Cannabinoid hyperemesis syndrome (CHS) is a chronic disorder characterized by episodes of severe vomiting, alternating with asymptomatic or minimally symptomatic periods. The episodes of emesis tend to be disabling, negatively affecting quality of life. The disorder’s main characteristic is that it is associated with previous chronic heavy cannabis use. CHS is similar to cyclic vomiting syndrome (CVS), with the exception that the sustained cessation of cannabis use is expected to resolve the vomiting episodes.
The average time between the onset of acute CHS episodes and diagnosis ranges from three to six years, based on previously published cases. This delay in the diagnosis reflects a lack of awareness of the condition on the part of physicians. Delayed diagnosis of CHS results in frequent emergency room visits and increased healthcare costs, and the lack of effective treatment leads to poor patient outcomes. The diagnosis is challenging, and some authors have diagnosed CHS when patients had cyclic vomiting, in the context of chronic cannabis use, regardless of the duration of use or the potency of the product used.
The aim of this narrative review is to provide a detailed and critical analysis of current knowledge about CHS. The present document focuses on a thorough review of the literature on worldwide cannabis use, the role of the endocannabinoid system in the pathophysiology of CHS, diagnostic criteria, and current management of CHS.
El síndrome de hiperémesis cannabinoide (SHC) es un trastorno crónico caracterizado por episodios de vómito intenso alternados con periodos asintomáticos o mínimamente sintomáticos. Los episodios de emesis tienden a ser incapacitantes, afectando negativamente la calidad de vida. Su principal característica es que se asocia a un consumo previo intenso y crónico de cannabis. El síndrome de hiperémesis cannabinoide es similar al síndrome de vómitos cíclicos (SVC), salvo que se espera que el cese sostenido del cannabis resuelva los episodios de vómitos.
La duración media entre el inicio de los episodios agudos de SHC y el diagnóstico oscila entre los tres y seis años con base en los casos publicados previamente. Este retraso diagnóstico demuestra una falta de concientización de los médicos con respecto a esta enfermedad. El retraso diagnóstico del SHC da lugar a frecuentes visitas al servicio de urgencias, aumenta los costes de la asistencia hospitalaria y la falta de un tratamiento eficaz conduce a una mala evolución de los pacientes. El diagnóstico plantea considerables dificultades y algunos autores diagnostican el SHC en pacientes con vómitos cíclicos y consumo crónico de cannabis, independientemente de la duración o la potencia del consumo.
El objetivo de esta revisión narrativa es proporcionar un análisis detallado y crítico de los conocimientos actuales sobre el SHC. Este documento se centrará en una revisión bibliográfica detallada sobre el consumo de cannabis en el mundo, el papel del sistema endocannabinoide en la fisiopatología del SHC, los criterios diagnósticos y el tratamiento actual del SHC.
Over the past two decades, increasingly fewer adults have viewed cannabis use as risky. However, even though some subjects can use cannabis without harm, its use in others has been associated with gastrointestinal disorders, one of which is cannabinoid hyperemesis syndrome (CHS).1,2 CHS is a chronic disorder characterized by episodes of severe vomiting alternating with asymptomatic or minimally symptomatic periods and is associated with previous chronic heavy cannabis use. CHS was first described in 1996 in a 22-year-old man who presented with recurrent nausea and vomiting for a period of two years.3 CHS is similar to cyclic vomiting syndrome (CVS), except that sustained cessation of cannabis use is expected to result in the resolution of vomiting episodes. Episodes of emesis tend to be disabling, negatively impacting quality of life. The prevalence of cyclic vomiting has increased in the US, in parallel with increased cannabis legalization, and hence has been the subject of greater attention by the scientific community in recent years. Nevertheless, data on CHS pathophysiology, associated factors, and treatment remain unknown. Despite considerable heterogeneity in diagnosing the disease, the Rome Foundation established diagnostic criteria for CHS in 2016. Table 1 describes the Rome IV criteria for CHS.
Diagnostic criteria for cannabinoid hyperemesis syndrome according to the Rome IV consensus.
| ●Stereotyped vomiting episodes similar to those present in cyclic vomiting syndrome in terms of onset, duration, and frequency |
| ●Presentation following prolonged and heavy cannabis use |
| ●Clinical improvement after prolonged cessation of cannabis use |
| Criteria met during the past 3 months with onset of symptoms at least 6 months prior to diagnosis. |
However, there are considerable challenges involved in making the diagnosis. Some authors have diagnosed CHS when patients had cyclic vomiting, in the context of chronic cannabis use, regardless of the duration of use or the potency of the cannabis used. Others have diagnosed CHS based on the association with hot-water bathing, though this is not pathagnomonic.4 In addition, many patients are unwilling to stop cannabis use, given its purported benefits, representing a significant diagnostic challenge. CHS results in frequent visits to the emergency department and increased healthcare costs, and the lack of effective treatment leads to poor patient outcomes.
Although the use of cannabis dates back thousands of years, it remains unclear why CHS has only recently become evident in clinical practice.5,6 The use of high potency cannabis products that have been available to the public for the past two decades is postulated to result in hyperemesis.
The aim of this narrative review focuses on a detailed review of the literature on worldwide cannabis use, the role of the endocannabinoid system in CHS pathophysiology, diagnostic criteria, and current management of CHS.
Material and methodsWe conducted a narrative review that assessed and analyzed articles published in the national and international literature on the diagnosis and treatment of CHS. A cross search was carried out on MEDLINE, EMBASE, Web of Science, and Scopus, for all available studies from January 1996 to September 2024, utilizing the following terms and abbreviations in Spanish and English: vomiting, hyperemesis, syndrome, cannabinoids, epidemiology, diagnosis, treatment, guidelines, consensus, and review.
The most relevant articles were identified, and they included technical reviews, systematic reviews, meta-analyses, clinical guidelines, and clinical trials on CHS. Information from observational studies, case series, case reports, abstracts, and intervention studies on patients with CHS were also reviewed. From 313 article abstracts found in the search, information from 147 full-length articles was included. Articles that could not be obtained in full were excluded.
Cannabis use prevalence in North AmericaCannabis is a genus of flowering plants (Cannabis sativa, C. indica, and C. ruderalis) grown for its fiber (hemp) and its use as a medicinal and recreational drug.7 The plant produces a group of chemicals called cannabinoids, whose concentration is highest in the flowers and fruit of the female plant, which when dried, constitutes the most widely consumed form of cannabis.8,9
Cannabis use in the United States of AmericaCannabis has been used in the United States (US) since the 1800s, with public perception about its potential harm decreasing dramatically over time.10 In 1996, US laws accepted the legal use of cannabis for medical reasons. Following this, Colorado became the first state in the US to allow marijuana sales for recreational purposes on January 1, 2014.11 The prevalence of cannabis use in the US was 4.1% in 2001-2002, with an increase to 9.5% in 2012-2013.12,13 More than 52 million people in the US (19%) used cannabis at least once in 2021 (vs 11% in 2002), whereas daily consumption has increased from 18% to 27% among users.14,15 The increase in cannabis use in the US has been observed in all ages and across sexes.16–19 Adults with underlying comorbidities are also reported to be more likely to use cannabis, compared with those without medical conditions. 20,21
Cannabis use in CanadaGlobally, Canada is one of the countries with the highest prevalence of cannabis use. Taking cannabis for therapeutic purposes has been a growing phenomenon in that country.22 In 2001, Canadians could obtain cannabis for medical purposes through the Medical Marijuana Access Regulations, which allowed individuals to possess dried cannabis, when conventional medical therapies were deemed unsuccessful. In 2016, laws permitted the legal sale of dried cannabis, cannabis oils, and fresh cannabis materials and allowed individuals to personally produce or designate someone to grow their own cannabis, and in 2018, the Canadian government legalized the recreational use of cannabis for adults. The Canadian Cannabis Survey estimates that 13% of Canadians aged 16 years or older, take cannabis to manage symptoms associated with a disease or health condition and the highest usage rates are in young adults 20-24 years of age, with 51% reporting having used cannabis in the past year.23
Cannabis use in MexicoIn Mexico, cannabis is the most commonly used illicit substance. In 2016, the Mexican National Institute of Public Health reported that prevalence of lifetime cannabis use was 8.6%. The prevalence of cannabis use for adult males (14%) was higher than for adult females (3.7%). In adolescent males, the prevalence of cannabis use increased from 2.1% in 2002 to 5.8% in 2016, and in female adolescents, it increased from 0.2% in 2002 to 4.8% in 2016. More recently, cannabis consumption was legalized in Mexico in 2021.24 A 2023 Mexican survey reported that 654 (20.7%) subjects identified themselves as active consumers of cannabis. Of those consumers, 74.3% reported using cannabis for recreational purposes, whereas the remaining subjects used it for medical reasons. The majority of cannabis users reported consuming it on a weekly basis, with 42.7% using it 1-2 times per week, and 35.6% using it 3-6 times per week. Only 16.2% reported daily consumption. A minority consumed cannabis on a monthly basis (2.3%) and 3.2% used it 3-6 times per month.25
Endocannabinoid system and cannabinoid receptorsThe endocannabinoid system (ECS) is a widespread neuromodulator system that plays an important role in the regulation of nausea, vomiting, and stress. This system is not restricted to the brain, and is involved in processes, such as modulating immune tolerance,26 gastrointestinal motility, visceral pain, nausea, and vomiting.27 The ECS consists of cannabinoid receptors type 1 (CB1) and type 2 (CB2), along with endogenous ligands (endocannabinoids) and enzymes responsible for endocannabinoid synthesis and degradation. The endocannabinoids, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), activate the two G-protein-coupled membrane cannabinoid receptors (CB1 and CB2), as well as several other non-CB-receptor types. CB1 receptors are densely distributed in areas of the brain, such as the dorsal vagal complex (the nucleus of the solitary tract, area postrema, and dorsal motor nucleus of the vagus), which is part of the emetic circuitry. The CB1 receptors are also found on dopaminergic, noradrenergic, and other transmitters containing neurons in the regions of the brain involved in the control of nausea and vomiting.
CB2 receptors are located throughout the gut, mainly in myenteric and submucosal neurons, and in non-neuronal cells, such as epithelial cells.28 The CB2 receptors are located mainly in inflammatory and epithelial cells, and to a lesser extent, in myenteric and submucosal neurons.29,30
Antiemetic properties of cannabisDelta-9-tetrahydrocannabinol (THC) and cannabidiol are thought to be the two important constituents of cannabis, though there are dozens of other related molecules, with a variety of actions that are typically found at low levels in the plant.7,9,31 THC is the major psychoactive component of cannabis and is thought to be responsible for the majority of its central effects, and activates both the CB1 and CB2 receptors. Its action via the CB1 receptors located in areas of the brain that are associated with the regulation of nausea, vomiting, and the stress response is thought to be responsible for its antiemetic properties.32–34 Cannabidiol is not psychoactive, and unlike THC, acts primarily on the 5HT1A receptors. However, it has actions in the brain and gut that can modulate the effects of THC, and it is a known antiemetic in animals.35–37
Understanding of the ECS has now been broadened to include an expanded repertoire of signaling molecules and receptors, through the inclusion of the endovanilloids and the transient receptor potential vanilloid-1 (TRPV1).38 Since the endovanilloids are also involved in the regulation of emesis, the TRPV1 agonist, capsaicin, is a potential therapeutic for CHS.38–40
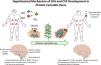
The paradox of chronic heavy cannabis use: increased risk of hyperemesisHeavy chronic cannabis use, in some people, leads to recurrent nausea and vomiting and abdominal pain, and presumably to CHS. Those symptoms are the opposite of what has been outlined above and hence represent a paradoxical effect of cannabis. Temporary relief from said symptoms can be obtained from hot baths and showers, but standard anti-emetic therapy is not particularly effective.41–43 The mechanisms underlying the paradoxical effects are not known, but are speculated to be due to a downregulation of cannabinoid receptors caused by high exposure to ligands. The latter, together with chronic stress in genetically predisposed subjects, appears to have a synergistic effect on the loss of ECS homeostasis, and consequently, the development of CVS and CHS (Fig. 1).44
Role of the endocannabinoid system in the regulation of nausea and vomiting. Proposed pathophysiology that could explain the development of cannabinoid hyperemesis syndrome.
Patients with CHS frequently exhibit a unique compulsive behavior with multiple hot baths or showers that commonly provide temporary symptom relief.45 One of the first few reports of hot-water bathing was by Allen et al.46 in a case series of 18 patients who had recurrent episodes of vomiting and chronic cannabis use. Though this hot-water bathing pattern was thought to be pathognomonic of cannabis use and CHS, the unique bathing behavior has also been reported in 50% of patients with CVS who do not use cannabis.47
Hot-water bathing behavior has been reported to result in marked, albeit temporary, improvement in nausea, vomiting, abdominal pain, and symptoms associated with panic. Symptoms often return within 10-30 minutes after hot-water bathing, which might explain why patients take up to 20 hot showers a day or sit in a hot bath as long as possible. In extreme cases, patients will even resort to checking into a hotel to ensure a continuous supply of hot water. Compared with occasional cannabis users or non-users, regular cannabis users (> 4 times/week) are more likely to use very hot water and remain in baths for longer periods of time to feel the relief provided by the hot water.48 Furthermore, regular users were noted to have a higher burden of GI symptoms and more anxiety, compared with occasional and non-users. About 4% of patients that present with hot-water bathing behavior can even sustain burns, called erythema ab igne, as a result. This hot-water bathing behavior in CHS is not understood and how it contributes to improving symptoms is unclear. The ECS is recognized to be engaged during response to stressful stimuli49: an increase in endocannabinoids occurs within several minutes of exposure to the stress and usually returns to the baseline value within an hour.50 Those data lead to the hypothesis that hot-water exposure may relieve the symptoms of nausea and vomiting because of increased endocannabinoid release and the subsequent activation of CB1 receptors. However, to the best of our knowledge, no one has examined the effects of hot-water exposure on endocannabinoid levels.
In addition, proposed mechanisms include increased core temperature via effects on CB1 receptors in the thermoregulatory centers in the hypothalamus, skin vanilloid-1 receptor stimulation, and blood flow shift from viscera to skin.46 A second target is the TRPV1 receptor, also known as the capsaicin or vanilloid receptor.51 The TRPV1 receptors are distributed in various parts of the gastrointestinal tract and vagal afferents that innervate the stomach and intestine, and are also distributed in the enteric nervous system. Those receptors are activated by heat (temperature > 43 °C) and capsaicin and by high concentrations of anandamide. This suggests that hot-water bathing does not act via TRPV1 receptors, which would require scalding temperatures. Thus, we speculate that mobilized anandamide could initially activate but then desensitize TPRV1, resulting in widespread symptom improvement. Understanding the mechanisms and specific characteristics of this hot-water bathing pattern in CHS will help determine the pathophysiology in CHS and CVS, potentially aiding in the development of targeted therapies for these complex entities.
Cannabinoid hyperemesis syndrome vs. cyclic vomiting syndromeDespite having a similar clinical picture (persistent nausea, abdominal pain, and repetitive vomiting), there are areas of difference between CVS and CHS. It is proposed that CHS may resolve after definitive cessation of cannabis use, although this has been difficult to ascertain, given the lack of long-term follow-up in most of these patients, except for the case series by Allen et al.46 A study by Venkatesan et al.52 demonstrated cannabis use patterns in CVS (Fig. 2).
Cannabis use patterns in patients diagnosed with cyclic vomiting syndrome.
Detailed information about the duration, frequency, and potency of cannabis use in CHS is scarce. More importantly, most reports on CHS have little or no follow-up, making it difficult, if not impossible, to ascertain whether cannabis was truly the cause of the hyperemesis. In a systematic review, the median duration of cannabis use before the onset of CHS symptoms was 42 months and the median age of starting cannabis use in patients with CHS was 16 years.40 Duration of cannabis use was reported to be ≤ 1 year for 25.1%, 2-5 years for 36.3%, 6-10 years for 16.8%, and ≥ 11 years for 21.8% of patients. Cannabis use frequency was > 1 time per day in 23.7%, daily in 47.9%, weekly in 19.4%, and < 1 time per week in 2.4%.40 In addition, a recent systematic review by Venkatesan et al.4 identified 25 case series and 101 individual case reports of patients identified with CHS.43,45,46,53–119 They reported a significant heterogeneity in the criteria used to define CHS cases. The diagnosis of CHS was made based on multiple criteria, including the hot-shower bathing pattern, cyclic vomiting, and cannabis use. The authors of that systematic review highlighted the lack of details in the literature, making interpretation difficult, as well as the use of cyclic vomiting with hot-shower bathing patterns as pathognomonic features of CHS, which most likely led to an overestimation of CHS. The authors also highlighted the need for systematic studies on cannabis use in CVS, in relation to hyperemesis.
Clinical presentation and diagnosis of CHSCHS is a chronic disorder of gut-brain interaction included in the classification of functional gastrointestinal disorders in the 2016 Rome IV criteria.62
CHS is characterized by cyclic vomiting, nausea, and abdominal pain, and in some cases it is associated with prolonged hot-water bathing behavior.43,46 Although the hot-water bathing pattern is not pathognomonic of CHS,120 it is significantly associated with CHS.47 In a systematic review of patients diagnosed with CHS, the mean duration of cannabis use before symptom onset was 6.6 years, daily use occurred in 68%, and hot-water bathing was reported in 71% of patients.4
The Rome IV criteria 121 are the most widely accepted diagnostic criteria for CHS, but they are mostly qualitative in nature. Other researchers have attempted to generate more quantitative criteria to differentiate CHS from CVS. As part of their systematic review, the eight members of the CVS Practice Guidelines Committee detailed features of cannabis use for helping CHS to be diagnosed more accurately, and included duration of cannabis use prior to CHS onset, frequency of use, and amounts of cannabis used.4 Based on those data, the following clinical diagnostic criteria for CHS and elements to be identified in a patient’s history were proposed by that committee4:
- 1
Clinical features: stereotypic episodic vomiting resembling CVS, in terms of onset, and with a frequency of 3 or more times annually.
- 2
Cannabis use patterns: duration of cannabis use > 1 year before symptom onset; frequency > 4 times per week, on average.
- 3
Cessation of cannabis use: resolution of symptoms after a period of abstinence from cannabis use for at least 6 months, or at least equal to the total duration of three typical vomiting cycles.
The approach to the diagnosis of CHS also requires a good clinical history and anatomic evaluations, with an upper gastrointestinal endoscopy and/or computed tomography scan of the abdomen to exclude outlet obstruction and other organic causes of vomiting.
An extensive diagnostic evaluation should be avoided unless there are specific features that suggest an alternative diagnosis. Other differential diagnoses that physicians might consider include rumination syndrome, gastroparesis, CVS, pregnancy, migraine, and functional chronic nausea and vomiting syndrome.122
Treatment in CHSIn general, CHS treatment is similar to CVS treatment and can be divided into abortive and preventive therapies. Experts agree that CHS is a subset of CVS and recommend that patients with a pattern of CVS and cannabis use be offered the same treatment as those with CVS.
For long-term management (prophylactic therapy), counseling to achieve the cessation of marijuana use and tricyclic antidepressants (TCAs), such as amitriptyline, are the mainstay of therapy.123
Abstaining from cannabis use is necessary for successful treatment of CHS. Moreover, advising patients with CHS to stop cannabis use immediately may be associated with significant withdrawal symptoms and high frequency of recidivism. Hence, advising slow reduction in frequency and switching to lower potency products (less THC) might be a more successful strategy in a clinical setting.
Abortive therapyEvidence for treatment in CHS is limited to case series and small clinical trials supporting the use of topical capsaicin, benzodiazepines, haloperidol, promethazine, olanzapine, and ondansetron, among others, for acute vomiting attacks.5,46,124–126
Topical CapsaicinTopical capsaicin cream has shown short-term success as an adjunctive therapy in patients suffering from CHS.39 Topical capsaicin (0.1%) cream may improve symptoms by activation of TRPV1 receptors, but data are limited. Topical capsaicin is applied to the upper abdomen and may reduce the need for antiemetics.127–129 Capsaicin may cause redirection of blood flow from the enteric system to the skin, leading to activation of TRPV1 receptors and reducing substance P.130 A randomized clinical trial examined topical capsaicin (0.1%) versus placebo cream applied to the abdomen and the authors reported a 46% reduction in nausea from the baseline in the capsaicin arm versus 24.9% in the placebo arm. However, vomiting or abdominal pain (the cardinal features of CHS) were not evaluated.128 But a recent retrospective study found a modest improvement in abdominal pain after two hours of capsaicin application.131 A retrospective cohort study showed that length of stay in the emergency department was modestly reduced by a median of 22 minutes (201 vs 179 min, p = 0.33) with topical capsaicin at different percentages (0.025-0.1%) applied to the abdomen. Furthermore, patients received fewer additional medications, if capsaicin was utilized (4 vs 3 doses, p = 0.015).132
Notably, the case series using capsaicin cream have a small patient sample cohort; as a result, efficacy of capsaicin should be evaluated in larger prospective cohorts. 133
DroperidolDroperidol is a short-acting dopamine antagonist used as an antiemetic and antipsychotic agent.133 A systematic review conducted by Furyk et al.134 examined the role of droperidol in the management of CHS. It was found that 0.625-2.5 mg of intravenous (IV) droperidol showed statistically significant differences in the visual analog scale (on a line from 0 to 100, with zero corresponding to ‘no nausea’ and 100 to ‘unbearable nausea’) compared with placebo in 48 patients.134 Current evidence has shown that droperidol administration for CHS patients resulted in a shorter length of stay in the hospital, decreased necessity of other antiemetics (ondansetron and metoclopramide), and demonstrated a significant decrease in nausea severity from the baseline value, when compared with placebo.135
BenzodiazepinesA case series of four treatment-resistant patients with CHS examined benzodiazepines (clonazepam) as a treatment option. The study revealed that 1 to 2 doses of 0.5 mg of oral clonazepam led to rapid cessation of symptoms, complete symptomatic relief, and discharge within 24 hours after administration.136 It should be noted that the evidence for benzodiazepine use in CHS is limited. Its mechanism of action is the enhancement of gamma-aminobutyric acid activity, which is the major inhibitory neurotransmitter in the central nervous system. That mechanism reduces anticipatory nausea and vomiting.137 In addition, benzodiazepines decrease CB1 receptor activation in the frontal cortex, enhancing its antiemetic effect.138
HaloperidolHaloperidol has been used in the management of nausea and vomiting in severe CHS cases.135,136 A case report by Inyat et al.139 showed that with 2 mg of IV haloperidol (Haldol, Janssen Pharmaceuticals, Raritan, New Jersey, USA) a 27-year-old patient had clinically significant improvement without any adverse reactions. His compulsive hot bathing and gastrointestinal symptoms began to diminish following two subsequent doses of 2 mg of IV haloperidol. The patient reported complete resolution of refractory nausea, vomiting, and abdominal pain after administration of the above schedule and revealed no recurrent symptoms at follow-up at one month.139 A case series carried out by Witsil and Mycyk140 found 5 mg of IV haloperidol resulted in successful relief of nausea and vomiting for 4 patients in the emergency department. A recent randomized controlled trial conducted by Ruberto et al.126 examined haloperidol and ondansetron use for CHS. A dose of 0.05 or 0.1 mg/kg of haloperidol was superior to ondansetron in decreasing nausea and vomiting and there was less use of rescue antiemetics, as well as a shorter stay in the emergency department. However, there were two return visits for acute dystonia, both in the 0.1 mg/kg dose haloperidol group.126 Notably, the majority of evidence found for haloperidol came from case series, limiting its generalizability.139–141
PropranololRichards and Dutzak142 presented a case report that examined an extreme case of CHS in the emergency department. The patient had intractable nausea, vomiting, and abdominal discomfort that was unresponsive to standard antiemetics. One milligram of IV propranolol led to rapid termination of the nausea and vomiting. The treating emergency physician chose propranolol and its dose, based on experience and successful outcomes in CVS patients. The evidence for propranolol use is limited, as that was the only case we found in literature.
AprepitantAprepitant is a neurokinin 1 receptor antagonist and is FDA-approved to treat chemotherapy-induced nausea and vomiting in the US. A case report by Parvataneni et al.143 described a 30-year-old female with intermittent nausea and vomiting who was unresponsive to conventional antiemetics, such as ondansetron and metoclopramide. She was discharged symptom-free 24 hours after the administration of aprepitant. However, in that case, the patient reported intermittent nausea and vomiting for four years and chronic marijuana use a few times a week for four years. She did not meet the clinical symptom resolution criteria after abstaining from cannabis use for at least 6 months and might have had CVS.
Prophylactic therapyProphylactic treatment is recommended for patients with moderate/severe disease (those with ≥ 4 episodes per year of severe nausea and vomiting that lead the patient to seek medical attention in the emergency department or require hospitalization). TCAs are first-line drugs for the prophylaxis of CHS and CVS and have been shown to reduce the duration, severity, and frequency of episodes, as well as the number of emergency department visits and hospitalizations.144 Amitriptyline is usually started at a low dose of 25 mg and gradually increased in increments of 10 mg each week to a target dose of approximately 100 mg at night in adults. TCAs can cause QT interval prolongation, so an electrocardiogram should be performed at baseline and during TCA titration to monitor it.145 Data gleaned from mostly retrospective and open label trials provide evidence that amitriptyline is effective in approximately 70% of patients.146
Clinical and economic impact of CHS and CVSCVS and CHS are associated with a significant healthcare burden and costs. Delayed and/or misdiagnosis and treatment can lead to increased frequency of seeking care in the emergency department or increased number of hospitalizations, with their consequent economic impact. The hospitalization outcomes of patients with CVS, obtained from a nationwide inpatient sample database from January 2010 to December 2011, were recently examined. The study included 20,952 CVS patients and 44,262 non-CVS patients and the CVS patients tended to be younger, male, and white, compared with the non-CVS patients. In addition, CVS was significantly associated with comorbidities (dysautonomia, migraine, anxiety, marijuana use, irritable bowel syndrome, gastroparesis, gastroesophageal reflux disease, asthma, cigarette smoking, and hypertension). CVS patients also had a greater number of gastrointestinal diagnostic studies, lower mortality, and shorter length of stay. The entire CVS cohort incurred about $4,000,000.00 USD in total hospital costs in the 2 years analyzed.147
Chen et al.148 reported that patients diagnosed with CVS had significantly higher annual total health care costs ($57,140 vs $14,912) than non-CVS controls. They also reported that total health care costs were four-times higher for patients with CVS, in relation to non-CVS controls.148 Marshall A et al. showed that the mean cost of hospitalization was significantly higher post-legalization ($18,714 vs $7,460), p < 0.0005, even after adjusting for medical inflation ($18,714 vs $8,520), p < 0.001.149
ConclusionsCannabinoid hyperemesis syndrome (CHS) is an emerging, complex entity, considered to be a subset of CVS and is expected to increase in prevalence with increased legalization and use of high-potency cannabis products. CHS can lead to high healthcare resource utilization, causing enormous economic losses for both the healthcare system and the patient. Currently, the treatment of CHS is mainly based on the available pharmacological evidence on the management of CVS and on small case series. There is a need to better understand the role of cannabis in CHS and its pathophysiology, which will help inform patients and the medical community about the effects of cannabis use on gastrointestinal health.
Ethical considerationsThe present article is a narrative review of cannabinoid hyperemesis syndrome. No animal or human experiments were performed or involved during the course of the review, so ethical committee authorization was not needed. No personal data that would allow identification of patients are published in the present article; therefore, informed consent was not requested for publication of this narrative review.
Financial disclosureNo financial support was received in relation to this study/article.
The authors declare that there is no conflict of interest.