Cyclic vomiting syndrome (CVS) is a disorder characterized by recurrent and unpredictable episodes of intense vomiting, interspersed with periods of apparent wellbeing. This disorder, which primarily affects children and adolescents but can persist into adulthood, has recently been the subject of extensive study and analysis in the medical literature. The aim of the present review is to examine the most important aspects of the epidemiology, pathophysiology, subtypes, diagnostic criteria, and current management of CVS. Even though the exact etiology remains unknown, genetic factors (polymorphisms), nervous system alterations and autonomic dysregulation, and environmental factors (use and abuse of cannabinoids) are postulated as possible triggers. CVS has significant diagnostic challenges, given that there is no specific test for confirming its presence. Thorough evaluation of symptoms and the ruling out of other possible causes of recurrent vomiting are required. Management of CVS typically involves a multidisciplinary approach. Pharmacologic options are explored, such as antiemetics and preventive medications, as well as behavioral and psychologic support therapies. Treatment personalization is essential, adapting it to the individual needs of each patient. Despite advances in the understanding of CVS, it remains a significant clinical challenge. This disorder impacts the quality of life of those affected and their families, underscoring the ongoing need for research and the development of more effective treatment strategies.
El síndrome de vómitos cíclicos (SVC) es un trastorno caracterizado por episodios recurrentes e impredecibles de vómitos intensos, separados por períodos de bienestar aparente. Este trastorno, que afecta principalmente a niños y adolescentes, aunque puede persistir en la edad adulta, ha sido objeto de un extenso estudio y análisis en la literatura médica recientemente. El propósito de esta revisión es revisar los aspectos más importantes de la epidemiología, la fisiopatología, los subtipos, los criterios diagnósticos y el manejo actual del SVC. Aunque la etiología exacta sigue siendo desconocida, se postulan factores genéticos (polimorfismos), alteraciones del sistema nerviosos y desregulación autonómica, y ambientales (uso y abuso de cannabinoides) como posibles desencadenantes El SVC presenta desafíos significativos en términos de diagnóstico, ya que no existe una prueba específica para confirmar su presencia. Se requiere una evaluación minuciosa de los síntomas y la exclusión de otras posibles causas de vómitos recurrentes. En cuanto al tratamiento, el manejo del SVC suele adoptar un enfoque multidisciplinario. Se exploran opciones farmacológicas, como antieméticos y medicamentos preventivos, así como terapias conductuales y de apoyo psicológico. La personalización del tratamiento es esencial, adaptándolo a las necesidades individuales de cada paciente. A pesar de los avances en la comprensión del SVC, sigue siendo un desafío clínico significativo. Este trastorno impacta la calidad de vida de los afectados y sus familias, destacando la necesidad continua de investigación y desarrollo de estrategias de tratamiento más efectivas.
Cyclic vomiting syndrome (CVS) is a chronic disorder characterized by episodes of uncontrollable vomiting that are interspersed with asymptomatic or minimal symptom periods.1 CVS was described by Heberden in 1806 as a pediatric disease specified by recurrent vomiting and influenced by psychologic disorders.2 However, it is currently known that the syndrome can start in adulthood, with a similar clinical behavior in the two age groups.3
Vomiting episodes tend to be incapacitating, negatively impacting quality of life. Therefore, CVS has been the subject of greater attention by part of the medical community in recent years, making substantial advances regarding its pathophysiology, associated factors, and treatment. Despite these advances, CVS continues to be a little-recognized entity at present by gastroenterologists. This was observed in a cohort of patients that met the Rome criteria for the entity, of whom only 4% were diagnosed with CVS.4 These data highlight the poor recognition of the syndrome, resulting in diagnostic delay and the consequently greater frequency of visits to the emergency room, increased health costs, and suboptimum treatment, with poor results for the patient.3,5,6
Thus, our aim was to carry out a detailed bibliographic review of the epidemiology, pathophysiology, subtypes, diagnostic criteria, and current management of CVS.
Material and methodsA review that evaluated and analyzed articles on the diagnosis and treatment of CVS, published in the national and international bibliography, was carried out. A cross-database search was conducted on MEDLINE, Embase, Web of Science, and Scopus, for all studies available from January 2000 to December 2022, utilizing the following terms (in English and Spanish): vomiting, cyclic, nausea, syndrome, hyperemesis, cannabinoids, pediatrics, epidemiology, diagnosis, treatment, guidelines, consensuses, and review.
Utilizing their criteria, the reviewers identified the most relevant articles on the theme. Technical reviews, systematic reviews, meta-analyses, and clinical guidelines on CVS, as well as information from observational studies, case series, case reports, and intervention studies on patients with CVS, were included in the search. The bibliography was organized, and the review was divided into the following sections: epidemiology, pathophysiology, subtypes, diagnostic criteria, and current management of CVS. Of the 416 abstracts found in the search, information from 124 articles in full was included. Articles that could not be seen in their entirety were excluded.
EpidemiologyCVS affects both children and adults. A study on an adult population showed a prevalence of 2%, 1%, and 0.7% in the United States, the United Kingdom, and Canada, respectively.7 Likewise, an Argentinian study reported a prevalence of 1% in adolescents.8 The prevalence of CVS is unknown in the Mexican population but a study conducted on Mexican school-age children evaluated the prevalence of gut-brain axis disorders, and found a 0.3% prevalence of CVS in that selected group.9
CVS is more frequent in women and the mean adult age at presentation is 37 years. A study that evaluated 101 adults diagnosed with CVS showed that 86% of the cohort were women.3 Regarding race, 78% were White, 17% were African-American, and 5% were Hispanic.3
CVS is associated with numerous comorbidities, such as migraine, generalized anxiety disorder, panic disorder, major depression, autonomic dysfunction, and cannabis use.10–12 The probability of presenting with a psychiatric comorbidity in adults with CVS is 40-times higher than in the general population, and the most frequent are anxiety (69%) and depression (48%). This underlines the need for conducting a biopsychosocial evaluation to obtain better patient results.13 Another important finding is that approximately 40% of patients with CVS use, or have occasionally used, cannabis, reporting symptom improvement. In contrast, daily or prolonged cannabis use is associated with hyperemesis.14,15
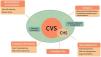
PathophysiologyVarious mechanisms and comorbidities have been described that interact with each other, giving rise to combinations that lead to cyclic emesis. The proposed pathophysiologic mechanisms of CVS are (Fig. 1):
Possible pathophysiologic mechanisms of CVS. Current data suggest that CVS is explained by a combination of genetic and host factors that lead to emesis. CHS is thought to be a subset of CVS and can be explained by the development of symptoms in genetically predisposed individuals.
CHS: cannabinoid hyperemesis syndrome; CVS: cyclic vomiting syndrome; ECSS: endocannabinoid signaling system; HPA: hypothalamic-pituitary-adrenal.
* Figure adapted from reference.67
A greater frequency of mitochondrial polymorphisms (16519T and 3010A) has recently been identified in pediatric patients with CVS, compared with controls,16–18 but it was not found in Asian pediatric populations or in adults.19,20 This variability may be due to the small sample sizes, involuntary bias during patient recruitment, and the differences in allelic frequencies between the different ethnicities.
Endocannabinoid signaling system alterationsThe endocannabinoid signaling system plays an important role in CVS.21,22 This system is formed by endogenous ligands, N-arachidonoylethanolamide, 2-arachidonylglycerol (endocannabinoids), and two cannabinoid G-protein coupled receptors (cannabinoid receptor type 1 [CNR1] and type 2 [CNR2]), which regulate gastrointestinal motility.23,24 The CNR1 and CNR2 receptor agonists prevent vomiting, and in contrast, the CNR1 receptor antagonists cause vomiting. The polymorphisms in the CNR 1 rs806380 gene are associated with a greater risk for CVS. In addition, the serum concentration of endocannabinoid-related lipids, N-oleoylethanolamine and N-palmitoylethanolamide, is higher in the symptomatic phase of CVS and those lipids are positively correlated with several CVS symptoms. These data support the hypothesis that chronic cannabis use causes an increase in stress responses during CVS,21 and show that the variations in the endocannabinoid pathway lead to a higher risk of developing CVS.
Autonomous nervous system dysregulationThe autonomous nervous system plays an outstanding role in emesis. A series of brainstem nuclei receive information from the vagus and sympathetic afferent nerves during emetic stimulation. Efferent signals are created that start the stereotyped and coordinated muscle actions involved in vomiting. Many adults with CVS have sympathetic nervous system dysregulation.10,25,26 Studies have reported increases in sympathetic tone in patients with CVS, recorded through various tests of autonomic function, such as postural adjustment ratio, heart rate variability, tilt table test, and sudomotor tests.10,27–29 The most frequent finding in these reports was an adrenergic anomaly, in addition to coexisting abnormalities of vagal cholinergic function. Based on these studies that describe dysautonomic characteristics in patients with CVS, researchers have posited the hypothesis that this imbalance can cause patients to be more susceptible to producing an uncontrolled response to central emetic signals.27
During the inter-episodic phase of the disease, patients with CVS can present with accelerated gastric emptying in up to 59% of cases.30 In fact, accelerated gastric emptying can be an indirect marker of the underlying autonomic dysregulation in CVS. Importantly, the presence of delayed gastric emptying should not be interpreted as gastroparesis, especially in the context of typical episodic vomiting. Therefore, carrying out gastric emptying tests for diagnosing CVS is not recommended.
This observed dysregulation of the autonomous nervous system has implications in the treatment of CVS, such as vagal neuromodulation with percutaneous electrical nerve field stimulation,31 which improves symptoms, modulating the vagal function in patients with gut-brain axis gastrointestinal disorders and those with CVS.32 In addition, neuromodulation has been shown to predominantly benefit the subgroup of patient that have deficient vagal function.33
AllostasisHomeostasis cannot completely explain the autonomic changes that accompany purely cognitive and emotional events. A more complete conceptual framework for autonomic control that accounts for them is known as allostasis.34 Allostatic regulation requires “central commands” that orchestrate changes in autonomic and motor activity. These central orders can ultimately proceed from the cerebral cortex and a network of central nuclei essential for autonomic regulation. In the framework of allostatic regulation, cognitive events, the anticipation of behavioral changes, and physiologic needs and the associations learned, shape the autonomic control patterns through changes in neuronal activity. Sleep deprivation, mood disorders, chronic stress, and early adverse life events can lead to allostatic regulation alteration through neural plasticity. Such maladaptive neural plasticity could lead to abnormal patterns of autonomic and neuroendocrine acitivity.35,36 The fact that vomiting and retching during the emetic phase of CVS are mainly produced when the stomach is empty, implies that the allostatic feedback mechanisms are disordered, which could give rise to a reduced threshold for vomiting and retching, regardless of physiologic necessity or peripheral information. This neural system could be abnormally developed to be activated through a low threshold in the presence of mental and/or physiologic stimuli, possibly analogous to anticipatory tachypnea patterns in patients with panic attacks.37 The prediction of this model would be that any factor that acutely alleviates stress or anxiety in patients with CVS could improve centrally driven vomiting patterns.
Neuronal hyperexcitability can be a common link between CVS and other central nervous system (CNS) disorders.38–40 Hyperexcitability can be a consequence of genetic variants in the structure and function of the ion channel and/or neurotransmitter receptors or can be the result of an aberrant development of neuronal circuits. Studies that describe alterations in the functional connectivity of the cerebral networks, particularly within the networks that involve the amygdala and insular cortex, can better explain the role of brain anomalies in CVS.41 In a case-control study, patients with CVS showed increased functional integrity of salience intrinsic connectivity to the middle/posterior insula, a key region of the brain for nausea and viscerosensory processing.41 It is possible that this neuronal hyperexcitability and a lower threshold for triggering specific patterns within the CNS predispose to the triggering of stereotypic vomiting episodes in CVS.
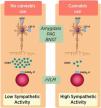
Association with cannabisCannabis has been used for centuries for both recreational and medicinal purposes. Its active ingredient is Δ 9-tetrahydrocannabinol (THC), a CNR1 agonist, and cannabidiol. THC acts on the CNR1, but cannabidiol has no psychotropic activity and acts on the 5-hydroxytryptamine 1A (5-HT1A) receptors.42 There is clinical data showing that cannabis has antiemetic properties and is frequently used by patients with CVS to alleviate the symptoms of nausea and vomiting, lack of appetite, and stress.14 Contrastingly, several case series on cannabinoid hyperemesis syndrome (CHS) have implied that the chronic use of cannabis causes vomiting.43–45 Approximately 20% of patients with CHS use cannabis ≥ 4 times a week, whereas the rest are occasional users. How cannabis use perpetuates symptoms is CHS must be determined. Cannabinoid use is independently associated with increases of sympathetic nerve flow.46,47 This influence on sympathetic activity occurs through the inhibition of the activity of the descending gamma-aminobutyric acid (GABA)ergic neurons.48–51 Since this descending GABAergic input ultimately inhibits the activity of the rostral ventrolateral medulla (rVLM) neurons,52 the chronic cannabinoid inhibition of this input would be thought to increase the activity of the target rVLM neurons (Fig. 2). The GABAergic reduction in the nucleus of the solitary tract could lower the vomiting threshold.
Experts consider that CHS is a subgroup of CVS. Based on similar clinical presentations and the lack of objective tests that demonstrate causality, it is hypothesized that chronic cannabis use can unmask symptoms in genetically predisposed individuals and cause hyperemesis.14 In addition, THC concentration in cannabis products has increased exponentially over time, which could also explain the paradoxical effects of cannabis use being an antiemetic at low doses and proemetic in high concentrations.53
Clinical subtypes of cyclic vomiting syndromeRecognizing the different CVS subtypes is possible through a detailed clinical history. An individualized therapeutic approach for each subgroup will result in better short-term and long-term results.
Catamenial cyclic vomiting syndromeCatamenial CVS is characterized by its association with the onset of menstrual periods. This subtype highlights the role of the hormonal influence on CVS, and said association is described in case series.54–56 A similar association between migraine and menstrual periods suggests a pathophysiologic parallelism.57 This triggering mechanism can reflect the sensitivity to the decrease of estrogens that is produced right before menstruation. Treatment with gonadotropin-releasing hormone (GnRH) analogues, estrogen supplements, oral contraceptives with low estrogen doses or progesterone alone achieves satisfactory prevention, suggesting the importance of maintaining adequate hypothalamic-pituitary axis regulation in CVS.58,59 The recognition of this subtype highlights the importance of a deeper understanding of the pathologic mechanisms of CVS, consequently providing highly efficacious personalized treatment.
Cannabinoid hyperemesis syndromeWe underline the importance of CHS as the differential diagnosis of CVS, given that the pharmacologic treatment will be different for each entity. The majority of patients with CVS arrive at the emergency room with episodes of vomiting, nausea, and abdominal pain.60 These symptoms are the same as those suffered by the patient with CHS, and so clinical differentiation can be difficult. The fact that intensity and chronicity of cannabis use are greater in CHS is a key differentiation point. In addition, symptom relief following abstinence helps define CHS, according to the Rome IV criteria (Table 1).
Cannabinoid hyperemesis syndrome diagnostic criteria, according to the Rome IV consensus1
| Cannabinoid hyperemesis syndrome diagnostic criteria |
|---|
| Stereotypic vomiting episodes similar to those present in cyclic vomiting syndrome, in terms of onset, duration, and frequency |
| Presentation after prolonged and excessive cannabis use |
| Clinical improvement after stopping prolonged cannabis use |
| * Criteria met for the past three months with symptom onset at least six months before diagnosis |
Table adapted from the reference: Stanghellini et al.1
Anxiety and depression are frequent comorbidities of CVS. A study found a 47% prevalence of anxiety in children with CVS.11 In adults, the prevalence of anxiety varies widely, from 15% to 84%, and was significantly associated in a multivariate analysis.61,62 Similarly, up to 78% of adults with CVS have been reported to present with mild-to-severe depression.62 Although the direct pathophysiologic mechanisms of anxiety or depression in CVS remain nuclear, the elevated incidence of these disorders suggests that emotional regulation plays an important role in the pathophysiology and development of CVS. For example, a considerable number of patients with CVS have indicated “anticipatory anxiety” as a possible triggering factor, as well.11,63 Patients that do not tolerate uncertainty also experience a greater negative impact on quality of life, regardless of the true frequency of the attacks of CVS experienced.64 These patients can especially benefit from cognitive-behavioral therapy. Lastly and interestingly, patients with CVS frequently have panic attacks. Both panic disorder and CVS follow a similar temporality pattern and share clinical signs of sympathetic nervous system activation, suggesting they can share certain pathophysiologic mechanisms.63 Different experts posit that previous adverse experiences or chronic stress can be risk factors for developing CVS,65 given that such events can affect neuronal processing and alter allostatic regulation, predisposing certain patients to the syndrome.65–67 Due to the high prevalence of mood disorders in CVS, recent clinical guidelines suggest offering some type of “mind-body” therapy, such as meditation, relaxation therapies, or even cognitive-behavioral therapy, to the majority of patients.68
Diet-related diseaseAlthough there is currently little scientific evidence for establishing a relation between specific foods and CVS, the consumption of chocolate, cheese, and monosodium glutamate has been described as a possible trigger of CVS attacks.60,69 Curiously, those same foods have long been associated with migraine,70 and several mechanisms can explain the associations. Specific smells and possibly flavors can precipitate migraines, and the neuronal codification of those stimuli may contribute to starting an attack of CVS.71 The possibility that food allergies can act as triggers for CVS has also been suggested. In a case series of patients with this CVS phenotype, the episodes were resolved by avoiding the food whose specific sensitivity was confirmed through tests.72 Even though such tests are still limited, they suggest that environmental exposures, especially those related to ingested foods, can be relevant therapeutic goals.
Clinical phases of cyclic vomiting syndromeCVS has four phases described in detail by Fleischer et al. and are shown in Fig. 3.63
- 1
Inter-episodic phase. In this phase, patients are relatively symptom-free, but approximately 30% of adults present with inter-episodic dyspepsia and nausea, which should not rule out the CVS diagnosis.
- 2
Prodrome phase. The prodrome phase precedes the emetic phase and is reported in approximately 61-93% of adult patients.3,63,73 Symptoms can include nausea, abdominal pain, and multiple autonomic symptoms, such as diaphoresis, intolerance to cold and heat, sialorrhea, pallor, and lethargy. Patients can also experience panic attacks. This phase lasts 30 to 90minutes and is an important window for the use of abortive medications.64,74,75
- 3
Emetic phase. The prodrome phase tends to progress to an emetic phase if untreated and is characterized by severe and incessant vomiting and retching. Abdominal pain is seen in 80% of patients and does not rule out the diagnosis of CVS.27 Many patients claim to feel relief when their stomach is empty and sometimes they attempt to induce vomiting. This habitual conduct should not be confused with feigned behavior. The emetic phase can be incapacitating, and patients are often incapable of communicating or ambulating. Patients may not show signs of dehydration until an advanced phase of the episode.
- 4
Recovery phase. Once the vomiting has ceased, patients slowly tolerate oral intake before returning to their normal routine.63 The frequency of CVS episodes varies from every two-to-three weeks to three times a year. Episode duration can vary from hours to several days.54
The clinical history is essential in the diagnosis of CVS, which is characterized by episodes of incoercible vomiting, alternating with periods of few or no symptoms.
First, a thorough interrogation should be carried out to determine the risk factors for developing CVS and accompanying symptoms. Even though vomiting is the distinctive feature of CVS, abdominal pain is observed in up to 80% of patients.76 Other symptoms are photophobia, sensitivity to sound, and autonomic symptoms (diaphoresis, sialorrhea, pallor, and lethargy).27 Patients also describe the behavior of “drinking and gulping down” and drink large quantities of water and then try to induce vomiting, which they say gives them relief (this should not be misinterpreted as a psychologic/psychiatric disorder).63 In addition, anatomic/organic causes that produce a similar symptomatology must be ruled out. Laboratory tests, such as complete blood count, blood chemistry, complete serum electrolytes, and serum amylase should be ordered. Imaging studies, such as computed tomography of the abdomen, should be carried out, as well as upper endoscopy, to rule out any obstruction at the level of the gastrointestinal tract (Fig. 4).
The definitive diagnosis is currently made in patients that meet the Rome criteria (Table 2)1 and in whom other causes of emesis have been ruled out through a detailed clinical history and the previously mentioned tests.
Cyclic vomiting syndrome diagnostic criteria, according to the Rome IV consensus1
| Cyclic vomiting syndrome diagnostic criteria |
|---|
| Stereotypic vomiting episodes; acute onset and duration of less than one week |
| At least three episodes in the past year and two episodes within the past six months (with at least a one-week interval) |
| Absence of nausea and vomiting between episodes but other mild symptoms can present between episodes |
| Personal or family history of migraine |
| * Criteria met for the past six months with symptom onset at least 12 months before diagnosis |
Table adapted from the reference: Stanghellini et al.1
The differential diagnosis is broad because symptoms can be due to gastrointestinal (malrotation, gastroparesis), intracranial (intracranial masses, hydrocephaly), or abdominal (renal colic) disorders, or to metabolic (fatty acid oxidation or urea cycle disorders, mitochondrial dysfunction) or drug/toxin (CHS) alterations.77 Laboratory, radiographic, and endoscopic tests detect a substantial number of these differential diagnoses.
Acute hepatic porphyrias should be considered in the evaluation of all patients, especially 15 to 50-year-old women with recurrent intense abdominal pain accompanied by nausea, vomiting, and neuropathy. These entities are congenital metabolism errors of the blood group and include acute intermittent porphyria, hereditary coproporphyria, variegate porphyria, and 5-aminolevulinic acid dehydratase deficiency porphyria.78 Acute intermittent porphyria is the most common type, with an estimated prevalence of one per 100,000 inhabitants.79,80
All patients with hepatic porphyrias should be advised to avoid triggers, such as stress, excessive alcohol and/or tobacco use, and calorie deprivation, given that they are factors that induce messenger RNA and delta-aminolevulinate synthase 1 protein expression in the hepatocytes.81–84 This differential diagnosis is mentioned in numerous articles, and a recent study on the prevalence of acute hepatic porphyrias in patients diagnosed with CVS was recently evaluated. Acute hepatic porphyria was not detected in any of the 234 patients analyzed.85
TreatmentGeneral measures and life-style modificationIt is necessary to avoid the triggers identified by the patient (insomnia, fasting, stress). In addition, the presence of mood disorders should be looked for, to correctly refer the patient to a psychologist or psychiatrist. Anxiety, panic, and depression disorders are frequent in adults with CVS62,63 and the symptoms of said disorders can influence the precipitation of CVS episodes.
Migraine is closely related to CVS.1,86 The prevalence of migraine in adults with CVS varies from 13% to 70%.63,87 In addition, 57% of adult patients with CVS (with or without migraine) have a first and/or second degree relative with migraine or migraine variants.63 Whether the treatment of migraine independently improves the evolution of CVS is not clear but many medications are efficacious prophylactic therapies (such as tricyclic antidepressants [TADs] and antiepileptics) or abortive therapies (such as triptans) for both conditions. Anecdotally, when CVS and migraines coexist, both have been observed to respond to TADs, but at different doses, obliging the physician to increase the amount of the drug, even when symptoms of one of the disorders have disappeared.
Given that chronic cannabis consumption is associated with the lack of treatment response, its use should be suspended.88 Patients usually benefit from a multidisciplinary approach that includes the gastroenterologist, psychologist/psychiatrist, and nursing personnel familiarized with the syndrome.
A unique characteristic of CVS is the conduct of bathing with warm water that provides temporary symptom relief.89 Even though this behavior is associated with cannabis use, it is also observed in 50% of patients that do not use cannabis.75
Prophylactic therapyDuring the inter-episodic phase, treatment is prophylactic and is recommended in patients with moderate/severe disease, i.e., in patients with > 4 episodes per year of nausea and severe vomiting that make the patient seek help at the emergency service or require hospitalization. TADs are the first-line drugs for CVS prophylaxis and have been shown to reduce the duration, severity, and frequency of episodes, as well as the number of emergency room visits and hospitalizations.90 Amitriptyline, the TAD of choice, is usually started at a low dose of 25mg and is gradually increased to a target dose of approximately 100mg at night in adults. TADs can cause a prolonged QT interval, and so an electrocardiogram should be taken at the start and during the titration of the TAD to monitor it.91
Alternative prophylactic therapiesIn patients that do not tolerate TADs, anticonvulsants, such as zonisamide or levetiracetam, can be considered. In a noncontrolled study of 20 patients that did not tolerate TADs and that were started on zonisamide (mean dose of 400mg/day) or levetiracetam (mean dose of 1,000mg/day), 75% had a moderate clinical response, whereas 20% had complete symptom remission.92 Topiramate, another anticonvulsive and antimigraine agent, can also be used as prophylactic treatment.93 It is usually started with 25mg per day and the dose is increased 25mg every week, until reaching 100mg per day. Topiramate can slightly increase the risk for kidney stones, therefore optimized liquid intake should be recommended to patients. It can also cause a decrease in bicarbonate levels, and so patients should be supplemented if levels are shown to be low.
AprepitantAprepitant is a highly selective high-affinity antagonist of substance P/neurokinin 1 receptors.94 It binds to the receptors of the nucleus of the solitary tract that mediate the emetic motor reflex. It has been utilized to prevent chemotherapy-induced vomiting, as well as postoperative nausea and vomiting. Even though the plasma half-life is nine to 13hours, clinical effects can last for three to five days.95 The significant inhibitors and inducers of the CYP3A4 system increase and decrease serum aprepitant levels, respectively, and should be avoided if possible.96 It should be used with caution in patients with severe liver disease. A clinical trial was conducted on the prophylactic use of aprepitant for treating children and adolescents presenting with CVS that was refractory to conventional treatment.97 The trial included adolescents weighing more than 60kg that were treated with 125mg twice a week. In an intention-to-treat analysis, 82% of the patients had complete resolution or partial response (> 50% decrease in frequency and intensity) of episodes at 12 months. According to those criteria, 19% had complete response and 63% had partial response. Based on the data in children and adolescents and the clinical experience of experts, aprepitant is currently recommended as an alternative prophylactic treatment in patients that do not respond to or do not tolerate standard therapy with amitriptyline or topiramate. The suggested dose is 125mg twice a week for adults weighing > 60kg, and 80mg twice a week for adults weighing 40-60kg. Aprepitant is expensive, which should be considered when prescribing it.
Coenzyme Q10Its use is recommended as monotherapy or in combination with prophylactic medications. Its recommendation is based on studies that signal mitochondrial dysfunction as part of the pathogenesis of CVS.98,99 Patients with migraine show reduced mitochondrial respiratory complex function, which most likely is present in CVS as well, albeit there are no studies confirming that assumption.100
Coenzyme Q10 (Co-Q10) is a natural hydrophobic compound derived from steroids that acts as an electron transporter between complex 1 or 2 and complex 3 of the mitochondrial respiratory chain. A retrospective study compared the efficacy of Co-Q10 with amitriptyline in children with CVS.101 Prophylactic treatment with Co-Q10 showed an overall efficacy of 68%, with no side effects.101 A dose of 10mg/kg of Co-Q10 per day, divided into two doses, until reaching 200mg twice a day, is recommended. For refractory cases, the suggestion is to measure Co-Q10 levels in blood and increase the dose to reach a serum level of 3mg/L.
Abortive medicationsTriptansSumatriptan is a serotonin agonist approved for the treatment of migraine by the US Food and Drug Administration.102 It binds to the 5-hydroxytryptamine 1B (5-HT1B) and 5-hydroxytryptamine 1D (5-HT1D) receptor subclasses in the meninges, producing dural blood vessel constriction. The efficacy of sumatriptan in migraine is due to multiple places of action (vascular, neural, and central) and the injectable or intranasal presentations are utilized. There are reports on the efficacy of sumatriptan in abdominal migraine, a disorder classified in the childhood periodic syndrome subgroup.103,104 Although the mechanisms that cause CVS are not well-defined, there is a clear clinical, familial, therapeutic, and probably pathogenic, parallelism with migraines.69 In a study carried out on a pediatric population, greater treatment efficacy was shown with injectable sumatriptan, compared with intranasal administration. Likewise, there was a greater response in patients with a family history of migraine.105 Importantly, neither injectable sumatriptan nor the nasal spray are available in Mexico, and so studies are needed to document the efficacy of oral treatment. A case report by Okumura et al. described 100% efficacy in the last five prodromes of a patient that received oral sumatriptan, suggesting an efficacy similar to that of other administration routes.106
Serotonin antagonistsOndansetron is a selective 5-hydroxytryptamine type 3 (5-HT3) receptor antagonist.107 It acts by blocking vagal afferent 5-HT3 receptors at the peripheral and central level in the chemoreceptor trigger zone of the area postrema in the medulla oblongata. These actions cause a decrease in circulating serotonin at the chemoreceptor trigger zone, reducing the symptoms of nausea and vomiting in affected patients.107,108 Ondansetron is metabolized in the liver by CYP3A4, CYP1A2, and CYP2D6, and excreted by the kidneys.109 Numerous data show the efficacy of this agent in nausea and vomiting induced by chemotherapy and in the postoperative period.110,111
The expert committee of the American Neurogastroenterology and Motility Society and the Cyclic Vomiting Syndrome Association recommend ondansetron as a first-line agent for aborting an episode of CVS. According to experts, the combination of 5-HT3 receptor antagonists with other abortive agents, such as anxiolytics and phenothiazines, for sedation may be more effective in aborting an episode than monotherapy.
A baseline electrocardiogram to check for a long QT interval is recommended in adults before starting treatment with ondansetron. A dose of 8mg of the drug is recommended at the start of the prodrome, in combination with other abortive medications, such as triptans and anxiolytics. Oral administration should be avoided, given that patients tend to be unable to tolerate oral preparations during an episode and the fact that medication absorption is unpredictable during an episode of vomiting.
AprepitantAprepitant as abortive therapy is recommended in patients that do not respond to standard treatment (sumatriptan and ondansetron) and in patients that have a defined prodrome or predictable periodicity (an episode that is related to the menstrual cycle). Aprepitant can be started one or two days before the predicted start of the emetic phase. Utilizing a standard dosing regimen of 125mg, 80mg, and 80mg, on three consecutive days, is recommended. The first dose should be taken as soon as possible in the prodrome and before the appearance of vomiting.
A clinical trial was conducted on oral aprepitant as an abortive treatment for CVS in children and adolescents that did not respond to standard treatment.97 At 12 months, the recurrent abortive regimen significantly reduced the duration of episodes, the number of vomiting episodes, and the number of hospital admissions.
Emergency room management during the emetic phaseDespite prophylaxis, patients with CVS can have intermittent vomiting attacks that need to be treated at the emergency service. A survey of adults with CVS showed that the median number of visits to the emergency room due to disease-related symptoms was 15 (range: 1-200). Relevantly, many patients tend to receive only a partially efficacious treatment due to lack of awareness of the disorder.112,113 Thus, all patients with CVS are recommended to have an individualized treatment plan for improving results (Table 3).114
Treatment protocol for patients diagnosed with CVS in the emergency room
| Name of the patient: |
|---|
| Is there a previous diagnosis of CVS? |
| Somatometry and vital signs |
| HR, AT, temperature, capillary refill, mucosal hydration aspect, physical examination |
| Laboratory tests |
| Complete blood count, blood chemistry, liver function tests, serum lipase |
| Imaging studies |
| At the criterion of the emergency room physician. Requested when there is clinical suspicion of additional involvement or involvement different from the emetic phase of CVS. |
| Treatment |
| Crystalloid solutions |
| IV saline solution bolus if the patient shows clinical signs of dehydration. |
| Maintenance hydration: mixed solution (glucose 5%/saline solution) 3 mL/kg/hour IV in continuous infusion. |
| Antiemetics |
| Ondansetron (8 mg IV, single dose), consider administering every 4-6 hours PRN |
| Diphenhydramine (50 mg IV, single dose) |
| Metoclopramide (100 mg IV, single dose) |
| Benzodiazepines for treating anxiety episode |
| Lorazepam (1-2 mg IV), consider administering every 4 hours PRN |
| In case of association with migraine episode |
| Abortive: nasal sumatriptan (20 mg) or administer 6 mg subcutaneously (presentations not available in Mexico). |
| Alternative: administer triptans orally. |
| Pain management |
| Ketorolac (30 mg IV, single dose) if pain lasts > 60 minutes, administer 15 mg every 6 hours on two occasions (maximum dose 60 mg/day). |
| Consider using opioids in cases of severe abdominal pain. |
| In cases of severe abdominal pain that is refractory to conventional management, administer ketamine IV in continuous infusion. |
CVS: Cyclic vomiting syndrome; HR: heart rate; AT: arterial tension.
Table adapted from the reference: Frazier et al.115
During the acute phase, a combination of antiemetics and benzodiazepine sedation appears to be the best option because the latter drugs are used for alleviating anxiety and promoting sleep. First-generation antihistamines, such as injectable diphenhydramine, can be used.115,116
In patients with abdominal pain, the analgesic of choice is intravenous ketorolac. If the pain is severe, treatment with opioids should be considered. If the pain is refractory to those treatments, the use of intravenous ketamine in infusion has recently been described as an option.117–119
In addition, enabling the patient to rest in a quiet and darkened environment and maintaining good hydration with crystalloid solutions, even when the patient shows no signs of dehydration, appear to be beneficial. Altogether, this is the preferred approach for ending the emetic phase of CVS.113
Future directionsNeuromodulationThe increasing understanding of the pathophysiology of CVS has brought about advances in its treatment. It is widely known that the vagus nerve plays an important role in nausea, vomiting, and pain.120 Some of the vagal nerve branches are situated in the external ear and directly project from there to the brainstem, together with other cranial nerves.121 Auricular stimulation with transcutaneous electrical nerve stimulation is efficacious in pediatric patients with gut-brain axis gastrointestinal disorders and is currently approved by the US Food and Drug Administration for the treatment of irritable bowel syndrome in children. Typical treatment is four weeks of auricular neurostimulation with four electrodes attached to the auricle that administer nonpainful wavelength frequencies.32 A recent study that evaluated the efficacy of auricular neurostimulation in children with CVS showed a decrease in abdominal pain and improvement in anxiety and sleep.122
Calcitonin gene-related peptide antagonistsStudies on migraine have shown that the calcitonin gene-related peptide is released during an acute migraine attack and sumatriptan normalizes its levels.123 This led to the production of calcitonin gene-related peptide antagonists and the use of monoclonal antibodies against the receptor of said peptide. Several studies have shown they are efficacious for reducing the number of days of both episodic and chronic migraines.124 Their role has not been studied in CVS, but given their shared pathophysiology, they should be taken into account for future projects.
Long-term efficacy of treatment with coenzyme Q10 in pediatric patients (COENZYME trial)The main goal of this trial is to compare the frequency of vomiting episodes per year observed during the first year after starting treatment with coenzyme Q10 with the frequency observed the year prior to starting treatment, in children with CVS. The trial is currently in development.
ConclusionsCVS is a chronic and incapacitating disorder associated with the main comorbid factors of migraine and cannabinoid use. At present, the diagnosis of CVS is frequently delayed, and so the goal of this review is to raise awareness about the syndrome in the medical community to favor its timely diagnosis and treatment.
Ethical considerationsThe authors declare this article contains no personal information that could identify the patients.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.