despite not having clear statistics, the mortality rate can vary from 26.7 to 84% of cases in developing countries.15 On the other hand, spontaneous recovery can present in 45% of patients, depending on disease etiology.21
Definition and clinical characteristics of acute liver failureDifferent stages are distinguished within the spectrum of the clinical behavior of ALF. The first encompasses acute liver damage or acute liver injury (ALI), a term that refers to liver function test abnormalities,10 with no progression to HE. This condition can progress to ALF or be resolved with no sequelae.
Etiology plays a determining role in disease outcome. For example, spontaneous recovery and TFS, in cases of paracetamol-induced ALF, reach approximately 70%, whereas in hepatitis A virus infection, they reach only 56%, and in complications related to pregnancy, they reach 83%. In contrast, entities such as autoimmune hepatitis, drug-induced liver injury, acute hepatitis due to hepatitis B virus, and indeterminate causes show an unfavorable outcome, with the reported spontaneous recovery rate equal to or less than 35%.21,22
The definition of ALI is not uniformly established. In a prospective study that included 386 patients with the disease, defined according to its etiology (paracetamol-related: INR≥2 and ALT≥10 times the upper limit of normal; non-paracetamol-related: INR≥2, ALT≥10 times the upper limit of normal and bilirubin ≥ 3.0mg/dL), Koch et al. found that progression to an adverse outcome (ALF, death, or liver transplant) was associated with the etiology of ALI (non-paracetamol-related), bilirubin levels (>3mg/dL), INR (>1.7), serum paracetamol levels (>60mg/dL), and the duration of jaundice (>3 days).23
Transition to the ALF stage is characterized by the development of HE that is specified by cognitive decline (deficiencies in attention, reaction time, disorientation, inadequate behavior, somnolence, confusion, and unconsciousness) and neuromuscular function deterioration (bradykinesia, asterixis, dysarthria, ataxia, hyperactive deep tendon reflexes, nystagmus, etc.). Three causes with fulminant manifestations that could be associated with a pre-existing liver disease, such as Wilson’s disease, the reactivation of chronic infection due to hepatitis B virus, and autoimmune hepatitis, must be considered.16,23
The definition of ALF varies worldwide, but in the United States and Europe, the most accepted definition is that of a disease lasting < 26 weeks, in a patient with no pre-existing liver disease or cirrhosis, and associated with any grade of HE or coagulopathy (INR≥1.5).10
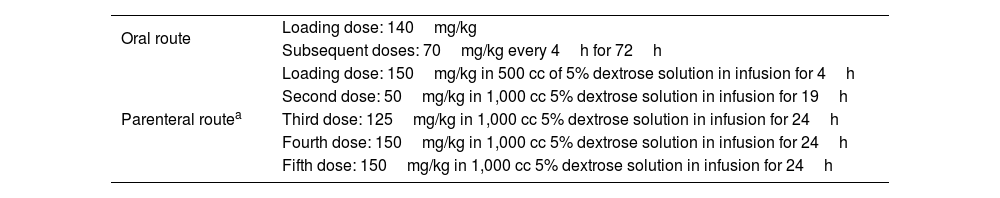
The length of time of disease progression provides information for associating etiology, complications, prognosis, and supportive medical treatment. In 1993, O’Grady et al.6 proposed one of the most accepted systems for describing disease progression and estimating its outcome. This classification is based on the time of the appearance of jaundice and the development of HE, subdivided into 3 groups: hyperacute < 7 days; acute 7-28 days; and subacute 4-12 weeks (Table 2).24 Even though this classification is the most widely used, it appears more logical to group acute and subacute into a single syndrome, because their speed of progression commonly overlaps, whereas patients with hyperacute liver failure have a distinct disease evolution and pattern.1
O’Grady system classification, clinical manifestations, and prognosis in acute liver failure subtypes
| Manifestations | Hyperacute | Acute | Subacute |
|---|---|---|---|
| Time interval from jaundice to encephalopathy, weeks | 0-1 | 1-4 | 4-12 |
| Coagulopathy severity | Severe | Moderate | Mild |
| ICH grade | Moderate | Moderate | Mild |
| LT-free survival | Good | Moderate | Poor |
| Suggested etiology | Paracetamol, HAV, HEV | HBV | DILI |
ALF: acute liver failure; DILI: Drug-induced liver injury; HAV: hepatitis A virus; HBV: hepatitis B virus; HEV: hepatitis E virus; ICH: intracranial hypertension; LT: liver transplantation.
In addition to the O’Grady classification, there are others used worldwide for classifying ALF. An example is the classification proposed by Bernuau et al.25 in France in 1986, which distinguishes between fulminant liver failure (<2 weeks) and subfulminant liver failure (from 2 to 12 weeks). Another classification, presented in 1999 by Tandon et al.,26 under the auspices of the International Association for the Study of the Liver, defines ALF as acute if it develops in fewer than 4 weeks; its subdivisions are hyperacute (fewer than 10 days), fulminant (from 10 to 30 days), and subacute (from 5 to 24 weeks). On the other hand, in 2011 in Japan, Mochida et al.27 proposed a classification that distinguishes between acute (fewer than 10 days) and subacute (from 11 to 56 days) ALF.
Despite the variations in the time intervals and definitions, there is a general consensus that patients that experience a rapid development of HE from symptom onset, also show a greater probability of responding favorably to medical treatment.
Acute liver failure etiologiesSudden and severe liver damage in ALF can have different causes, such as drug toxicity, viral infections, autoimmune disorders, genetic disorders, vascular problems, malignancies, and metabolic alterations (Table 3). To better comprehend the causes of ALF, we can classify them into 2 main categories: those triggered by paracetamol and those not related to paracetamol.
General causes of acute liver failure
| Viral | Hepatitis A, B (±D), C, and E |
| CMV, EBV, herpes virus, HHV-6, parvovirus B19, parainfluenza, and VZV | |
| Drug-induced liver injury (dose-dependent) | Paracetamol |
| Antibiotics/antivirals: amoxicillin-clavulanate, ciprofloxacin, minocycline, dapsone, doxycycline, TMP/SMX, didanosine, ketoconazole, efavirenz, abacavir | |
| Antiepileptics: valproic acid, phenytoin, CBZ | |
| Antituberculosis agents: isoniazid, rifampicin, pyrazinamide. | |
| Antihypertensive: methyldopa, hydralazine, labetalol | |
| NSAIDs: diclofenac, ibuprofen, indomethacin, naproxen | |
| Anesthetics: halothane | |
| Herbal medicine and supplements | |
| Others: amitriptyline, statins, amiodarone, methotrexate | |
| Drug-induced liver injury (synergism) | Ethanol+paracetamol, |
| Barbiturates+paracetamol | |
| Isoniazid+rifampicin | |
| Genetic/metabolic | Wilson’s disease |
| Galactosemia | |
| Hemochromatosis | |
| α1-antitrypsin deficiency | |
| Tyrosinemia | |
| Diseases related to pregnancy | Pregnancy-related fatty liver |
| HELLP syndrome | |
| Preeclampsia/eclampsia | |
| Vascular | Budd-Chiari syndrome |
| Hepatic artery thrombosis | |
| Veno-occlusive disease | |
| Other | Malignancy |
| Reye’s syndrome | |
| Autoimmune hepatitis | |
| Leptospirosis, malaria |
CBZ: carbamazepine; CMV: cytomegalovirus; EBV: Epstein-Barr virus; HHV-6: human herpesvirus 6; NSAIDs: nonsteroidal anti-inflammatory drugs; TMP/SMX: trimethoprim sulfamethoxazole; VZV: varicella zoster virus.
Paracetamol poisoning (PP) is a worldwide problem, especially due to the increased use of narcotics and paracetamol. PP is often considered to be associated with intentional use related to attempted suicide.28 The safe therapeutic dose of paracetamol is up to 4,000mg every 24h. However, a dose higher than 10-15g in a 24h period, generally associated with suicide attempts, or the prolonged use of high doses (>10g) can induce ALI. Situations such as fasting, malnutrition, the concomitant use of alcohol, and advanced age can be predisposing factors to hepatotoxicity.10,29
PP is the main cause of ALF worldwide, but the mortality rate is low, compared with other causes. Factors that are associated with the severity of hepatotoxicity due to paracetamol are the increase in concomitant and chronic alcohol use, fasting, age, and nutritional status, which are factors that reduce the intrahepatic glutathione reserves.24 When PP is suspected, serum measurements of the drug can be useful for risk stratification. Its determination and correlation with the Rumack-Matthew nomogram is useful for guiding therapy and evaluating outcomes. Relevantly, negative or low detection of paracetamol should be interpreted with caution, given that levels can be underestimated, even when there is already established liver damage.28,30
PP is characterized by a significant increase in aminotransferases, variable INR, and moderate-to-normal bilirubin levels. There can be concomitant lactic acidosis, acute kidney injury, hypoglycemia, and rapid neurologic decline within the first 72h, with marked cerebral edema. In this etiology in particular, TFS is close to 70%, even in patients with grade 3-4 HE.28,30–32 The treatment of choice in paracetamol-induced ALF is the antidote with oral or parenteral NAC (Table 4).10,16 In Mexico, the most common presentation of NAC is in effervescent tablets for oral administration. Nevertheless, both oral and intravenous administration are equally effective, and the latter is preferred in other regions due to its better tolerability and ease of administration. It is crucial to dissolve the tablets in water and administer the solution within one hour from its preparation. If the patient vomits during that window of time, administering the dose again is recommended.33,34
N-acetylcysteine administration regimen
| Oral route | Loading dose: 140mg/kg |
| Subsequent doses: 70mg/kg every 4h for 72h | |
| Parenteral routea | Loading dose: 150mg/kg in 500 cc of 5% dextrose solution in infusion for 4h |
| Second dose: 50mg/kg in 1,000 cc 5% dextrose solution in infusion for 19h | |
| Third dose: 125mg/kg in 1,000 cc 5% dextrose solution in infusion for 24h | |
| Fourth dose: 150mg/kg in 1,000 cc 5% dextrose solution in infusion for 24h | |
| Fifth dose: 150mg/kg in 1,000 cc 5% dextrose solution in infusion for 24h |
Non-paracetamol-related causes of ALF can be idiosyncratic or related to drugs, viral hepatitis, autoimmune hepatitis, and dengue, among others. Identifying the cause, quickly starting specific treatment according to etiology, and providing supportive management are essential for improving outcome (Table 5). In the study conducted by Lee et al., they included 1,147 patients with ALF, to evaluate factors related to mortality and spontaneous resolution, discriminating by etiology. They identified groups with different outcomes. Good outcome, with a significant probability of spontaneous resolution, was observed in cases of paracetamol poisoning (60-70%), hepatitis A (50-70%), and pregnancy (75%). Poor outcome, albeit with a certain possibility of spontaneous resolution, was seen in autoimmune hepatitis (15%), hepatitis B (20%), drug poisoning (20%), and cases of indeterminate etiology (20-30%).35
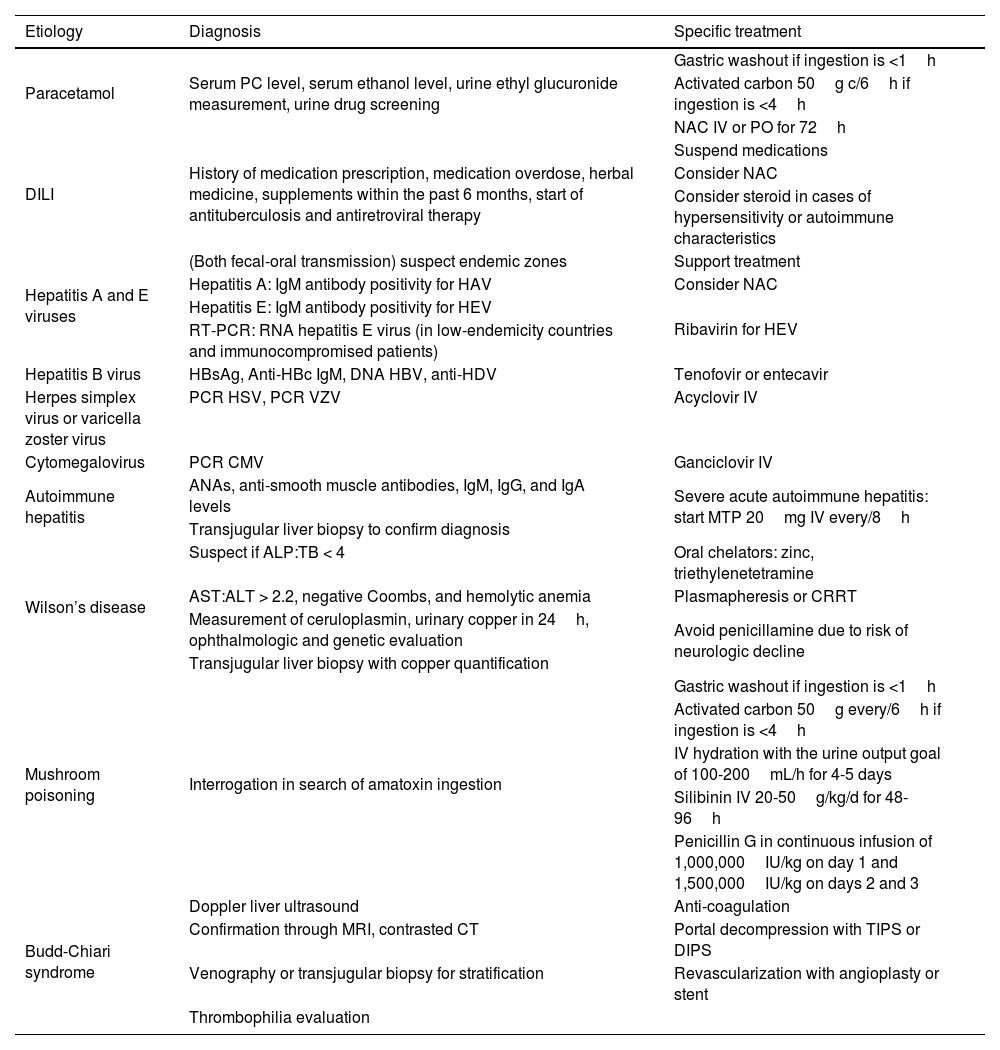
Evaluation and treatment recommendations in adults with acute liver failure (most frequent causes)
| Etiology | Diagnosis | Specific treatment |
|---|---|---|
| Paracetamol | Serum PC level, serum ethanol level, urine ethyl glucuronide measurement, urine drug screening | Gastric washout if ingestion is <1h |
| Activated carbon 50g c/6h if ingestion is <4h | ||
| NAC IV or PO for 72h | ||
| DILI | History of medication prescription, medication overdose, herbal medicine, supplements within the past 6 months, start of antituberculosis and antiretroviral therapy | Suspend medications |
| Consider NAC | ||
| Consider steroid in cases of hypersensitivity or autoimmune characteristics | ||
| Hepatitis A and E viruses | (Both fecal-oral transmission) suspect endemic zones | Support treatment |
| Hepatitis A: IgM antibody positivity for HAV | Consider NAC | |
| Hepatitis E: IgM antibody positivity for HEV | Ribavirin for HEV | |
| RT-PCR: RNA hepatitis E virus (in low-endemicity countries and immunocompromised patients) | ||
| Hepatitis B virus | HBsAg, Anti-HBc IgM, DNA HBV, anti-HDV | Tenofovir or entecavir |
| Herpes simplex virus or varicella zoster virus | PCR HSV, PCR VZV | Acyclovir IV |
| Cytomegalovirus | PCR CMV | Ganciclovir IV |
| Autoimmune hepatitis | ANAs, anti-smooth muscle antibodies, IgM, IgG, and IgA levels | Severe acute autoimmune hepatitis: start MTP 20mg IV every/8h |
| Transjugular liver biopsy to confirm diagnosis | ||
| Wilson’s disease | Suspect if ALP:TB < 4 | Oral chelators: zinc, triethylenetetramine |
| AST:ALT > 2.2, negative Coombs, and hemolytic anemia | Plasmapheresis or CRRT | |
| Measurement of ceruloplasmin, urinary copper in 24h, ophthalmologic and genetic evaluation | Avoid penicillamine due to risk of neurologic decline | |
| Transjugular liver biopsy with copper quantification | ||
| Mushroom poisoning | Interrogation in search of amatoxin ingestion | Gastric washout if ingestion is <1h |
| Activated carbon 50g every/6h if ingestion is <4h | ||
| IV hydration with the urine output goal of 100-200mL/h for 4-5 days | ||
| Silibinin IV 20-50g/kg/d for 48-96h | ||
| Penicillin G in continuous infusion of 1,000,000IU/kg on day 1 and 1,500,000IU/kg on days 2 and 3 | ||
| Budd-Chiari syndrome | Doppler liver ultrasound | Anti-coagulation |
| Confirmation through MRI, contrasted CT | Portal decompression with TIPS or DIPS | |
| Venography or transjugular biopsy for stratification | Revascularization with angioplasty or stent | |
| Thrombophilia evaluation |
ALF: acute liver failure; ALP: alkaline phosphatase; ALT: alanine aminotransferase; ANAs: antinuclear antibodies; AST: aspartate aminotransferase; CRRT: continuous renal replacement therapy; CT: computed tomography; DILI: drug-induced liver injury; DIPS: Direct intrahepatic portocaval shunt; HAV: hepatitis A virus; HBV: hepatitis B virus; HDV: hepatitis D virus; HEV: hepatitis E virus; HSV: herpes simplex virus; IV: intravenous; MRI: magnetic resonance imaging; MTP: methylprednisolone; NAC: N-acetylcysteine; PC: paracetamol; PO: oral route; RT-PCR: real-time polymerase chain reaction; TB: total bilirubin; TIPS: transjugular intrahepatic portosystemic shunt; VZV: varicella zoster virus.
Source: Shingina et al.10
Treatment with NAC outside of the non-paracetamol-related group has been considered an option in certain cases (drug-induced liver injury, hepatitis A virus, and autoimmune hepatitis). The use of NAC has increased in the last 10 years and has been related to an increase in TFS, particularly when used early and in cases of HE<grade 2, given that a significant effect has not been seen in higher grades of HE.36
The role of liver biopsy in the initial approach has not been well-defined and has a high risk for complications. Its performance is justified in specific situations, such as suspected autoimmune hepatitis, viral infections like herpes simplex, and when malignancy is suspected. In those cases, the transjugular rather than the percutaneous route is preferred, in order to minimize the risk for bleeding.36
Acute liver failure in pregnant womenALF during pregnancy can arise from specific causes associated with the gestational status during the third trimester or in the immediate postpartum period, such as hemolysis, elevated liver enzymes, and low platelet (HELLP) syndrome and acute fatty liver of pregnancy (AFLP), in addition to causes seen in nonpregnant individuals.
The incidence of ALF during pregnancy has been reported in approximately 3% of pregnancies. There are significant differences between AFLP and HELLP syndrome. HELLP syndrome is associated with a history of pregnancies complicated by hemolysis, elevated liver enzymes, and low platelet count, numerous pregnancies, and maternal age extremes, and usually manifests after 20 weeks of pregnancy. Its main laboratory test characteristics include hemolysis, thrombocytopenia, hypertension, and proteinuria, rarely progressing to ALF. Ultrasound can reveal normal images, as well as infarctions, hematomas, or capsular rupture, with a maternal and fetal mortality rate of 1 and 11%, respectively. On the other hand, AFLP presents more frequently in first pregnancies, numerous pregnancies, and male fetuses, typically after 24 weeks of pregnancy. Its laboratory test results include coagulopathy, encephalopathy, hypoglycemia, and jaundice. Fat infiltration is revealed in ultrasound examination. AFLP leads to maternal and fetal mortality of 7-18 and 9-23%, respectively. As with other diseases related to pregnancy, the resolution of pregnancy can contribute to improving the liver condition.37
HELLP syndrome and AFLP each account for approximately 25% of the causes of ALF during pregnancy. The remaining 50% are due to etiologies that present in nonpregnant individuals, with a predominance of paracetamol-related and viral and autoimmune causes. The presence of ALF that is associated with the HELLP syndrome and AFLP is an obstetric emergency that requires multidisciplinary management. This includes a prompt resolution of the pregnancy, supportive management described further ahead, and evaluation for liver transplant in patients that do not improve after childbirth.10
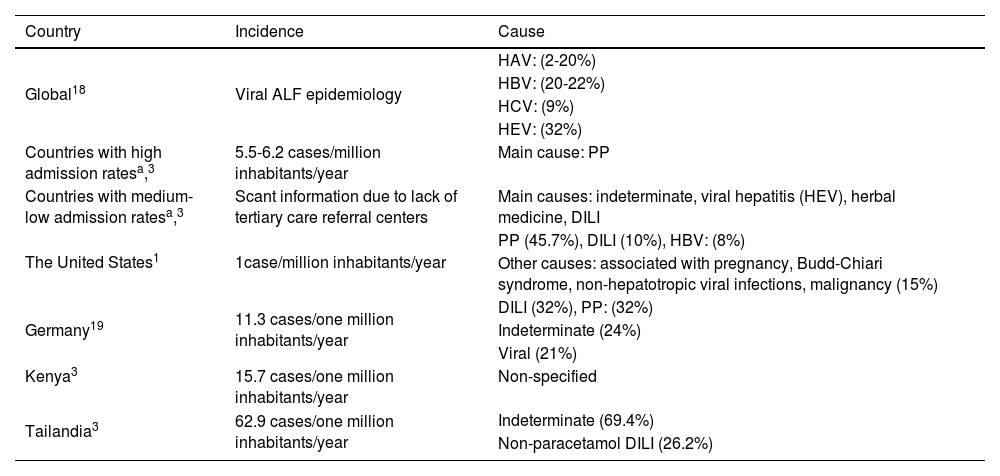
Complications and management of acute liver failureThe comprehensive management of patients with liver failure is complex. It should include strict monitorization in an intensive care unit, a multidisciplinary approach involving intensivists, hepatologists, nephrologists, and infectologists. Medical support, focusing on ensuring hemodynamic stability and evaluating disease severity, is considered initial treatment, while at the same time carrying out a detailed analysis of the etiology. The most frequent and serious complications of ALF are related to neurologic, infectious, and hemodynamic alterations, and in the most severe cases, to the development of multiple organ failure (Fig. 1).
Cerebral edema and intracranial hypertensionIntracranial hypertension is characterized by an increase in intracranial pressure above 20-25mmHg. It is a complication of ALF that is linked to unfavorable prognosis, with a mortality rate of 35 to 40%. Therefore, the timely identification and treatment of this complication is crucial.38
The presence of cerebral edema is rare in HE grades 1-2 and is present in 25-35% of patients with grade 3 HE, and 75% with grade 4 HE.39 In the context of ALF, intracranial hypertension appears to be related to hyperammonemia and early astrocytic swelling that causes a loss of intracranial blood flow autoregulation. Levels of arterial ammonia >150μmol/L are directly proportional to the increase in intracranial pressure (ICP), which can progress to encephalic herniation, hemodynamic instability, and death.40
Ultrasound monitoring is among the strategies for detecting intracranial hypertension. Its measurement of the diameter of the sheath of the optic nerve is significant, if it is above 0.48cm. This measurement is related to an ICP>20mmHg and can be utilized to monitor and evaluate treatment response.41 Transcranial Doppler ultrasound can evaluate blood flow and identify patients with cerebral hypoperfusion, as well as measure the pulsatility index that correlates with ICP and predicts outcome.42–44
Invasive monitoring of intracranial pressure is an option in patients with grade 3-4 HE but its generalized use is not recommended, given that it has not shown a decrease in mortality, and so it is restricted to selected patients in centers with experience in its placement and use.10 Real-time exact measurements are achieved with intraparenchymal monitoring, whereas the application of intraventricular, subdural, or epidural catheters provides indirect measurements. An epidural catheter is less invasive and has a lower complication rate (infection and bleeding).45 The prophylactic use of recombinant Factor VIIa, fresh frozen plasma, and desmopressin prior to the placement of intraparenchymal monitoring is an option for reducing the bleeding risk to <5%.22,46,47Table 6 describes the general measures for cerebral edema in the patient with ALF.
Intensive care management of cerebral edema in the patient with acute liver failure
| General | Laboratory tests: CBC, serum electrolytes, coagulation times, fibrinogen, Factor V, lactate and ammonia every 8-12h |
| Capillary blood glucose monitoring every 1-2h | |
| Stress ulcer prophylaxis with PPI | |
| Cultures (blood culture, urine culture, sputum culture) and chest x-ray on admission, and evaluation every 48-72h | |
| Neuroprotection | Neurologic evaluation (Glasgow coma scale, West Haven) every 1-2h |
| Headboard > 30-35%. | |
| Avoid depressants and sedatives (benzodiazepines, opioids, antihistamines). | |
| Use of short-acting medications (midazolam, propofol) in case of agitation or for AMV in case of grade 3-4 HE | |
| Minimize manipulation and stimulation (endotracheal aspiration, Valsalva maneuvers). | |
| Intracranial hypertension management | Head CT in grade 3 or 4 HE (rule out intracranial bleeding) |
| Invasive monitoring of ICP is generally not recommended, only in highly selected patients | |
| Use of osmotic agents: | |
| - Hypertonic saline solution: goal of serum Na 145-150mmol /L, avoid increases >8mmol of Na in 24h | |
| - Mannitol 0.5-1g/kg IV bolus for 5min. Monitor serum osmolarity every 4h (stop if > 320 mOsm/L) | |
| Evaluate permissive hyperventilation PaCO2 28-30mmHg for short periods in cases with no response to previous therapy |
AMV: assisted mechanical ventilation; CBC: complete blood count; CT: computed tomography; HE: hepatic encephalopathy; ICP: intracranial pressure; IV: intravenous; PaCO2: partial pressure of carbon dioxide; PPI: proton pump inhibitor.
An option for reducing neurologic complications in ALF is to decrease the production of arterial ammonia and increase its purification. Another option is the use of CRRT, which is effective for reducing the concentration of circulating ammonia, maintaining metabolic and thermal stability, mainly in the patients that develop acute kidney injury and oliguria with fluid overload. CRRT has been associated with better survival in ALF, especially in HE>grade 3.48–50
CoagulopathyINR prolongation is a characteristic that presents in all cases of ALF, according to its definition, but importantly, associated bleeding is rare (5-10%). In this setting, the liver reduces the synthesis of coagulation factors (II, V, VII, IX, and X) and anticoagulant factors (proteins C and S), and the fibrinolytic system is altered. Despite the fact that an increased INR is a prognostic factor, the INR value is not directly linked to the risk for bleeding. In a study by Agarwal et al.,22 there was a lack of correlation between the INR and viscoelastic tests in patients with ALF. The use of rotational thromboelastography has been proposed for improving the correlation of the coagulation statuses in that population and its association with complications and incidences of bleeding.51
Other frequent hemostatic alterations are thrombocytopenia and hypofibrinogenemia. The routine administration of blood products, such as fresh frozen plasma, is not recommended for correcting alterations in the coagulation panel in the absence of bleeding. To the contrary, it can have a negative impact on outcome, exacerbating pulmonary and/or cerebral edema and masking the true INR and factor V values. The indication for prophylactic blood product transfusion is recommended only prior to the performance of invasive procedures and according to the protocols of each center. In general, platelet administration for maintaining levels > 50,000/mm3 or correcting fibrinogen to levels above 100mg/dL is considered.52
InfectionsInfections in patients with ALF occur in up to 80% of patients and are a significant cause of death. Therefore, strategies for prevention and timely treatment must be developed. Up to 20% of patients can have concomitant fungal infections, increasing morbidity and mortality.53 The most frequent infections include pneumonia, urinary tract infections, infections associated with intravenous devices, and bacteremia. Symptomatology can be absent and liver and/or neurologic function decline may be the only diagnostic clue of infection. Carrying out directed serial cultures and imaging studies, with a low threshold for starting empiric therapy with antibiotics, is recommended.54
Nutrition and metabolic supportMetabolic alterations are frequent in ALF and energy expenditure is increased by 18 to 30%, compared with healthy individuals. Starting nutritional support, according to the status of the patient, is imperative, and the enteral route is the preferred first option. Support can be carried out orally in cooperative patients with low HE grades, or through an enteral catheter in cases of high HE grades and invasive mechanical ventilation. Increased protein support does not have an impact on HE progression, and so a dose of 1.0-1.5g/kg per day is recommended. Hypoglycemia, caused by the decrease in the liver glycogen stores should be actively monitored with glucometers every 1 to 2h. If present, support with continuous 10% dextrose infusion should be started for maintaining glycemia values between 150-180mg/dL. Lastly, fluid and electrolyte deficiencies (phosphorus, magnesium) should be monitored, and if present, replaced.10
Other therapiesDespite the advances in supportive medical management in patients with liver failure, high morbidity and mortality persists. Thus, treatment options are required that can be bridging therapies for those patients, spreserving liver function while they are on the transplant waitlist or when transplantation is not viable.
In such settings, a new supportive treatment modality has been proposed, which is high volume plasma exchange therapy (PLEX). The first randomized control study describing the usefulness of this therapy was reported in 2016 by Larsen et al.55 Their results showed that treatment with high-volume PLEX improved the in-hospital survival rate without liver transplantation, compared with standard medical therapy (59 vs 48%, respectively; p=0.0083). However, no difference in survival without liver transplant during hospitalization was observed (26 and 36%, respectively; p=0.17). Those authors reported a significant decrease in systemic inflammatory response and organ dysfunction markers in the group treated with PLEX. Subsequent studies have supported the idea that PLEX can have positive effects on the modulation of the inflammatory response, organ function, and the cerebral metabolic rate of oxygen in patients with ALF, which could lead to improved clinical results and a decrease in the morbidity and mortality associated with the condition.56,57 In general, there is information on the potential benefit of high-volume PLEX, but more studies are needed to better define its role in the management of ALF. Therefore, a generalized recommendation cannot be made at present.
Determination of severity and prognosis in acute liver failureALF is the most serious of the liver diseases. The mortality rate with supportive medical treatment fluctuates around 60% and its clinical course tends to be insidious, sudden, and uncertain. Since the introduction of orthotopic liver transplantation (OLT) into the treatment algorithm, overall survival results have improved. Nevertheless, the main benefits of OLT in this group of patients are limited due to several factors. They include disease severity at the time of evaluation (irreversible brain damage or multiorgan failure), as well as the lack of organ availability at the precise moment.58
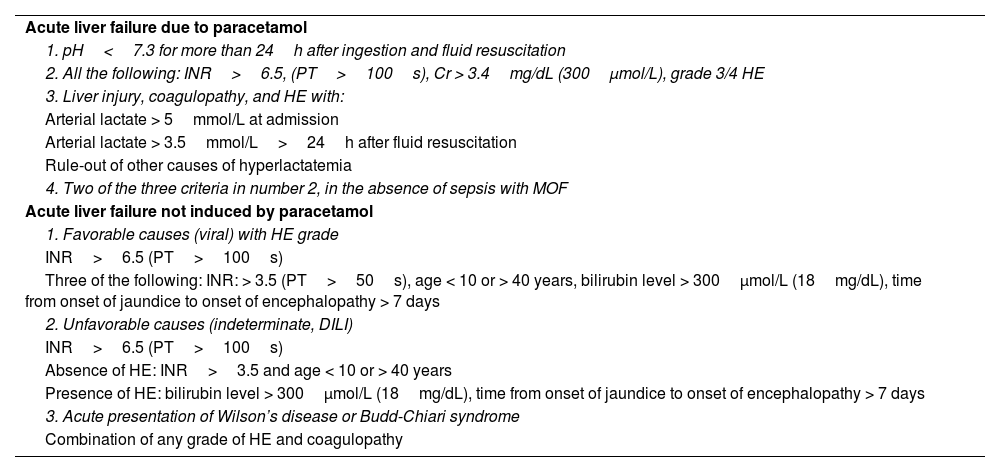
The goal of prognostic markers is to differentiate between patients with the probability of survival with only medical therapy (without the need for OLT) and patients with a poor prognosis in whom OLT should not be delayed. The main scales for determining disease severity are the King’s College Hospital criteria (KCC)59 and the Clichy-Villejuif criteria,60 among others.61
Currently, the majority of transplantation centers use the KCC (Table 7), which have 68-70% sensitivity and 82-92% specificity, as well as an 80% positive predictive value in paracetamol-related ALF and 70-90% for other etiologies.62
Updated King’s College Hospital criteria
| Acute liver failure due to paracetamol |
| 1. pH<7.3 for more than 24h after ingestion and fluid resuscitation |
| 2. All the following: INR>6.5, (PT>100s), Cr > 3.4mg/dL (300μmol/L), grade 3/4 HE |
| 3. Liver injury, coagulopathy, and HE with: |
| Arterial lactate > 5mmol/L at admission |
| Arterial lactate > 3.5mmol/L>24h after fluid resuscitation |
| Rule-out of other causes of hyperlactatemia |
| 4. Two of the three criteria in number 2, in the absence of sepsis with MOF |
| Acute liver failure not induced by paracetamol |
| 1. Favorable causes (viral) with HE grade |
| INR>6.5 (PT>100s) |
| Three of the following: INR: > 3.5 (PT>50s), age < 10 or > 40 years, bilirubin level > 300μmol/L (18mg/dL), time from onset of jaundice to onset of encephalopathy > 7 days |
| 2. Unfavorable causes (indeterminate, DILI) |
| INR>6.5 (PT>100s) |
| Absence of HE: INR>3.5 and age < 10 or > 40 years |
| Presence of HE: bilirubin level > 300μmol/L (18mg/dL), time from onset of jaundice to onset of encephalopathy > 7 days |
| 3. Acute presentation of Wilson’s disease or Budd-Chiari syndrome |
| Combination of any grade of HE and coagulopathy |
Cr: creatinine; DILI: drug-induced liver injury; HE: hepatic encephalopathy; INR: international normalized ratio; MOF: multiorgan failure; PT: prothrombin time.
The prognostic score proposed by the Acute Liver Failure Study Group (ALFSG), the ALFSG index, includes HE grade, ALF etiology, vasopressor use, total bilirubin levels, and INR, and is a better predictor of TFS than the MELD or KCC scores.63 However, it must be pointed out that prospective validation of this model is still pending.
Evaluation of liver transplantation in acute liver failureALF that does not respond to supportive treatment is a clear indication for liver transplantation. It is currently the reason for approximately 4 to 8% of all transplants, according to that reported by the Scientific Registry of Transplant Recipients and the European Liver Transplant Registry.32,64
The transplantation decision should be made in a time lapse not over 3 days, through the evaluation of a multidisciplinary team, with the intention of preventing the clinical deterioration that would contraindicate a liver transplant.65 A comprehensive evaluation should include dynamic imaging of the liver and biliary tree and an echocardiogram, within the first 12 to 24h, to confirm the suitability of the patient for transplantation and evaluate the patient’s cardiopulmonary status. The candidates that meet the transplantation criteria are given priority on the waitlist, but if they have either significant clinical deterioration or notable improvement, their removal from the waitlist is considered.66
In 2020, the United Network for Organ Sharing adopted a new prioritization system for patients with ALF, categorizing them as having high short-term mortality and situating them in priority 1A (Table 8).67
Criteria for 1A priority on the transplantation list (UNOS)
| Patients with acute liver failure |
| Presence of hepatic encephalopathy within the first 56 days from symptom onset |
| Admission to the intensive care unit |
| No pre-existing chronic liver disease |
| Presence of any of the following: |
| Mechanical ventilation |
| Continuous renal replacement therapy |
| INR≥2 |
| Patients with recent liver transplantation |
| Primary graft dysfunction or hepatic artery thrombosis within the first 7 days post-transplant with AST>3,000U/L and one of the following parameters: |
| Arterial pH≤7.3 |
| Venous pH≤7.5 |
| INR≥2.5 |
| Lactate ≥ 4mmol/L |
AST: aspartate aminotransferase; HE: hepatic encephalopathy; INR: international normalized ratio; UNOS: United Network for Organ Sharing.
In Mexico, the criteria proposed by the Centro Nacional de Trasplantes are the basis for enrolling a patient with ALF. They consist of a) no evidence of previous liver disease; b) patients in the intensive care unit with the following conditions: neurologic decline with any HE grade < 8 weeks of progression, INR ≥ 2, and need for renal replacement therapy; and c) can be supported by the KCC or Clichy-Villejuif criteria.
Patients registered on the liver transplant waitlist can be removed from it if they present with clinical improvement with spontaneous resolution or they have deterioration and a contraindication for transplant. The relevant contraindications to take into account can be classified into 3 main categories. First, medical contraindications and poor prognosis factors of active malignancy, HIV/AIDS infection, heart failure with reduced left ventricular ejection, dependence on ventilation support with an inspired fraction of oxygen greater than 90%, positive end-expiratory pressure, acute respiratory distress syndrome and concomitant acidosis, hemodynamic instability requiring the use of more than 2 vasopressors, uncontrolled sepsis, and confirmed invasive fungal infection; second, the presence of irreversible neurologic damage, such as encephalic herniation and severe intracranial bleeding; and lastly, the existence of psychosocial barriers, such as the lack of a support network and social support, uncontrolled substance abuse disorder, and a low probability of adherence to medical follow-up and pharmacologic treatment.67 The majority of these are classified as relative contraindications, with the exception of irreversible neurologic damage, which is an absolute contraindication.
Ethical and psychosocial factorsPsychologic assessment and the evaluation of the social environment can be challenging due to the neurologic decline developed by patients, mainly in the presence of advanced HE. Inquiry into transplant acceptance, family support network, resources, and possible adherence to immunosuppressive treatment is important. ALF etiology, such as attempted suicide or intravenous drug use, can involve social stigmas, requiring accurate evaluations with respect to justice and equity.
Post-transplantation survival in acute liver failureSurvival rates reported in Europe and the United States range from 79 to 84% in the first post-transplant year, 75% at 5 years, and 72% at 10 years. Male sex, donor age above 60 years, recipient age above 50 years, ABO incompatibility, and small graft size are factors that have been identified as predictors of mortality.32,61 The most frequent causes of death within that time period are infections (mainly fungal infections), neurologic complications, and multiple organ failure.68 In addition, patients that during their time on the waitlist required supportive measures (mechanical ventilation, vasopressor use, blood product transfusion, and a high number of medical and drug interventions due to neurologic complications) have worse survival.11,19
ConclusionsThe timely detection of patients with ALD is essential, given that the start of early treatment and opportune referral to a transplantation center can significantly improve survival. ALF is a rare entity, whose main cause in developed countries is PP, whereas the principal causes in middle and low-income countries are acute hepatitis virus, especially hepatitis A virus and hepatitis E virus, and indeterminate etiologies. An increase in transplant-free survival in ALF, regardless of its presentation with or without acute kidney injury, has been observed through the use of CRRT. Optimizing supportive medical management in the areas of intensive care, carrying out continuous monitoring, and rapidly identifying complications of ALF, especially cerebral edema, infections, and coagulopathy, are all crucial. In the initial evaluation, prognostic transplantation-free survival scales should be utilized to identify patients that should begin to be evaluated for a liver transplant. In general, long-term survival results in patients that undergo liver transplantation due to ALF are favorable.
Ethical considerationsThis article is based on a thorough bibliographic review, and so does not involve direct patient participation or the need for approval by an institutional ethics committee. Importantly, no identifiable personal data have been utilized and we have taken measures for protecting the privacy of any information that could have been used in the bibliographic review.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.