Functional dyspepsia (FD) is a highly prevalent condition characterized by upper gastrointestinal symptoms with no apparent organic cause. It is a complex and multifactorial disease that frequently overlaps with other disorders of gut-brain interaction. It is recurrent, has a variable therapeutic response, and affects patient quality of life. Our aim was to formulate good practice recommendations for the management of FD through a consensus review of the disease, updating and complementing the 2017 consensus on dyspepsia from the Asociación Mexicana de Gastroenterología (AMG).
MethodsSixteen experts summoned by the AMG carried out a literature review (2017–2024) and formulated good clinical practice recommendations for the diagnosis and treatment of FD. They were discussed until reaching a consensus, and the most recent evidence on the theme was evaluated, utilizing the GRADE system.
ResultsTwenty-three good clinical practice recommendations for the management of FD were developed that addressed the following aspects: (1) definition, pathophysiology, and epidemiology; (2) diagnosis; (3) nonpharmacologic treatment; (4) Helicobacter pylori eradication; (5) antisecretory and anti-acid therapy; and (6) prokinetics and neuromodulators.
ConclusionsFD is one of the most frequent gastrointestinal conditions seen in daily practice. We present good clinical practice recommendations for the specific management of this disorder, taking into account the most recent advances that complement and update the consensus on dyspepsia published by the AMG in 2017.
La dispepsia funcional (DF) es un padecimiento de alta prevalencia que se caracteriza por la presencia de síntomas del tracto gastrointestinal superior sin causa orgánica evidente. Es una enfermedad compleja y multifactorial, que frecuentemente se traslapa con otros trastornos de la interacción cerebro intestino; es recurrente, tiene una respuesta terapéutica variable y afecta la calidad de vida de quienes lo padecen.
ObjetivoElaborar recomendaciones de buena práctica para el manejo de este trastorno, que surjan de una revisión consensuada de la DF, que actualicen y complementen el consenso sobre la dispepsia de la Asociación Mexicana de Gastroenterología (AMG) 2017.
MétodosDieciséis expertos convocados por la AMG, realizaron una revisión de la literatura (2017–2024) y elaboraron recomendaciones de buena práctica clínica en el diagnóstico y tratamiento de la DF, que fueron discutidas hasta alcanzar consenso, calificando la evidencia más reciente sobre el tema con base en el sistema GRADE.
ResultadosSe elaboraron 23 recomendaciones de buena práctica clínica para el manejo de la DF que abarcaron las siguientes áreas: (1) definición, fisiopatología y epidemiología; (2) diagnóstico; (3) tratamiento no farmacológico; (4) erradicación de Helicobacter pylori; (5) antisecretores y terapia contra el ácido; y (6) procinéticos y neuromoduladores.
ConclusionesLa DF es uno de los padecimientos digestivos más frecuentes en la práctica diaria. Presentamos las recomendaciones de buena práctica en el manejo específico de este trastorno, tomando en cuenta los avances más recientes, que complementan y actualizan el consenso sobre la dispepsia publicado por la AMG en 2017
Functional dyspepsia (FD) is a disorder of gut-brain interaction (DGBI) that affects a substantial portion of the general population and is characterized by upper gastrointestinal symptoms, which after the appropriate diagnostic evaluation, have no apparent organic cause.1 This condition is a challenge for the physician due to its complex pathophysiogenesis, frequent overlapping with other gastrointestinal syndromes, recurrent pattern, and variable therapeutic response.
In 2016, a group of gastroenterologists belonging to the Asociación Mexicana de Gastroenterología (AMG) carried out a consensus on dyspepsia that was published a year later.2 Since then, greater knowledge of this disease has been developed and new therapeutic alternatives have appeared, justifying the creation of a new document to update the 2017 consensus. In March 2024, the AMG summoned a working group to carry out a review, evaluate evidence, and formulate good practice recommendations, specifically focusing on functional dyspepsia and its diagnosis and treatment.
This document aims to present a consensus review on the current status of FD, through the formulation of good clinical practice recommendations for the diagnosis and treatment of FD, updating and complementing the 2017 consensus on dyspepsia, integrating the new scientific evidence published internationally for its practical application.
MethodsThis expert review was commissioned by the AMG. The working group participants were selected based on their recognized academic, teaching, research, and healthcare trajectory with a special interest in dyspepsia and DGBIs. The same method employed for the formulation of the AMG’s 2017 Mexican Consensus on Dyspepsia was utilized.2 Working subgroups were formed in the following areas: (1) definition, pathophysiology, and epidemiology; (2) diagnosis; (3) nonpharmacologic treatment; (4) Helicobacter pylori (H. pylori) eradication; (5) antisecretory and antacid therapy; and (6) prokinetics and neuromodulators. The members of each subgroup carried out a bibliographic review utilizing “functional dyspepsia”, combined with the following terms: “epidemiology”, “incidence”, “prevalence”, “pathophysiology”, “inflammation”, “microbiota”, “diagnosis”, “differential diagnosis”, “treatment”, “therapy”, “management”, “review”, “guidelines”, and “meta-analysis” as search terms, as well as their Spanish equivalents. The search was conducted in PubMed on literature from January 2016 to May 2024, and then complemented by one of the coordinators (RCS) with works up to September 2024. All publications in English and Spanish were included. Preference was given to consensuses, guidelines, systematic reviews, and meta-analyses, but not limited to them. Complementary searches in the archives of the Revista de Gastroenterología de México and all publications the coordinators considered relevant up to September 2024 were also carried out.
After reviewing each theme, statements with good clinical practice recommendations were formulated and sent to all members of the group for their electronic discussion. The second version of the statements was discussed at a face-to-face meeting in Guadalajara, Jalisco (Mexico), in May 2024. At that meeting, the quality of evidence sustaining each statement was established, employing the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.3Table 1 describes the GRADE system codes. The third version of the statements underwent a final electronic round of anonymous voting. The votes were issued, utilizing a 5-point scale: (A) in complete agreement; (B) in partial agreement; (C) uncertain; (D) in partial disagreement; and (E) in complete disagreement. The final statements agreed upon follow below.
GRADE systema.
| Quality of evidence | Code |
|---|---|
HighFurther research is very unlikely to change our confidence in the estimate of an effect.
| A |
ModerateFurther research is likely to have an important impact on our confidence in the estimate of an effect and is likely to change the estimate.
| B |
LowFurther research is very likely to have an important impact on our confidence in the estimate of the effect and is likely to change the estimate.
| C |
Very lowAny estimate of effect is very uncertain.
| D |
GRADE: Grading of Recommendations Assessment, Development and Evaluation.
FD is a chronic and heterogeneous disorder characterized by proximal gastrointestinal tract symptoms, in the absence of metabolic or structural diseases detectable through an appropriate diagnostic evaluation that includes upper gastrointestinal endoscopy.
Quality of evidence: A.
Level of agreement: 81% in complete agreement; 19% in partial agreement.
FD is defined as the presence of chronic symptoms originating from the gastroduodenal region and is mainly characterized by 4 cardinal symptoms: postprandial fullness, early satiety, epigastric pain, and epigastric burning sensation, symptoms which may or may not be related to food intake.1,2 When the patient presents with gastroduodenal symptoms, but has not yet been studied, a definitive diagnosis cannot be made, and should be classified as uninvestigated dyspepsia. Organicity is impossible to rule out, based only on symptomatology; if the conditions of the individual or his/her risk factors warrant it, studies for evaluating the symptoms should be carried out, including upper gastrointestinal endoscopy.4 Therefore, the diagnosis of FD requires the presence of symptoms in the absence of organic disease that is endoscopically confirmed.1,2
After an appropriate evaluation, approximately 80% of patients with dyspepsia have been reported to have no organic, systemic, or metabolic alterations that explain their symptoms; these are the patients that should be categorized as having FD.5 However, there are certain exceptions to those considerations in clinical practice. Even though endoscopy is considered mandatory for diagnosing FD, that does not apply to young people that have no risk factors or alarm symptoms and who can be approached through the strategies discussed further ahead in the Diagnosis section of this document. Another controversial exception is H. pylori infection, whose presence does not rule out the diagnosis of FD, despite being an organic condition. This will be discussed in the “Helicobacter pylori infection” statement.
In recent years, symptom-based diagnostic criteria have been developed and perfected, with the intention of making a direct, positive diagnosis. The latest version of the Rome criteria (Rome IV), which are the most widely accepted for defining and classifying the different DGBIs, defines FD as a disorder characterized by at least one of the following symptoms: postprandial fullness, early satiety, and epigastric pain or burning sensation, with no signs of an organic disorder that could explain the symptomatology.1 Both conditions must be met and the symptoms have to be present for the past 3 months (for one or more days per week) and have started at least 6 months prior to diagnosis.6,7 Other symptoms, such as nausea, belching, and bloating, can be present in patients with FD and are considered part of the spectrum. Their presence may reflect shared pathophysiologic mechanisms, such as altered gastroduodenal motility and hypersensitivity.8 Despite the absence of structural abnormalities in the gastrointestinal tract, patients with FD experience a decrease in their quality of life and productivity. This disease also produces elevated healthcare costs, for both the patient and the healthcare systems.9
PathophysiologyThe pathophysiology of FD is multifactorial. The symptoms are produced by a complex interaction of dietary, genetic, allergic, infectious, inflammatory, and psychologic comorbidity factors, among others.
Quality of evidence: B.
Level of agreement: 94% in complete agreement; 6% in partial agreement.
The mechanisms involved in the production of dyspeptic symptoms are multiple; they tend to coexist, can enhance each other, and are not mutually exclusive. They include an increase in permeability in the duodenal mucosa, low-grade inflammation, altered immune system signaling and activation, gastric accommodation abnormalities, hypersensitivity, and hypervigilance, among others.10,11
The Rome IV criteria (as will be addressed in the following sections) classify patients with FD into those with postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS).1 In the subjects with PDS, a close relation between symptoms and altered gastric accommodation has been reported.10,12 Gastric emptying has been found to be significantly delayed in at least 35% of patients with FD, and is involved in nausea, vomiting, and the uncomfortable feeling of fullness. However, great disparity between the subgroups has been described, making the patterns nonconclusive.8,9,13 Nitric acid synthase inhibition in healthy volunteers has been shown to lead to gastric accommodation suppression and the sensation of early satiety. That alteration in response to foods is a key element in the pathology of FD, particularly in patients with PDS, which is present in 40% of cases.9,12,14
Gastric acid has been implicated as a causal agent of FD. The infusion of acid in the stomach induces dyspeptic symptoms in healthy adults and increases them in patients with dyspepsia.9,12 Hypersensitivity to acid in the duodenum is associated with nausea, and hypersensitivity to gastric distension is associated with postprandial fullness and belching.10–12
Acute infectious gastroenteritis doubles the risk for presenting with FD (odds ratio [OR] 2.54, 95% confidence interval [CI] 1.76–3.65) and signs of microinflammation in the duodenum, such as the presence of eosinophils and mast cells, have been reported.10,15,16 Recent studies have shown that alterations in the function of the duodenal epithelial barrier and low-grade infection are correlated with the infiltration of immune cells and the activation of localized immunity that can cause mechanical and chemical hypersensitivity. Duodenal eosinophil density and degranulation grade have been associated with early satiety.9,15,16
There are numerous reports on the role of the gut microbiota in the pathophysiology of FD. Alterations in its composition can affect intestinal permeability and influence symptom development and exacerbation.17,18 In recent years, there is evidence underlining the importance of duodenal microbiota alterations in producing dyspepsia. A systematic review that included 9 studies comparing the duodenal microbiota of 391 subjects with dyspepsia and 132 controls with no dyspepsia, identified a significant increase in the abundance of the Fusobacteria phylum and the Alloprevotella, Corynebacterium, Peptostreptococcus, Staphylococcus, Clostridium, and Streptococcus genera. In contrast, there was a pronounced decrease in the Actinomyces, Gemella, Haemophilus, Megasphaera, Mogibacterium, and Selenomonas genera in the patients with FD. There was also a negative correlation in the changes in relative abundance between Streptococcus and Prevotella that was correlated with symptom severity in the patients with FD. Those duodenal microbiota alterations are associated with greater symptomatic burden, which in turn, affects quality of life.18 These findings suggest that alterations in the duodenal microbiota can be crucial for FD symptom appearance and expression, emphasizing the significant role of the duodenal microbiota in the gut-brain interactions in FD.
Adverse childhood experiences (such as physical, mental, or emotional abuse) have a deep impact on the physical and mental health of persons in the long term, increasing the risk for developing visceral hypersensitivity in adulthood, and are recognized as an important part of DGBI pathophysiogenesis. Population studies have found that a history of physical or sexual abuse in childhood, as well as psychologic stress, are significantly more prevalent in subjects with dyspepsia and 37% of them are reported to present with hypersensitivity to gastric distension.7,9 Because the central nervous system and the immune system are not completely mature at birth and continue maturing during the postnatal period, the hypothesis of a bidirectional interaction between the central nervous system and the immune system has been posited, in which stressful factors in childhood are fundamental in priming individuals for later adult psychopathology. Preclinical studies have indicated that stress can cause the intestinal dysbiosis that alters central nervous system function and behavior, reinforcing the concept of the bidirectional communication of the gut-brain axis.19 Psychologic stress increases duodenal permeability through mast cell activation, mediated by the release of the hormone, corticotropin, resulting in increased permeability and microinflammation.
Neuroimaging studies in patients with FD have shown anomalies in several regions of the brain, such as the frontal cortex and somatosensory cortex.10,20 Sleep disorders, insufficient exercise, irregular food intake patterns and different dietary components have been associated with FD.19,21 The intake of fat and other foods aggravates symptoms in some patients.21,22
Certain genetic alterations have been related to FD, such as the GNB3 825 > T, SCL6A4 5HTTLPR, and CCK-1R 779 > C polymorphisms. A meta-analysis found that the minor allele (T) in GNB3 825C > T was associated with increased susceptibility to EPS.23
The relation between food allergies and inflammatory cell infiltration into the gastroduodenal mucosa is a subject of debate.10,24
EpidemiologyThe worldwide prevalence of FD has been reported at 10–40% of the general population.
Quality of evidence: A.
Level of agreement: 69% in complete agreement; 31% in partial agreement.
The prevalence of FD varies widely due to different factors, such as the criteria utilized to define its presence, the populations studied, and the methods used for interviewing subjects, among others. A survey conducted on an open population from 3 different countries, utilizing the Rome IV criteria, showed that the prevalence of FD was 10% (8% in the United Kingdom, 12% in Canada, and 12% in the United States); 61% of those surveyed had PDS, 18% EPS, and 21% overlap of both types.25 A study conducted in 4 Latin American countries (Argentina, Brazil, Colombia, and Mexico) that analyzed internet surveys applied to more than 8,000 patients, utilizing the Rome IV criteria, reported a prevalence of FD between 6.59 and 10.6% and a predominance of PDS and women.26
The population studies that have utilized prior endoscopy for establishing the diagnosis of FD provide better prevalence estimates in the community. Only 30% of adults with uninvestigated dyspepsia and no alarm symptoms have significant lesions detected through endoscopy.27 Studies conducted in Scandinavian countries and Italy have reported a prevalence in those patients between 10 and 16%.7 In Mexico, no studies have been conducted that evaluate dyspepsia through endoscopy in open populations.
A systematic review and meta-analysis of 44 studies, representing 80 independent adult populations, included 256,915 participants from 40 countries worldwide and established an overall prevalence of FD of 8.4% (95% CI 7.4–9.5). Prevalence was highest utilizing the Rome I criteria (11.9%, 95% CI 5.1–25.4), whereas it was the lowest, with the Rome IV criteria (6.8%, 95% CI 5.8–7.9).28 FD was more frequent in developing countries than in developed countries (9.1 vs 8.0%) and in women than in men (9.0 vs 7.0%). The analysis revealed that prevalence has decreased over time, from 12.4% (1990–2002) to 7.3% (2013–2020). The variation in prevalence appears to depend on the country, economic level, geographic region, and sex, with a general tendency to decrease.
The incidence of FD has not been studied nearly as much. A Belgian report whose data were obtained from a morbidity registry network based on general practice established an incidence of 109/100,000 inhabitants, analyzing more than half a million registers over 2 decades.29
FD is more frequent in women, in individuals with a high body mass index, in middle-aged adults, and in subjects with psychologic comorbidities. Smoking, nonsteroidal anti-inflammatory drug use, previous acute gastroenteritis symptoms, H. pylori infection, and antibiotic use are among the predisposing factors.2,8,25,26,29
Recognizing functional dyspepsiaDiagnostic criteria and overlap with other disordersDiagnostic criteria based on symptoms and the description of subgroups that make up functional dyspepsiaThe symptoms that characterize FD are epigastric pain, a burning sensation in the epigastrium, postprandial fullness, and early satiety. FD is classified into 2 subtypes: epigastric pain syndrome and postprandial distress syndrome.
Quality of evidence: B.
Level of agreement: 100% in total agreement.
Previous versions of the Rome IV criteria introduced minor changes to improve symptom specificity and define the minimal thresholds that enabled the frequency and severity of each individual symptom to be more accurately established. Symptoms must be at least severe enough to be identified as “bothersome”, clinically defined as “sufficiently severe to affect daily activities”. The semiquantitative definition of “bothersome” can also be used if symptom intensity is estimated at 2 points or higher on a 5-point scale. Likewise, the proposal of a minimal frequency for distinguishing persons with the disease was proposed. Thus, the limits for symptom frequency were based on data indicating that no more than 5% of the normal population would experience each symptom with said frequency.1
The diagnosis of FD requires the presence of one or more of the following 4 symptoms: epigastric pain, epigastric burning sensation, postprandial fullness (severe enough that it affects daily activities), or early satiety (severe enough that it prevents finishing a regular-sized meal). The symptoms should be present at least once a week over the past 3 months and have started at least 6 months prior to diagnosis.
EPS typically manifests as epigastric pain and/or epigastric burning sensation but there can also be postprandial bloating, nausea, and belching. Said pain can be caused or ameliorated by food intake or can present during fasting. Likewise, symptoms are not modified by bowel movements or flatulence.
In PDS, in addition to postprandial fullness and/or early satiety, patients report having postprandial epigastric pain or burning sensation, epigastric distension, excessive belching, and nausea. Symptoms that are modified by bowel movements or flatulence should not be considered part of dyspepsia and the presence of vomiting indicates the possibility of a different problem.
When obtaining the clinical history, the patient should be directly asked about alarm features or risk factors for organic diseases, and possible side effects from other treatments should be ruled out. FD is diagnosed when no organic etiology is identified that could explain the symptoms and the definitive diagnosis almost always requires the performance of upper gastrointestinal endoscopy, except in the cases previously described, which will be elaborated upon further ahead.1,2,8
Regarding the Spanish version of this document, the use of the term “distrés” posprandial, which mirrors the English term, “postprandial distress”, was discussed by the working group in the preparation of these recommendations, because in a strict sense, distrés is not a Spanish word. Even though we consider that plenitud (fullness) and saciedad (satiety) describe this subgroup of subjects with FD well, we decided to use the word distrés to concur with the most recent Rome criteria. Likewise, we use the term síndrome de distrés posprandial (SDP) (the equivalent of the English “postprandial distress syndrome [PDS]”) to refer to the subgroup with FD that is predominantly characterized by postprandial plenitud (fullness) and saciedad (satiety).
Overlap with other gastrointestinal disordersPatients with FD commonly present with other disorders of gut-brain interactions.
Quality of evidence: A.
Level of agreement: 100% in total agreement.
It is well-known that some patients with FD can have more than one DGBI, most likely because they share pathophysiologic mechanisms. This has mainly been reported in patients seen at referral centers or tertiary care hospitals.8,26,30
Even though the Rome criteria have demonstrated their usefulness, especially for epidemiologic studies, they do not take overlapping disorders into account. Symptom overlap can complicate the diagnosis, and consequently, treatment. Optimum management of those cases can require the collaboration of different medical specialties.31
Reports from several Asian countries state that more than 80% of patients with FD have overlap with another DGBI. Based on factor analysis studies of symptom clustering, an Asian Pacific Association of Gastroenterology working group has proposed 4 groups of FD with symptom overlap:32
- a)
Gastroesophageal reflux disease (GERD)-FD: sensation of epigastric burning or postprandial fullness associated with belching, acidity, and possibly with dysphagia.
- b)
Irritable bowel syndrome (IBS)-EPS: epigastric pain or burning sensation associated with food intake that improves after a bowel movement or flatulence, and that was preceded by a change in the number of bowel movements.
- c)
IBS-PDS: fullness sensation, bloating, belching, and flatulence.
- d)
Constipation-FD: pain or discomfort in the superior part of the abdomen associated with less frequent or harder-consistency bowel movements.
The prevalence of each one of those 4 groups was calculated through a survey of the members of an expert panel, recognizing that there could be other possible overlap between the different DGBIs.32
Asian and Western studies have found an overlap of FD and GERD symptoms of 40–50%: EPS has been associated with nonerosive GERD, and PDS more frequently overlaps with functional heartburn. IBS has been found to be 8-times more frequent in persons with FD. In Mexico, the survey-based SIGAME 2 study, which evaluated 1,000 subjects in an open population, found overlap between dyspepsia and GERD in 8.1% of those surveyed, dyspepsia and IBS in 6.5%, and GERD, dyspepsia, and IBS in 3.1%.33 A study conducted on a retrospective cohort of 308 individuals with refractory functional constipation found that 38.6% had concurrent FD.34 A survey applied to residents of Olmsted county (Minnesota, USA) showed a prevalence of rumination syndrome and FD of 5.8% and 7.1%, respectively, but overlap was 3.83-times greater than that expected by chance.35 The identification of overlap between FD and other DGBIs could represent additional therapeutic opportunities, as well as contribute to the knowledge about possible shared pathophysiologic factors.
Recommendations for the diagnosis of functional dyspepsiaEndoscopyThe performance of proximal gastrointestinal endoscopy is necessary for diagnosing FD.
Quality of evidence: A.
Level of agreement: 100% in total agreement.
According to the currently accepted definition of FD (Rome IV), dyspeptic symptoms due to organic alterations must be ruled out.1 Based on that definition, endoscopy is useful and necessary for detecting possible organic causes of dyspepsia and the procedure is considered mandatory for the diagnosis of FD.8 Nevertheless, it is important to distinguish between the management of uninvestigated dyspepsia and the diagnosis of FD, concepts that are often confused and overlap.
It is well-known that most patients with uninvestigated dyspepsia have no significant endoscopic findings, including the presence of malignant tumors.12,27 Therefore, the majority of clinical guidelines and consensuses currently recommend endoscopy when patients with uninvestigated dyspepsia present with risk factors (including age) or alarm symptoms.10,36 The low prevalence of clinically relevant gastroduodenal and esophageal lesions seen through endoscopy, added to the high cost and inherent risks of an invasive procedure, have led to a change in the recommendations of different clinical guidelines, regarding the indications for endoscopy in patients with uninvestigated dyspepsia, seeking a more cost-effective approach at the individual and social levels.8,10 A recent systematic review and meta-analysis that included 15 clinical trials and 41,763 participants37 showed that more than 85% of the endoscopies performed on patients with dyspepsia were normal and gastroesophageal cancer was a very rare finding (< 0.4%). The most frequent lesion viewed endoscopically in patients with dyspepsia was erosive esophagitis (accumulated prevalence 11.0%, 95% CI 8.9%–13.2%), followed by peptic ulcer (accumulated prevalence 4.4%, 95% CI 2.5%–6.7%). Upon comparing the prevalence of lesions between subjects with and without dyspepsia, only peptic ulcer was more frequent in the former (OR 1.61, 95% CI 1.08–2.39).
The indications for performing endoscopy in patients with uninvestigated dyspepsia are8,10,38,39:
- a)
Recent onset of symptoms in patient ≥55 years of age.
- b)
Alarm features: significant weight loss, anemia, iron deficiency, thrombocytosis, gastrointestinal bleeding, persistent vomiting, progressive dysphagia, and odynophagia.
- c)
Risk factors in subjects that have not yet undergone endoscopy: first-degree relatives with esophageal or gastric cancer and relatives with genetic syndromes that increase the risk for cancer.
- d)
Refractory dyspepsia (with no response after first or second-line treatment or with recurrence), if they have not had a previous endoscopy.
From a cost-effectiveness perspective, different clinical guidelines do not agree on the starting age at which endoscopy should be performed as the initial approach to dyspepsia: it can be from 40 years of age in areas at high risk for gastric cancer, with some guidelines suggesting 60 years of age as the at-risk age. There is also debate as to whether or not to carry out endoscopy to rule out neoplasms in young patients with alarm symptoms, given that those data are often considered nonspecific, limiting their positive predictive value.33,35 However, there is evidence that alarm symptoms increase the risk of tumors by 2–3 times. Because Mexico is an intermediate-risk country for the development of gastric cancer, with an incidence of 6.3 cases/100,000 inhabitants,40,41 we consider the performance of endoscopy to be justified when there are alarm features, even when there is not a high risk of neoplasm, particularly if a timely diagnosis of incipient gastric cancer is sought.40
The Japanese guidelines emphasize that the physician can directly diagnose FD in cases in which organic disease is not suspected, based on clinical history, H. pylori infection status, and other initial detection criteria, thus indicating that endoscopy should only be used as an adjunct modality in the diagnosis of FD.10 In the Mexican environment, we consider endoscopy mandatory for making the diagnosis of FD because it is a DGBI that frequently presents with recurrent and refractory symptoms that overlap with other digestive symptoms. In such a setting, endoscopy not only enables the ruling out of malignant neoplasms, but also of benign organic disorders that can explain the symptoms.8
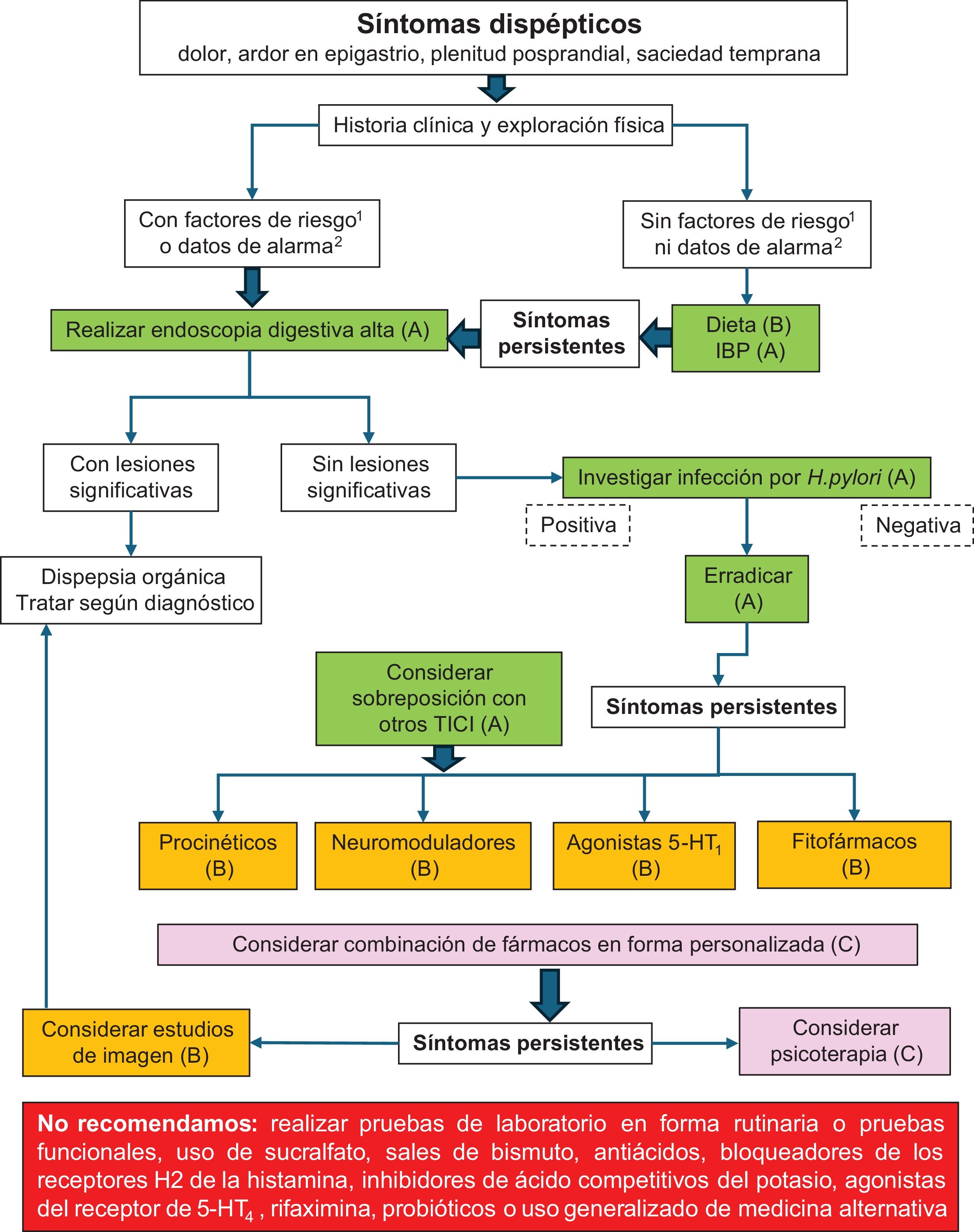
Thus, if no significant lesions are found in the upper gastrointestinal endoscopy performed on the patient with uninvestigated dyspepsia, the diagnosis of FD is made. Having an accurate diagnosis of FD is extremely relevant because it paves the way for different therapeutic options, once organic disorders are ruled out. Fig. 1 shows our proposed diagnostic algorithm for use in subjects with dyspepsia.
Dyspeptic symptoms.
(1) Risk factors: age >55 years, first-degree relatives with esophageal or gastric cancer, relatives with genetic syndromes that increase the risk for cancer.
(2) Alarm symptoms: significant weight loss, anemia, iron deficiency, thrombocytosis, gastrointestinal bleeding, persistent vomiting, progressive dysphagia, odynophagia.
The quality of evidence for each intervention is in parentheses.
When endoscopy is performed on patients with dyspepsia, taking biopsies is recommended in the following cases:4,12
- a)
Biopsies of relevant mucosal lesions.
- b)
Gastric biopsies, using the Sydney protocol to detect H. pylori infection, if it has not been previously ruled out.
- c)
Duodenal biopsies, in cases of positive serology for celiac disease. Biopsies for ruling out duodenal eosinophilia can also be considered in specific cases, according to the clinical context.
Two systematic reviews and meta-analyses42,43 showed an increase in the number of eosinophils and mast cells in patients with FD, when compared with subjects with no dyspepsia. However, this cannot be considered a disease biomarker because, even though it is a frequent finding in patients with postinfectious FD, there is no well-defined cutoff point and there is considerable overlap with other diseases.
Helicobacter pylori infectionWe recommend studying Helicobacter pylori infection in all patients with FD.
Quality of evidence: A.
Level of agreement: 63% in total agreement; 37% in partial agreement.
The approach to H. pylori infection in patients with dyspepsia tends to be confusing. The recommendation of searching for and treating the bacterium in patients with uninvestigated dyspepsia, known as the test-and-treat strategy, should not be confused with the management of infection in FD itself.2,8,36
As previously stated, the diagnosis of FD requires ruling out organic disorders, but the elevated prevalence of H. pylori infection and FD suggests that the two entities can coexist in the same patient, without necessarily indicating causality. As discussed in the Treatment section (“Helicobacter pylori eradication in functional dyspepsia”), H. pylori eradication can improve symptoms in a subgroup of patients with dyspepsia, even if endoscopy is macroscopically normal, but studies suggest that the symptomatic benefit is only achieved in the long term and not in all cases.36,44 FD that responds to eradication therapy has been called “H. pylori-associated dyspepsia”, which could be considered an organic disease, but strictly speaking, it can only be confirmed through long-term follow-up.8
A variable number of patients with FD do not achieve relief despite successful H. pylori eradication. In addition, even though superficial chronic gastritis can affect a variety of gastric functions, there is no evidence of gastric mucosal inflammation (with or without atrophy) being the cause of symptoms. Therefore, the mere detection of gastritis due to H. pylori does not rule out FD.
Because endoscopy is a necessary procedure for making the diagnosis of FD, biopsies should be obtained during the examination to evaluate the status of H. pylori at the time of the study.
Imaging studiesWe recommend that imaging studies be carried out in patients with FD whose symptoms are refractory to treatment or who present with alarm symptoms.
Quality of evidence: B.
Level of agreement: 81% in complete agreement; 19% in partial agreement.
FD is diagnosed based on the Rome IV criteria, evaluation of the personal medical history, the performance of endoscopy, when indicated, H. pylori detection, and occasionally, clinical laboratory tests or imaging studies.10 In the absence of alarm symptoms (see the “Endoscopy” statement), imaging studies are not recommended and treatment is usually started.2,8,36
There is some debate on the significance of abdominal pain in dyspepsia. In that group of patients, abdominal pain has been thought to possibly mask pancreatobiliary diseases, such as gallstones, choledocholithiasis, cholecystitis, or cancers of the pancreas or bile ducts. Two meta-analyses conducted by Kraag et al.45 and Berger et al.46 showed that only biliary colic was correlated with gallstones. Thus, in the context of dyspepsia, ultrasound is only recommended when the pain is located in the right hypochondrium or radiates into the back.8 On the other hand, computed tomography and magnetic resonance imaging are recommended when anatomic changes or organic lesions, such as tumors, are suspected.2
Functional testsWe do not recommend the routine use of gastrointestinal functional studies (satiety or gastric emptying tests) in the diagnosis of FD.
Quality of evidence: A.
Level of agreement: 94% in complete agreement; 6% in partial agreement.
Functional tests have been very useful for advancing the knowledge of the pathophysiology of FD but there is no evidence on their practical utility. Even though gastric emptying delay has been associated with dyspeptic symptoms,47 other studies have shown that this phenomenon occurs in patients with FD, as well as in healthy subjects.48,49 The possible overlap of the symptoms of gastroparesis and FD50 is a subject of debate; studies basing treatment on said overlap have produced inconsistent results.51 Moreover, recent studies on subjects with upper gastrointestinal symptoms have reported similar results in patients with FD or gastroparesis that are comparable to the results seen in normal subjects.8 Consequently, different medical associations recommend utilizing functional tests only in the context of clinical trials.8,10,36,38,39
Laboratory testsWe do not recommend the routine performance of laboratory tests in patients with FD.
Quality of evidence: B.
Level of agreement: 81% in complete agreement; 19% in partial agreement.
Laboratory exams as part of the diagnostic approach to dyspepsia should be based on the clinical suspicion of an organic condition but we do not recommend their generalized performance in all patients.38 Certain laboratory tests are useful for pointing toward organic causes or ruling them out.8 For example, a complete blood count can detect anemia and iron deficiency, alerting us as to possible underlying organic causes.
The role of enteric infections on the pathophysiogenesis of FD was described above. A meta-analysis showed that acute gastroenteritis was associated with a 2.5-times greater risk for developing FD, with a calculated prevalence of 9.55% (OR 2.54, 95% CI 1.76–3.65); Norovirus, Giardia intestinalis, Giardia duodenalis (previously Giardia lamblia), Salmonella spp., Escherichia coli O157, and Campylobacter spp. were the most frequently associated microorganisms.52 Due to the high prevalence of giardiasis (global prevalence 20–60%), especially in developing countries, and its association with FD, it could be worthwhile to study said infection in that context. In addition, there is evidence that giardiasis is associated with duodenitis (detectable through endoscopy and histopathologic analysis). Stool DNA testing for Giardia has a better diagnostic yield than aspirate examination or duodenal biopsies.53 Some cases of postinfectious dyspepsia appear to be related to the persistence of Giardia, particularly when associated with IBS, with its diagnosis enabling specific treatment.54
A study conducted in Mexico reported a high prevalence of celiac disease in patients with FD.55 However, a more recent publication from the same group of researchers that had a larger number of subjects found that celiac disease seroprevalence in blood donors with dyspeptic symptoms (detected through the PAGI-SYM validated questionnaire) was not different from that of controls without dyspepsia (1.15 vs 1.18%).56 Similarly, different systematic reviews and meta-analyses have shown that celiac disease seroprevalence in subjects with dyspepsia can be slightly higher, but not reaching statistical significance, compared with controls.57 Based on that evidence, we consider that routinely carrying out serologic testing to detect celiac disease in all patients with FD is not justified. In selected cases, such as patients with refractory dyspeptic symptoms and diarrhea or FD/IBS overlap, serologic testing for celiac disease and the detection of Giardia intestinalis with fecal antigens or PCR could be useful.
Recommendations for the treatment of functional dyspepsiaDietThere is no effective specific diet for the treatment of FD, thus dietary recommendations should be made individually, promoting a personalized diet.
Quality of evidence: B.
Level of agreement: 100% in complete agreement
The majority of subjects with dyspepsia recognize foods that trigger symptoms. Despite the fact that there are numerous pathophysiologic mechanisms that can explain this association, causality evidence is weak and relatively under-studied. Fats, wheat, and certain carbohydrates have been frequently pointed out as causing dyspeptic symptoms and spicy foods, coffee, and alcohol have been restricted or eliminated from their diet, either by patients themselves or by medical indication, but without objective bases.21,22 Most dietary advise is empiric and often leads to exclusion diets that reinforce patient perception of “damage” by or “intolerance” to certain foods, propitiating hypervigilance and symptom anticipation.14 Dietary habit modification, such as eating small-quantity meals several times a day and reducing the intake of fatty foods, are recommendations by experts but with insufficient scientific evidence.8 This can result in nutritionally unbalanced diets and even foment unnecessarily restrictive dietary behaviors. Exclusion diets can put patients at risk for developing avoidant/restrictive food intake disorder (ARFID) and the continued avoidance of foods can perpetuate pre-existing symptoms of ARFID.58 Notable overlap between DGBIs and ARFID has been described,59 and reports have found that 13–40% of patients with DGBIs meet all the criteria for ARFID or have clinically significant symptoms of ARFID.58
On the other hand, there are bidirectional relations between FD and eating disorders (EDs). More than 90% of individuals with unspecified EDs have been found to present with postprandial discomfort and nausea, whereas 94% of persons with anorexia nervosa are reported to meet the clinical criteria for FD (Rome IV).60,61 Gastric emptying and accommodation can be altered by the intake of large volumes of food within a short time period, possibly explaining the frequent presence of FD in patients with binge-eating and bulimia nervosa disorders.61 Even though there is no evidence of a cause-effect relation between EDs and DGBIs, the shared symptomatology must be correctly identified, with personalized treatment of each of the disorders.62
In addition, the efficacy of nutritional therapy in FD is marginal. In recent years, interest has centered on the possible effect on FD of a low fermentable oligosaccharide, disaccharide, monosaccharide, and polyol (FODMAP) diet, a fructose or lactose-restricted diet, a gluten-free diet, or the Mediterranean diet. Systematic reviews that analyze those dietary interventions have shown marginal effects on specific patient groups, but the lack of high-quality studies is also apparent.63,64 The low FODMAP diet has been the most widely studied in the treatment of FD,65–67 but initial findings show modest, nonsignificant benefit for most patients. Similarly, nonceliac gluten or wheat sensitivity has been associated with dyspeptic symptoms and duodenal eosinophilia, suggesting overlap of the 2 conditions.68 The accumulated evidence up to now indicates that those interventions could be reasonable in patients with FD that have overlap with IBS or abdominal distension and in patients that are interested in and motivated to adopt said dietary interventions for a 4 to 8-week test period.67
Fig. 1 shows our proposed treatment algorithm for managing the patient with dyspepsia.
Local-acting medicines and histamine H2-receptor antagonistsWe do not recommend the use of sucralfate, bismuth salts, antacids, or histamine H2 receptor blockers for the treatment of FD.
Quality of evidence: B.
Level of agreement: 94% in complete agreement; 6% in partial agreement.
At present, there are no adequate studies demonstrating the efficacy of antacids, bismuth salts, and mucosal protective agents, such as sucralfate, in FD.2,5 A systematic review that evaluated the effect of various mucosal protective agents and antacids,69 found a trend toward greater effectiveness of bismuth salts, compared with placebo, but with marginal statistical significance (p = 0.07). In that same review, 2 studies evaluating the effect of sucralfate on 246 patients showed a decrease in dyspeptic symptoms, compared with placebo, but without statistical significance. Antacids were not better than placebo in a randomized study that included 109 patients.69 A randomized study on 63 patients that received bismuth or sucralfate for 4 weeks, compared with the use of similar-appearing placebos, found no statistically significant difference in symptom relief between groups.70 Another controlled randomized study compared the efficacy of simethicone (105 mg/3 times a day), cisapride (10 mg/3 times a day), and placebo, for 8 weeks in 185 patients with FD. Both drugs improved the dyspeptic symptoms, albeit simethicone was significantly better than cisapride in the first 2 weeks of treatment.71 Despite being considered safe compounds, some of them can cause different side effects. For example, the excessive acute intake of bismuth, or its abusive use over a long period of time, can cause toxicity.72 Even though antacids and mucosal protective agents are a relatively economic and available option, there is no updated and quality evidence on them, preventing recommending their use in FD.
The use of histamine H2-receptor antagonists (H2RAs) for managing symptoms of dyspepsia has been evaluated in numerous clinical trials, but most of the studies were conducted before the emergence of the Rome criteria.38 A meta-analysis that included 12 good-quality studies showed that H2RAs reduced the relative risk (RR) of symptoms, compared with placebo (RR 0.79, 95% CI 0.71−0.98), with a number needed to treat (NNT) of 7.73 The antihistamine effect of those drugs appears to improve the duodenal eosinophilia related to PDS.32,73–75 In the recent Asia-Pacific guidelines for the management of FD and overlap, at least 2 studies stand out, in which ranitidine (combined with loratadine or hydroxyzine) improved duodenal eosinophilia and even predicted symptom response.32,74,75 Nevertheless, when H2RAs were compared with proton pump inhibitors (PPIs), there was a tendency to favor PPIs, regarding symptom improvement. And it should be kept in mind that the tachyphylaxis associated with H2RAs limits their prolonged use.39 Importantly, in 2019, ranitidine was removed from the market due to the detection of a potentially carcinogenic metabolite, even though famotidine continues to be marketed in Mexico.
Proton pump inhibitorsWe recommend the use of proton pump inhibitors for the treatment of FD.
Quality of evidence: A.
Level of agreement: 81% in total agreement; 19% in partial agreement.
PPIs are frequently used in the management of dyspeptic symptoms. Taking into account that the most frequently detected organic lesions in patients with uninvestigated dyspepsia are erosive esophagitis and ulcerative disease (both susceptible to treatment with PPIs) and that malignant lesions are rare,76–78 the empiric starting of treatment with PPIs has been suggested in that group of patients, as long as they do not present with alarm symptoms.76 However, this recommendation cannot be completely transferred to subjects with FD, without first considering various aspects.
A meta-analysis that included 7 controlled clinical trials and 3,725 patients found that PPIs were more effective than placebo for reducing symptoms in subjects with FD (NNT: 14.6, 95% CI 8.7–57.1),78 but the stratified analysis showed they were efficacious only in patients with “ulcerous-type” or “reflux-type” dyspepsia and not in patients with “dysmotility-type” dyspepsia. Another meta-analysis that included 25 controlled clinical trials and 8,453 participants analyzed the effect of PPIs versus placebo, H2RAs, or prokinetics for the relief of overall symptoms and quality of life in the patients with FD. PPI efficacy was found to be similar at low and standard doses, they were more efficacious than placebo (NNT: 11), and their effectiveness was slightly higher or similar to that of the H2RAS and slightly higher than that of the prokinetics.79 A population study conducted in India reported that symptom control through PPIs was different, according to the drug utilized,80 showing that the response to PPI treatment of FD is not completely homogeneous. Given this heterogeneity, there can be subgroups of FD patients that experience alterations in acid sensitivity and can directly benefit from PPI therapy.81
Different guidelines recognize that PPI use is an effective therapy in FD.8,36,38,39 Some authors suggest PPI use at low doses,38 and others suggest their use only in patients with symptom persistence after H. pylori eradication or in persons negative for the bacterium.36,39 The most recent evidence shows there are no significant differences between the different PPI types, high or low doses, or their effectiveness in FD subgroups.8,82 We suggest short-term treatment with a low dose of PPIs in FD patients. All patients chronically treated with PPIs should be checked at regular intervals to evaluate if there is truly a need to continue PPI therapy, or if possible, reduce the dose or suspend them completely, to prevent overprescription and favor a lower cost of this therapy.
Potassium-competitive acid blockersWe do not recommend the use of potassium-competitive acid blockers for the treatment of FD.
Quality of evidence: C.
Level of agreement: 44% in complete agreement; 37% in partial agreement, 19% uncertain.
Potassium-competitive acid blockers (P-CABs) are a new therapeutic class of drugs that selectively inhibit the proton pump through reversible blocking of the potassium channels.83 Due to their pharmacologic properties, they have important advantages over PPIs: they act rapidly and from the first intake, they raise the intragastric pH above 6 from day one, and they do not need to be taken before food ingestion. Vonoprazan was the first P-CAB to be approved and registered in Asia and the United States, whereas tegoprazan was the first to be approved and registered in Latin America.84,85 Even though there are numerous studies on P-CAB use in GERD, H. pylori eradication, gastric lesion prophylaxis, and peptic ulcer management, there is very limited evidence on their use in FD.85–88 A study on 43 patients with FD treated with vonoprazan 20 mg daily or placebo, for 4 weeks, reported reduced symptom intensity with the drug (45.3% vs 28.2%).86 Another study compared the efficacy of 10 mg daily (n = 48) versus acotiamide 100 mg/3 times a day (n = 37), for 4weeks, and found that epigastric pain and postprandial discomfort scores significantly improved in the two groups, with a similar degree of improvement in each score.87 In the most recently published open and noncomparative study, 173 patients with FD (Rome IV) were treated with tegoprazan 50 mg daily, reporting satisfactory symptom relief in 74.6% at 4 weeks and 86.7% at 8 weeks. There was also significant improvement at 4 and 8 weeks in the quality-of-life scales, compared with the initial scores, with no severe adverse events related to the drug.88
Thus, even though P-CABs are a promising option, current evidence is insufficient for recommending their use in FD.
ProkineticsWe recommend the use of prokinetics for the treatment of FD.
Quality of evidence: B.
Level of agreement: 69% in complete agreement; 31% in partial agreement.
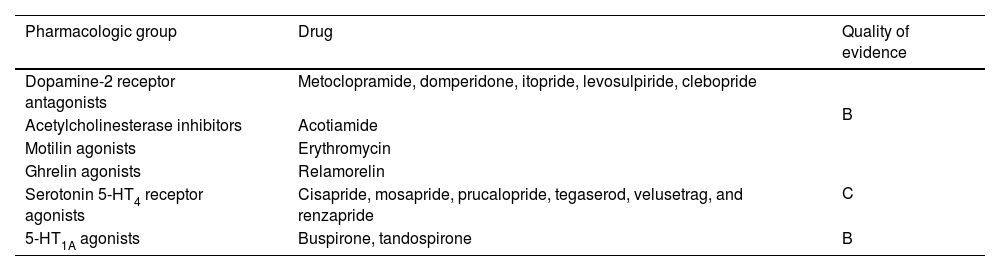
Because FD symptoms are associated with gastric motility abnormalities that include delayed emptying and a lack of accommodation after food intake, prokinetics are considered part of the therapeutic focus. However, the relation between symptom improvement and gastrointestinal motor function continues to be a subject of debate and their long-term efficacy is limited by side effects from some of them. Prokinetics are composed of different drug classes that improve gastrointestinal motor function, acting through different pathways, and include dopamine-2 receptor antagonists, acetylcholinesterase inhibitors, motilin agonists, and ghrelin agonists. Even though 5-HT1 and 5-HT4 agonists also have a prokinetic effect, they will be separately addressed further ahead due to their distinct effects and drug profile. Although we issue our recommendation treating prokinetics as a group, we recognize that the evidence sustaining their use in FD is not homogeneous (Table 2).
Classification of prokinetics and quality of evidence on their effectiveness in functional dyspepsia.
| Pharmacologic group | Drug | Quality of evidence |
|---|---|---|
| Dopamine-2 receptor antagonists | Metoclopramide, domperidone, itopride, levosulpiride, clebopride | B |
| Acetylcholinesterase inhibitors | Acotiamide | |
| Motilin agonists | Erythromycin | |
| Ghrelin agonists | Relamorelin | C |
| Serotonin 5-HT4 receptor agonists | Cisapride, mosapride, prucalopride, tegaserod, velusetrag, and renzapride | |
| 5-HT1A agonists | Buspirone, tandospirone | B |
A meta-analysis that evaluated prokinetics as a group and included 29 studies and 10,044 patients found they were significantly more effective than placebo in reducing FD symptoms, with a therapeutic gain of 14% over placebo, as well as a NNT of 7.89 Despite its good results, that study has been questioned, due to the heterogeneity of the trials it included and potential biases.
The dopamine-2 receptor antagonists (metoclopramide, domperidone, itopride, levosulpiride, and clebopride) reduce symptoms in patients with FD by favoring gastric emptying and increasing gastrointestinal motility.
Metoclopramide, the first D2-receptor antagonist, has prokinetic effects by acting as a 5-HT4-receptor agonist. Its easy passage through the blood-brain barrier is related to possible irreversible neurologic effects, resulting in a warning being issued, due to the induction of extrapyramidal symptoms.90 Different trials and a meta-analysis that compared the efficacy of metoclopramide with placebo and other drug therapies in FD described significantly improved symptoms in favor of the drug, underlining its limitations due to the potential adverse effects.90–94
In addition to having peripheral prokinetic properties, domperidone has an anti-emetic effect. Although there are few published studies, some of them indicate a significant reduction of dyspeptic symptoms with domperidone, compared with placebo, reaching results similar to those with metoclopramide in up to 76% of patients, but with fewer side effects. Nevertheless, only its short-term use is recommended.95 A Cochrane meta-analysis suggested that beneficial effects were achieved with a dose of 10−20 mg/3 times a day, compared with placebo, regarding the overall dyspeptic symptom rate.96 The effectiveness of domperidone in specific FD subgroups has not been analyzed due to the risk of a prolonged QT interval and greater risk of ventricular arrythmia.90
Itopride is a D2-receptor antagonist and cholinesterase inhibitor that promotes gastric contractility, increases lower esophageal sphincter pressure, and accelerates gastric emptying. Four of 6 clinical trials reported significant FD symptom improvement after 2–8 weeks of treatment with itopride, whereas 2 trials showed no improvement, compared with placebo.36,89,90,97 A study that evaluated the effects of itopride, utilizing validated measures reported by patients, described the efficacy of itopride, especially in patients with PDS and EPS overlap.98
Levosulpiride acts through the dopaminergic pathways that control gastrointestinal motility, and its serotonergic component (5-HT4) can also increase its therapeutic efficacy. Some studies have supported the efficacy of levosulpiride in controlling the symptoms of epigastric pain or discomfort, nausea, bloating, and aerophagia, as well as overall symptoms, in addition to having a favorable safety profile.99,100 A systematic review reported that the incidence of adverse events with levosulpiride was 11%, the majority of which were mild and rarely interrupted treatment. When compared with cisapride, levosulpiride showed similar efficacy in reducing gastric emptying times in a randomized trial.101
Clebopride is a nonselective benzamide with a great affinity for the D2, D3, and D4 receptors that acts as a dopamine-receptor antagonist. Even though it has been shown to be efficacious in symptom relief, there is limited evidence on its effectiveness in FD and it has not been updated in recent studies.102
Of the acetylcholinesterase inhibitors, there is broad evidence only on acotiamide, regarding its clinical utility in FD, basically due to its effect as a gastric prokinetic. Acotiamide improves the release of acetylcholine into the enteric nervous system, through muscarinic receptor antagonism and acetylcholinesterase inhibition. It has a low affinity for the 5-HT2, 5-HT3, and 5-HT4 serotonin receptors and the D2 receptors, compared with other prokinetics.103 In several clinical trials, acotiamide has been shown to significantly improve the fullness sensation, bloating, and early satiety, compared with placebo.104 A comparative placebo-controlled trial that evaluated symptom improvement, using a 7-point Likert scale, and emptying, through ultrasonography, before and after the intervention, showed symptom improvement in 31.6% of patients with acotiamide and in 16.7% with placebo.105 The most appropriate dose of acotiamide has been a subject of study. A meta-analysis that included seven comparative, placebo-controlled clinical trials utilized doses varying from 50 to 900 mg/3 times a day for different periods of time,106 whereas Tack et al.107 and Matsueda et al.108 utilized an additional 50 mg and 300 mg to the conventional dose of 100 mg of acotiamide. The dose of 100 mg/3 times a day showed better results, and so is considered the most appropriate in the treatment of FD.
Motilin agonists are drugs that imitate the action of this neurotransmitter, selectively interacting with its receptor, increasing lower esophageal sphincter pressure, stimulating gastric motility, and improving accommodation. Of that drug group, there is only evidence on erythromycin, a macrolide antibiotic, in FD.109 In a controlled clinical trial conducted on patients with FD and delayed gastric emptying, erythromycin did not significantly improve overall symptoms; there is also an important risk of tachyphylaxis with its use.110
Ghrelin agonists stimulate gastric motor function through the vagus nerve, and they have been related to motility and appetite regulation. Relamorelin, a ghrelin agonist, has been described as a promising drug in dyspepsia. Nevertheless, evidence on its use in FD is scarce and has contradictory results.111
Different guidelines recognize the use of prokinetics as an effective therapy in FD.8,36,38,39 Some authors suggest their use only in patients with persistent symptoms after H. pylori eradication or after treatment with PPIs.36 Others recommend their use as first-line treatment, especially in patients with PDS, because of the greater frequency of motor alterations in that group.39 However, studies have found a similar prevalence of gastric motor anomalies in PDS, EPS, and cases of overlap of the two subtypes. In general, the evidence supporting the use of prokinetics in FD is considered to have poorer quality, compared with other therapeutic options, added to the fact that not all of them are available in all countries.112 In Mexico, a wide range of prokinetics is available; if patients are adequately selected and these drugs are used appropriately, they have a good safety profile. Therefore, we consider them a therapeutic alternative in FD.
Serotonin 5-HT4 receptor agonistsWe do not recommend the use of serotonin 5-HT4 receptor agonists for the treatment of FD.
Quality of evidence: C.
Level of agreement: 56% in complete agreement; 38% in partial agreement, 6% uncertain.
The 5-HT4 receptor agonists (cisapride, mosapride, prucalopride, tegaserod, velusetrag, and renzapride) release acetylcholine from the myenteric plexus and stimulate smooth muscle contraction, accelerating gastric emptying. However, the wide distribution of those serotonin receptors contributes to their participation in a large number of functions that have yet to be completely studied, including visceral pain modulation.113
Cisapride, one of the first nonselective 5-HT4 receptor agonists used in patients with FD and gastroparesis, has been shown to accelerate emptying and potentiate gastric accommodation in healthy subjects. Nevertheless, the effects of cisapride on gastrointestinal symptoms are controversial, given that some studies show no significant differences due to elevated responses to placebo.114 It was taken off the market in the United States due to its arrhythmogenic potential, related to its affinity for the human ether-a-go-go (hERG) channel.115
Several selective 5-HT4 receptor agonists have been developed. Mosapride is used as a prokinetic agent in Asian countries but a controlled trial in Europe showed no efficacy in FD.116 In a controlled clinical trial that compared controlled-release mosapride with nortriptyline in patients with FD for 4 weeks, the two drugs had similar efficacy, not only in symptom relief but also in controlling anxiety and improving quality of life, regardless of the dyspepsia subtype.117 Prucalopride is a potent 5-HT4 receptor agonist with high specificity for that receptor and has been shown to improve gastric emptying, as well as small bowel and colonic transit in patients with idiopathic chronic constipation. Studies on healthy volunteers also indicate that prucalopride can increase gastric emptying with symptom relief after 120 min.118 Even though its potential efficacy in FD has been suggested, there are still no large-scale trials for evaluating its action on the condition. Tegaserod, a partial 5-HT4 receptor agonist that was originally developed for constipation-predominant IBS (IBS-C) and functional constipation, has been shown to have benefits in the treatment of FD. A randomized placebo-controlled trial reported 4.6% improvement in days of symptom relief after 6 weeks of treatment, compared with placebo; the effect of treatment was greater in patients with moderate or severe symptoms.119 Another study on women with FD that received concomitant treatment with PPIs for heartburn showed no statistically significant benefits.120 Tegaserod was taken off the market in 2008 due to a presumed increase in cardiovascular side effects but the US Food and Drug Administration recently approved its reintroduction for women under 65 years of age with IBS-C. Velusetrag and renzapride have not yet been evaluated in patients with FD.
A meta-analysis that included 10 comparative placebo-controlled clinical trials evaluated the efficacy of serotonin agonists in the treatment of FD. They were highly efficacious for symptom control, compared with placebo.121 However study selection was very heterogeneous, involving different drug classes, and included patients diagnosed through different criteria, impeding reaching clear conclusions.
Serotonin 5-HT4 receptor agonists cannot be recommended in FD because there is very little evidence supporting their use, and in some cases, there is none; their undesirable effects are also a limitation.
5-HT1A serotonin receptor agonistsWe recommend the use of 5-HT1A agonists in patients with FD.
Quality of evidence: B.
Level of agreement: 56% in total agreement; 44% in partial agreement.
The 5-HT1A agonists induce gastric relaxation, improving symptoms of FD in patients with altered accommodation and hypersensitivity to gastric distension.122 A controlled clinical trial in which subjects received buspirone 10 mg/3 times a day for 4 weeks significantly improved overall symptoms and the individual symptoms of early satiety, postprandial fullness sensation, and upper abdominal distension but did not show improvement in epigastric pain and burning sensation, signifying it could be more useful in the subgroup of patients with PDS.123 Tandospirone showed significant improvement in patients with FD in a 4-week comparative placebo-controlled clinical trial conducted in Japan.124 A randomized comparative study with placebo showed that tandospirone improved both gastrointestinal symptoms and anxiety in patients with FD.125 Those therapeutic effects have been suggested to be associated with the modulation of the neurotropic factor derived from the brain and inflammatory cytokines that were measured in said analysis.
Even though, compared with other drugs, there is a small amount of evidence that sustains the use of 5-HT1A receptor agonists in FD, we consider they can be a therapeutic option, and so we recommend their use in selected cases.
Helicobacter pylori eradication in functional dyspepsiaWe recommend prescribing eradication treatment in patients with FD that present with Helicobacter pylori infection.
Quality of evidence: A.
Level of agreement: 88% in total agreement; 12% in partial agreement.
A recent systematic review and meta-analysis included 29 clinical trials and 6,781 patients with FD that were positive for H. pylori. It provided high-quality evidence showing that eradication of the bacterium is a safe and effective treatment in that setting.126 The analysis showed that H. pylori eradication was superior to control treatment (antisecretory agents, prokinetics, with or without an antibiotic placebo, or only placebo) in curing symptoms (NNT: 14) and improving dyspeptic symptoms (NNT: 9). The patients with successful H. pylori eradication were cured or had symptom improvement, compared with the patients with unsuccessful eradication (NNT: 4.5). The therapeutic benefit lasted up to 12 months. Adverse effects in general (number needed to harm [NNH]: 3) and the adverse events that made it necessary to suspend treatment were more common with eradication treatment (NNH: 71).120 Other meta-analyses have reported that H. pylori eradication in FD reduces the risk of developing peptic ulcer or gastric cancer.127
The findings of this new evidence enable the following conclusions that support the abovementioned recommendation to be made:
- a)
H. pylori infection is the cause of FD, even though the mechanisms through which the infection produces dyspeptic symptoms are not yet clear. Its eradication is curative or improves symptoms.
- b)
H. pylori eradication should be considered the treatment of choice in a patient with dyspeptic symptoms, negative endoscopy and biopsies, or tests revealing H. pylori infection.
- c)
Successful H. pylori eradication treatment increases the rates of cure or improvement of symptoms in FD. Thus, utilizing eradication regimens with proven efficacy in the population to treat is imperative.
- d)
The posited concept is that FD that responds to eradication treatment could be considered an organic disease, called H. pylori-associated dyspepsia, as suggested in the Kyoto consensus128 and the Rome IV criteria.1
- e)
Even though adverse events with eradication treatment are more common, they are mild and rarely result in ending treatment.
- f)
Those meta-analyses included studies on populations of dyspeptic patients with both a low prevalence (the United States and Europe) and a high prevalence (Asian countries, such as China) of H. pylori infection and found the same beneficial effect of eradication treatment, suggesting that this strategy is effective regardless of the prevalence of the infection.
- a)
There are no controlled trials in Mexico through which the efficacy of H. pylori eradication in FD could be evaluated. However, given the evidence described above, we can recommend eradication treatment in Mexican patients with FD and H. pylori infection.
NeuromodulatorsWe recommend the use of neuromodulators for the management of FD.
Quality of evidence: B.
Level of agreement: 94% in complete agreement; 6% in partial agreement.
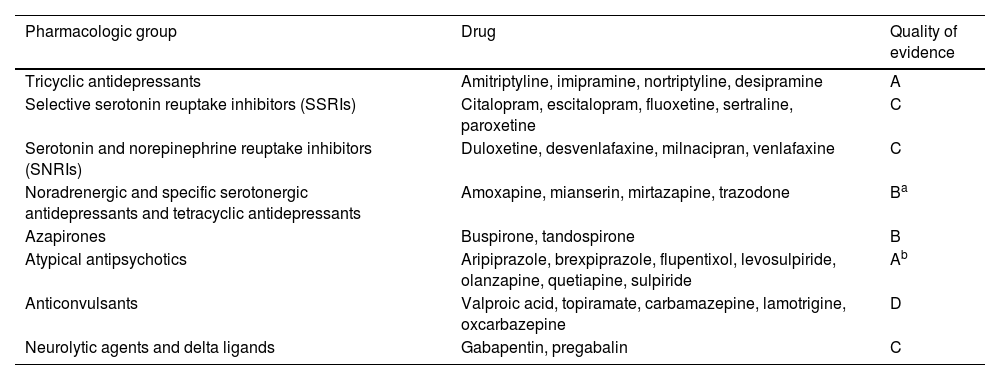
Neuromodulators are molecules that act regulating the activity of ion channels and membrane potentials in the neural cells. According to their pharmacologic group, they totally or partially stimulate or inhibit one or more pre and postsynaptic serotonergic, muscarinic, cholinergic, or noradrenergic receptors, they have effects on gastrointestinal motility and tone and gastric accommodation, and they are antinociceptive or have an effect on central pain processing.129,130 There are 8 drug groups and depending on the action site, they can be central (all except the delta-ligands) and peripheral (delta ligands).131 Even though our recommendation is made considering the neuromodulators a group, we recognize that the evidence sustaining their use in FD is not homogeneous (Table 3).
Classification of neuromodulators and quality of evidence, regarding their effectiveness in functional dyspepsia.
| Pharmacologic group | Drug | Quality of evidence |
|---|---|---|
| Tricyclic antidepressants | Amitriptyline, imipramine, nortriptyline, desipramine | A |
| Selective serotonin reuptake inhibitors (SSRIs) | Citalopram, escitalopram, fluoxetine, sertraline, paroxetine | C |
| Serotonin and norepinephrine reuptake inhibitors (SNRIs) | Duloxetine, desvenlafaxine, milnacipran, venlafaxine | C |
| Noradrenergic and specific serotonergic antidepressants and tetracyclic antidepressants | Amoxapine, mianserin, mirtazapine, trazodone | Ba |
| Azapirones | Buspirone, tandospirone | B |
| Atypical antipsychotics | Aripiprazole, brexpiprazole, flupentixol, levosulpiride, olanzapine, quetiapine, sulpiride | Ab |
| Anticonvulsants | Valproic acid, topiramate, carbamazepine, lamotrigine, oxcarbazepine | D |
| Neurolytic agents and delta ligands | Gabapentin, pregabalin | C |
Modified from reference125.
Initial studies and systematic reviews grouped neuromodulators into a single category or catalogued them as antidepressants or anxiolytics, showing heterogeneous results.132 Improved DGBI understanding has shown that there are important differences between groups, which have been confirmed in subsequent controlled studies and meta-analyses.132–137 Under this new vision as a group, neuromodulators have been shown to be useful in the treatment of FD, with a NNT of 6.132
Several studies have shown the usefulness of tricyclic antidepressants (TCAs) in FD, and they are the group with the best quality of evidence. Amitriptyline and imipramine have been shown to be superior to placebo and escitalopram in EPS, with a NNT of 6 for symptom improvement and a NNT of 7 for reducing pain scores.133 A controlled clinical trial in patients with EPS, that compared the effect of pantoprazole with low doses of amitriptyline (25 mg at night) for 4 weeks, showed significantly improved symptoms in the group treated with amitriptyline, but did not have an impact on the psychologic stress or anxiety scores.134 A study that included 107 patients with FD that was refractory to esomeprazole and domperidone analyzed the effect of imipramine versus placebo, showing significant improvement in the overall FD symptom scores, with a NNT of 4.135 Even though the increase in the dose of imipramine was gradual, a large number of the patients treated with the drug suspended it due to adverse effects, compared with placebo (18 vs 8%, respectively); the most common side effects were dry mouth, constipation, and somnolence. A comparative, randomized, controlled, clinical trial analyzing nortriptyline and duloxetine, found nortriptyline to be superior in symptom improvement in FD patients, albeit duloxetine was more effective for reducing anxiety.136 The most recent meta-analyses have confirmed the efficacy of TCAs in the treatment of FD, with low NNTs.73,132,137
In contrast, the selective serotonin reuptake inhibitors (SSRIs) and the serotonin and norepinephrine reuptake inhibitors (SNRIs) have not been superior to placebo, TCAs, or tetracyclic agents for symptom control in FD.132,138 Some guidelines consider this group second-choice for pain control when there is intolerance to TCAs, but there is insufficient evidence.130 Their use can be considered in patients with FD that have anxiety (SSRIs and SNRIs) or obsessive compulsive disorders or depression (SSRIs).
Of the noradrenergic and serotonergic tetracyclic agents, mirtazapine has been shown to be useful in PDS, particularly when associated with weight loss. Mirtazapine was better than placebo in overall improvement, early satiety, quality of life, and gradual weight gain; it appears to be related to an effect on fundic relaxation and gastric accommodation.139 A study that compared the effect of mirtazapine with nortriptyline in FD showed a significant decrease in epigastric pain, belching, bloating, and depression in the group treated with mirtazapine, but found no differences in anxiety.140 Despite those good results, mirtazapine efficacy was not statistically significant in the meta-analysis by Ford et al.132
Azapirones with a 5-HT1 receptor antagonist effect have been shown to be useful in PDS (buspirone) and in EPS (tandospirone).73 Tandospirone appears not only to have an effect on gastric motility but also has reduced anxiety scores in subjects with FD.125
Of the atypical antipsychotics, evidence on the treatment of FD is the best for levosulpiride and sulpiride. Both drugs have dual action, given that, in addition to being D2 receptor antagonists and partial D3 agonists, they are 5-HT1 agonists and 5-HT2 antagonists, acting as both prokinetics and neuromodulators. In the meta-analysis by Ford et al.,132 the group of atypical antipsychotics showed a risk for symptom persistence below 1 (RR 0.50, 95% CI 0.37−0.67), with a NNT of 3 and a NNH of 21.
There are no clinical studies on anticonvulsants in FD.
Delta-ligands belong to the group of anticonvulsants, but they have also been classified as peripheral-acting neuromodulators with anxiolytic activity. Pregabalin and gabapentin have been evaluated in case series, open studies, and comparative placebo-controlled clinical trials, as well as in combination with omeprazole, showing overall improvement and improved perception and symptom scores.141–143
Some neuromodulators appear to have an effect on more than one mechanism associated with pain, including gastroduodenal and visceral hypersensitivity, altered central pain processing, and gastric accommodation. The effect is cumulative over 6–8 weeks, after which it reaches its maximum clinical benefit, which can be limited due to side effects. Those effects can present at the start of treatment, for which doses should be gradually scaled, according to tolerance. Drug selection should take different factors into account, such as demonstrated effectiveness, the associated psychologic comorbidity, undesirable effects, and tolerance.
Probiotics and rifaximinWe do not recommend the use of probiotics or rifaximin for the treatment of FD.
Quality of evidence: C.
Level of agreement: 69% in complete agreement; 31% in partial agreement.
In FD, dysbiosis and mucosal barrier alterations have been shown to contribute to low-grade inflammation and to influence sensory dysfunction, causing dyspeptic symptoms that are modified by environmental factors, such as diet and medications, especially gastric acid secretion inhibitors.144–146 There is a growing interest in modulating the microbiota for achieving a therapeutic effect in FD, resulting in the use of probiotics and rifaximin.
Probiotics have been evaluated in numerous cohort studies and controlled clinical trials, utilizing specific strains and their combinations, exclusively, or together with conventional treatment with prokinetics and antisecretory agents.147,148 Specific probiotics have shown some physiologic or symptomatic benefit but the designs of those studies and a lack of clarity in the mechanisms of action impede solid and reproducible conclusions from being made. Prebiotic and probiotic efficacy was evaluated in a meta-analysis that only included controlled clinical trials.149 Those researchers concluded that probiotics were not associated with significant improvement in FD symptoms (RR 1.13, 95% CI 0.99–1.28, p = 0.67). A detailed analysis of those works revealed important differences in strain, dose, and treatment duration, as well as patient subgroups, clinical outcomes, and improvement definitions.
The potential usefulness of rifaximin for treating dysbiosis in patients with FD has been analyzed in different studies. A comparative placebo-controlled clinical trial found that antibiotic administration (400 mg/3 times a day for 2 weeks) alleviated overall dyspeptic symptoms in a significantly higher number of patients, compared with placebo (78% vs 52%, p = 0.02), as well as improved belching and postprandial bloating at 4 weeks, especially in women.150 Although the number of patients was relatively low, a relevant aspect of the study was that the active exclusion of subjects with symptoms suggestive of IBS or diagnosed with IBS, signifying that FD and IBS overlap did not influence the results. Another controlled clinical trial compared the effectiveness of rifaximin, mosapride, and their combination in the treatment of bacterial overgrowth in subjects with FD.151 Rifaximin reduced expired gases and relieved some symptoms, but the small number of patients, overlap with IBS, the high rate of abandonment, and the lack of a placebo group hindered the interpretation of the results. Another small open study, in which 21 patients with FD (with or without IBS) were consecutively treated with 550 mg de rifaximin/twice a day for 10 days, reported significant relief in symptom scores, finding no apparent influence of IBS on the results.152 The obvious study limitations hampered the result interpretation.
Given the current evidence, the potential of probiotics and rifaximin is undeniable, but more and better studies are needed before recommending their use in the treatment of FD, especially in patients with IBS overlap.
Herbal compounds and phytopharmaceutical drugsWe recommend the use of STW-5 and peppermint for the treatment of FD.
Quality of evidence: B.
Level of agreement: 63% in complete agreement; 31% in partial agreement, 6% uncertain.
A greater number of studies have been published in recent years on the potential usefulness of herbal products in certain DGBIs, mainly FD.153,154 The majority of these herbal products are preparations of several components, with a wide variety of effects on gastrointestinal function, which until now, lacked high quality evidence on their efficacy.155–157 However, formal research on herbal medicine has been resumed through phytopharmacology, which focuses on the study of standardized extracts of medicinal plants. Phytopharmaceuticals are medicines whose active substance contains the extract from a determined plant or a combination of different plants, roots, and vegetables, with a known mechanism of action. For example, Rikkunshito (composed of 8 roots and herbs) reduces dyspeptic symptoms by promoting adaptive relaxation and increasing gastric emptying. A recent meta-analysis that included 5,475 patients described greater efficacy with Rikkunshito, compared with Western treatment and placebo, significantly reducing the dyspeptic symptom score and improving gastric emptying.158
For several years, various phytopharmaceutical drugs have been marketed and are available for clinical use. One of them is the German STW-5, composed of 9 herbs and roots, that has been shown to improve FD symptoms by producing fundic relaxation and favoring gastric emptying. Several placebo-controlled clinical studies, as well as meta-analyses, have evaluated STW-5 in patients with FD, confirming its efficacy in symptom control after 4–8 weeks, with a good safety profile.159–162 This phytopharmaceutical drug has been described as “multipurpose” because of evidence on its effectiveness in the treatment of IBS, making it a practical alternative in patients with FD-IBS overlap.163–165
Peppermint oil, whose main active ingredient is menthol, has antispasmodic properties due to its capacity to block intestinal smooth muscle calcium channels. There is evidence of other possible mechanisms of action, such as visceral and central sensitivity modulation; antioxidant, antiparasitic, and antifungal effects; microbiota modulation; and direct anti-inflammatory effects.166,167 A meta-analysis that included 5 controlled clinical trials and 578 patients evaluated the usefulness of the combination of peppermint oil and caraway oil in the treatment of FD. There was significant relief in overall symptoms and epigastric pain (NNT of 3 for both aims) with good tolerance and no serious undesirable effects.168 Because peppermint oil has shown good clinical effects in IBS,169 it could be considered a good therapeutic option for patients with FD and IBS overlap.
Psychologic therapiesWe do not recommend the generalized use of psychologic therapy in subjects with FD, but it could be useful in patients with severe, refractory symptoms and psychologic comorbidities.
Quality of evidence: C.
Level of agreement: 88% in complete agreement; 12% in partial agreement.
Several types of psychotherapy, such as cognitive behavioral therapy, coping flexibility, and hypnotherapy, have been studied in patients with FD. Studies have suggested adding psychotherapy to the standard medical treatment of FD because it produces more favorable results than medical treatment alone.170–172 A study showed that cognitive behavioral therapy for stress management was effective in dyspeptic symptom control, compared with a control group.170 Another comparative study reported symptom and quality of life improvement in 59% of patients with FD treated with weekly hypnotherapy sessions for 3 months versus 41% of patients receiving conventional medical treatment versus 34% of patients that had only support treatment.172 A small pilot study that evaluated self-applied hypnotherapy, utilizing a completely automated audio program that did not require the participation of a therapist or physician, described significant improvement in FD symptoms, quality of life, and emotional wellbeing.173 A systematic review and meta-analysis that included 9 controlled studies found that psychologic interventions offered significant persistent benefit in reducing overall FD symptoms, but they were low-quality studies due to biases and heterogeneity.174 Another recent systematic review found that hypnotherapy in FD provided promising results but concluded that further better quality studies are needed before recommending this treatment in patients with FD.175
Psychologic therapies have important disadvantages: they require much time, and few expert therapists are available, making the therapies relatively expensive. The most recent evidence suggests that the most cost-effective therapy formats (by telephone, internet, group therapy, fewer therapies with a therapist, or self-administered therapies) can be efficacious for treating gastrointestinal disorders.176 Through adopting the available technologies, psychologic therapies can possibly become options that improve clinical results in the near future, even in refractory cases of FD.
Even though in this document we do not recommend the generalized use of psychologic therapy in subjects with FD, it is important to screen for psychologic factors in individuals with moderate, intense and/or refractory symptoms.177,178 Investigating specific factors, such as anxiety, depression, and adverse childhood experiences is essential, as well as evaluating sleep quality, given that there are shared mechanisms involved in the circadian rhythm, visceral hypersensitivity, and immune responses.179 Improving sleep quality and anxiety improves FD symptom scores.180 Increasing evidence suggests psychologic factors are the cause of symptoms in a subset of patients with DGBIs that are not explained by seeking medical attention, whereas in other cases, psychologic factors can be secondary to intestinal disease.181 A review of guidelines on the management of FD from the British Society of Gastroenterology emphasized that there are 4 main behavioral gut-brain interventions that can be efficacious in the treatment of overall FD symptoms: hypnotherapy, stress management, interpersonal psychodynamic informed psychotherapy, and cognitive behavioral therapy.182 On the other hand, psychologic factors predict treatment response, help select therapeutic options, and improve outcome and quality of life in persons with FD, giving great relevance to their identification and evaluation.
Personalized medicineWe recommend personalizing the treatment of FD and combining different therapeutic options according to the characteristics of each patient.
Quality of evidence: C.
Level of agreement: 100% in complete agreement.
FD has peculiar characteristics that make it a complex syndrome. Its pathophysiology is diverse, and in many cases, not yet completely understood. The prevalence of FD is elevated in the general population, signifying that it could coexist with other highly frequent organic conditions with similar symptoms, and it can also overlap with other DGBIs. Patients with overlap with other DGBIs have more intense symptoms, respond less efficiently to management, and have greater psychologic comorbidity.183,184 Even though FD is a chronic and benign stable condition, it is characterized by exacerbations and remissions of variable duration and intensity over time.185 Like other DGBIs, it has an elevated response to placebo, imposing special demands on the different therapeutic interventions.186 On the other hand, we should not forget that the treatment goals in FD are symptom control and improved quality of life.2
It is important to follow the direction research takes in the future, given that the new techniques of neural imaging, multiomics, and artificial intelligence are renovating the diagnosis and treatment of FD. Functional brain network characteristics determined through MRI could be useful as biomarkers for identifying patients with FD.187 Experimental studies have revealed characteristics of the microbiome, transcriptome, and metabolome that could regulate the low-grade duodenal inflammation in FD.188 Diagnostic models based on artificial intelligence have been developed that utilize brain activity and food preference images to distinguish individuals with FD from healthy controls.189 Artificial intelligence has also been used to develop prediction models that aid in better evaluating subjects with FD, predicting response, adapting to and adjusting treatment, and improving therapeutic efficacy.190,191
Taking the abovementioned into account, it is easy to understand that not all patients respond to the same management, that it is difficult to achieve therapeutic goals with a single intervention or drug, that treatment needs are not exactly the same for all patients, and even that treatment needs are not the same for the same patient over time. We recommend personalizing treatment and combining different therapeutic options according to the characteristics of each patient. Fig. 1 shows our proposed diagnosis and treatment algorithm for the management of dyspepsia.
ConclusionsFD is one of the most frequent gastrointestinal conditions in clinical practice. It is a complex disorder, with multiple pathophysiologic factors and diverse therapeutic options. In this consensus review, we present the advances achieved in this disease in recent years and issue good practice recommendations for its management, in an effort to complement and update the consensus document on dyspepsia published by the Asociación Mexicana de Gastroenterología in 2017.
Ethical considerationsThe authors declare that, in the development of this work: (1) no active investigation was conducted on any patient, thus informed consent was not required; (2) the work meets the current bioethical research regulations; (3) authorization by an ethics committee was not requested, given that the work is a thematic bibliographic review, in which no interventions involving subjects, biologic samples, medications, or healthcare products were performed; and (4) this document contains no personal information that could identify patients.
Financial disclosureThis document was carried out with the economic support of Laboratorios Carnot, partially covering the costs of the face-to-face voting meeting (transportation and housing). The authors received no fees for their participation.
Genaro Vázquez-Elizondo is a researcher for Laboratorio Chinoin, Laboratorio Carnot, and Novo Nordisk de México. He is an advisor for Chinoin, M8 Pharmaceuticals, and Eurofarma. He is a speaker for M8 Pharmaceuticals, Chinoin, Menarini, Alfasigma, Asofarma, Pfizer, Roche, Takeda México, Ferrer, Novo Nordisk, MSD, Laboratorio Carnot, and Eurofarma.
Octavio Gómez-Escudero is a speaker for the Chinoin, Alfasigma, Faes Pharma, and Carnot laboratories.
Enrique Coss-Adame is an advisor for Medtronic de México, Adium de México, Grunenthal de México and a speaker for Medtronic, Adium, Grunenthal, Alfa-Sigma, Sanfer, Medix, Megalabs, and Carnot.
Miguel Morales-Arámbula is a speaker for the Grünenthal, Menarini, Carnot, Eurofarma, ProMedCS, Praha, y M8 Pharma laboratories.
Eliana Carolina Morel-Cerda is a speaker for the Alfasigma and Megalabs laboratories.
Alejandra Noble-Lugo has been a speaker for the Alfasigma, Adium, AstraZeneca, and Ferrer laboratories. She has participated in the development of academic material and as a speaker for the Chinoin, Liomont, and M8 Pharma laboratories.
José María Remes-Troche is an advisor for and advisory board member of Asofarma, Carnot, Pro.Med.CS. Praha A.S, and PISA. He is a speaker for Asofarma, Abbot, Carnot, Chinoin, Ferrer, Johnson & Johnson, Medix, and Medtronic.
Omar Edel Trujillo-Benavides is a speaker for the Adium, Chinoin, Carnot, Grünenthal, and Medix laboratories.
Miguel Ángel Valdovinos-Díaz is a speaker for the Carnot, Asofarma, and Faes Farma laboratories.
The remaining participants declare they have no conflict of interest.