To determine the clinical, sociodemographic, and treatment characteristics of inflammatory bowel disease (IBD) in a Colombian population register.
MethodsA descriptive, analytic, observational, cross-sectional, multicenter study on patients with IBD from 17 hospital centers in 9 Colombian cities was conducted.
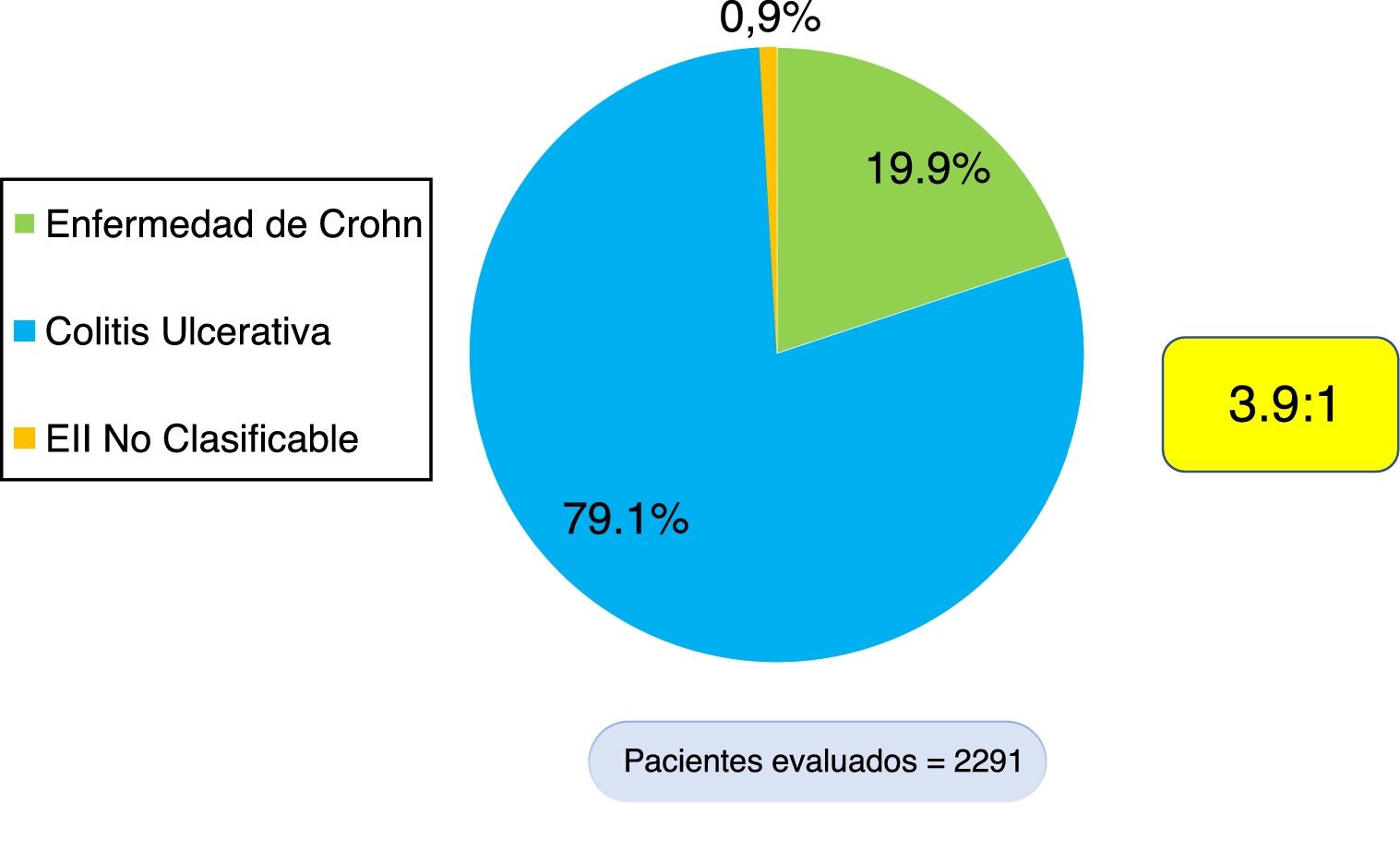
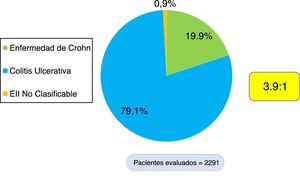
ResultsA total of 2,291 patients with IBD were documented, 1,813 (79.1%) of whom presented with ulcerative colitis (UC), 456 (19.9%) with Crohn's disease (CD), and 22 with IBD unclassified (0.9%). The UC/CD ratio was 3.9:1. A total of 18.5% of the patients with UC and 47.3% with CD received biologic therapy. Patients with extensive UC had greater biologic therapy use (OR = 2.78, 95% CI: 2.10-3.65, p = 0.000), a higher surgery rate (OR = 5.4, 95% CI: 3.5-8.3, p = 0.000), and greater frequency of hospitalization (OR = 4.34, 95% CI: 3.47-5.44, p = 0.000). Patients with severe UC had greater biologic therapy use (OR = 5.04, 95% CI: 3.75-6.78, p = 0.000), a higher surgery rate (OR = 8.64, 95% CI: 5.4-13.78, p = 0.000), and greater frequency of hospitalization (OR = 28.45, 95% CI: 19.9-40.7, p = 0.000). CD patients with inflammatory disease behavior (B1) presented with a lower frequency of hospitalization (OR = 0.12, 95% CI: 0.07-0.19, p = 0.000), a lower surgery rate (OR = 0.08, 95% CI: 0.043-0.15, p = 0.000), and less biologic therapy use (OR = 0.26, 95% CI: 0.17-0.41, p = 0.000).
ConclusionIn Colombia, there is a predominance of UC over CD (3.9:1), as occurs in other Latin American countries. Patients with extensive UC, severe UC, or CD with noninflammatory disease behavior (B2, B3) have a worse prognosis.
Determinar las características clínicas, sociodemográficas y tratamiento de la EII en un registro de la población colombiana.
MetodologíaEstudio observacional descriptivo, analítico, multicéntrico, de corte transversal de una cohorte nacional de pacientes con EII en 17 centros de nueve ciudades del país.
ResultadosSe documentaron 2,291 pacientes con EII, 1,813 (79.1%) con CU, 456 (19.9%) con EC y 22 con EII no clasificable (0.9%). La razón CU/EC es de 3.9:1. 18.5% de los pacientes con CU y 47.3% con EC han recibido terapia biológica. Los pacientes con CU extensa presentaban mayor uso de terapia biológica (OR = 2.78; IC 95%: 2.10-3.65; P = 0.000), mayor tasa de cirugía (OR = 5.4; IC 95%: 3.5-8.3; P = 0.000) y mayor frecuencia de hospitalización (OR = 4.34; IC 95%: 3.47-5.44; P: 0.000). Los pacientes con CU severa presentaban mayor uso de terapia biológica (OR = 5.04; IC 95%: 3.75-6.78; P = 0.000), mayor tasa de cirugía (OR = 8.64; IC 95%: 5.4-13.78; P = 0.000) y mayor frecuencia de hospitalización (OR = 28.45; IC 95%: 19.9-40.7; P = 0.000). Los pacientes con EC inflamatorio (B1) presentaban menor frecuencia de hospitalización (OR = 0.12; IC 95%: 0.07-0.19; P = 0.000), menor tasa de cirugía (OR = 0.08; IC 95%: 0.043-0.15; P: 0.000) y menor uso de terapia biológica (OR = 0.26; IC 95%: 0.17-0.41; P: 0.000).
ConclusiónEn nuestro país existe un predominio de CU sobre EC (3.9:1), como ocurre en otros países de Latinoamérica. Los pacientes con CU extensa y severa y con EC de comportamiento no inflamatorio (B2, B3) tienen peor pronóstico.
Inflammatory bowel disease (IBD) encompasses two distinct entities: ulcerative colitis (UC) and Crohn’s disease (CD). They are chronic inflammatory pathologies of the gastrointestinal tract that are not very common and primarily affect the colon and small bowel. Their clinical course is characterized by multiple relapses, and in recent years, a worldwide increase has been detected in the frequency of their appearance.1,2 The cause of IBD is unknown, but it results from a complex interaction between the genotype of the host, the gut microbiota, and environmental factors that trigger an alteration in the intestinal immune response.3 Historically, studies with a greater prevalence of IBD come from the Scandinavian countries, the United Kingdom, and North America. IBD affects approximately five million persons worldwide, which includes 1.4 million in the United States and close to three million in Europe.4 A systematic review of epidemiologic studies on IBD found a prevalence of UC of 4.9-505 per 100,000 inhabitants in Europe, 37.5-248.6 per 100,000 in North America, and 4.9-168.3 per 100,000 inhabitants in Asia. The prevalence of CD was 0.6-322 per 100,000 in Europe, 16.7-318.5 per 100,000 in North America, and 0.88-67.9 per 100,000 inhabitants in Asia. An increase in incidence over time was demonstrated in 75% of the studies on CD and 60% of the studies on UC.5 A more recent systematic review with 147 studies reported high prevalence rates for UC and CD in Europe (505 per 100,000 inhabitants for UC in Norway) and North America (286 per 100,000 inhabitants for UC in the United States), which have remained stable.6 Nevertheless, since 1990, population studies have shown an increase in incidence and prevalence in developing countries in Asia and South America, including Brazil and Mexico.6–9 The prevalence and incidence of UC and CD in Colombia was recently determined through information obtained from data cubes from the Comprehensive Social Security Information System (SISPRO, Spanish acronym) of the Colombian Department of Health and Social Security. For 2017 in Colombia, the prevalence of UC and CD in the adult population was 58.1/100,000 inhabitants and 8.9/100,000 inhabitants, respectively, and the incidence of UC and CD was 6.3/100,000 person/years and 0.74/100,000 person/years, respectively.10
Colombia has epidemiologic data on the behavior of IBD from local referral centers. In 1991 a case series was published that included 108 cases of IBD (98 UC and 10 CD) that were diagnosed between 1968 and 1990 in Bogotá.11 In 2010, at the Hospital Pablo Tobón Uribe in Medellín, the epidemiologic characteristics of 202 patients with IBD diagnosed between 2001-2009 were reported, with a distribution of 80.7% of patients with UC, 15.8% with CD, and 3.5% with IBD unclassified (IBD-U), for a UC:CD ratio of 5.1:1.12 The experience at the Clínica Universitaria Colombia in Bogotá with 165 patients was recently published, showing that 75.8% of the patients presented with UC and 24.2% with CD, for a UC:CD ratio of 3.1:1.13 However, we know of no combined data covering several regions of the country. Therefore, we decided to merge experiences and conduct a multicenter study to determine the epidemiologic, phenotypic, clinical, and current treatment characteristics of our Colombian patients with IBD, taking into account the global increase in the frequency of the disease, the availability of new diagnostic methods (through endoscopy, radiology, serology, and fecal markers), and the possibility of using new medical treatments in our country.
Materials and methodsType of studyAn analytic, observational, cross-sectional, multicenter study was conducted on a national cohort.
Study populationAll patients with IBD that were seen at the emergency room, were hospitalized, or were treated as outpatients at hospital referral centers from 9 Colombian cities were included. The participating referral centers and their corresponding cities and the number of patients provided by the national IBD register were: Hospital Pablo Tobón Uribe de Medellín (741), Cirujanos Unidos de Manizales (426), Fundación Santa Fe de Bogotá (329), Instituto Gastroclínico de Medellín (167), Clínica Colombia de Bogotá (151), Gastroadvanced de Bogotá y Medellín (115), MTG Servimed SAS de Bogotá (101), Endodigestivos de Pereira (91), Emdiagnóstica SAS de Bogotá (71), IMAT de Montería (64), Clínica Intermedios de Montería (46), Clínica La Misericordia de Barranquilla (41), Clínica La Carolina de Bogotá (23), LIMEQ de Tunja (14), Hospital Central de la Policía de Bogotá (11), Gastrosalud, Santa Marta (10), and Hospital Universitario del Caribe de Cartagena (6). There was a total of 2,407 patients, but 116 were eliminated from the database due to duplicate register. A high percentage of patients included in the register were from the region of the Andes and the Colombian Atlantic coast.
Within the study time frame, the first patient with IBD was documented in August 2001 and the last patient in July 2019. The diagnoses of UC and CD were taken from the clinical histories under the following ICD-10 codes: K50.0 Crohn’s disease of small intestine, K50.1 Crohn’s disease of large intestine, K50.8 Crohn’s disease of both small and large intestine, K50.9 Crohn’s disease, unspecified, K51.9 Ulcerative colitis, unspecified, K51.8 Other ulcerative colitis.
Diagnostic criteriaThe recent European Crohn’s and Colitis Organisation (ECCO) guidelines for the diagnosis of UC and CD state that there is no “gold standard” for diagnosing UC or CD and that it should be made through clinical, laboratory, imaging, endoscopic, and histopathologic findings. They do not recommend the use of genetic testing or serologic tests for making the diagnosis.14,15 The diagnostic criteria for UC16 used in the present study were based on the presence of three of the following four criteria, after ruling out infectious, ischemic, and neoplastic pathology: 1) a typical history with diarrhea and/or blood and/or mucus in stool for more than six weeks or in repeated episodes; 2) typical colonoscopic findings of granular, friable mucosa, with or without ulcerations; 3) histologic findings consistent with IBD resulting from acute or chronic inflammation, with cryptitis and crypt distortion, associated with lymphoplasmacytic infiltrate, and with no granulomas; and 4) no suspicion of CD from small bowel radiologic studies, ileocolonoscopy, or biopsies. UC severity was defined utilizing the Truelove and Witts classification17 and disease extension was determined by colonoscopic findings and defined according to the Montreal classification.18
We based CD diagnosis on the presence of two or more of the following criteria: 1) typical symptoms, including abdominal pain, diarrhea, and weight loss for more than six weeks; 2) macroscopic appearance at endoscopy or during surgery of segmental, discontinuous, or patchy lesions, with or without rectal involvement, aphthous ulcers, fissures, or penetrating or strictured lesions; 3) radiologic evidence of small bowel stricture, segmental colitis, or the presence of fistulas; and 4) histologic evidence of focal or transmural inflammation or epithelial granulomas with giant cells.19 The location and behavior of CD were determined according to the Montreal classification.18
Patients that did not meet the previously established criteria for UC and CD, despite their clinical, radiologic, endoscopic, histologic, and serologic findings, were identified as having IBD-U.18,20 Cases in which there was doubt, or patients that did not meet the IBD diagnostic criteria, were excluded from the register.
Data collectionA database was created in Excel, collecting the following information from each patient to be analyzed: 1) IBD type (UC, CD, and IBD-U); 2) age at diagnosis; 3) sex; 4) anatomic extension of UC; 5) greater disease activity grade in UC; 6) location of CD; 7) behavior of CD; 8) accumulated medical treatment (5-ASAs, steroids, immunosuppressants, biologic therapy); 9) surgical treatment; 10) hospitalization frequency; and 11) death.
Statistical analysisA univariate analysis was initially carried out that utilized absolute and relative frequencies for the qualitative variables and mean and standard deviation or median and interquartile range (25th-75th percentile) for the quantitative variables, after verification of the normality supposition.
The quantitative variables were dichotomized, and the decision was made to compare proportions utilizing the chi-square test of independence and calculating the odds ratios (ORs), with their respective 95% confidence intervals. Statistical significance was set at a p<0.05 and the SPSS version 21 (Universidad CES license) and Epidat version 3.1 software were employed.
The research was considered no risk, given that it exclusively involved information sent by the researchers from their clinical practices, guaranteeing the confidentiality and privacy of the data collected.
Ethical considerationsThe project researchers adhered to the international principles stated in the Declaration of Helsinki revised by the World Medical Association in 2013 at the General Assembly in Fortaleza, Brazil, and Resolution 008430 from the Health Department of Colombia in 1993. According to the resolution, the present study is no risk because it is a review of patient clinical histories and guarantees the confidentiality and privacy of the data collected.
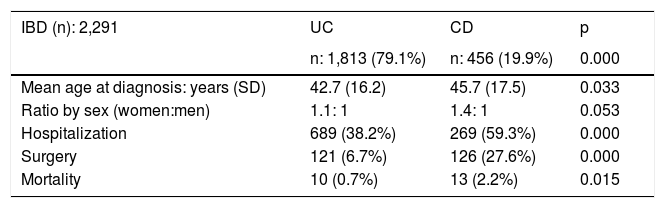
ResultsEpidemiologic characteristicsAn analytic, descriptive, observational, cross-sectional, multicenter study was conducted on a national Colombian cohort that systematically included 2,291 patients that met the diagnostic criteria of IBD, seen at 17 different hospital centers in nine cities (Fig. 1). Of those patients, 1,813 (79.1%) were diagnosed with UC, 456 (19.9%) with CD, and 22 (0.9%) with IBD-U, resulting in a UC:CD ratio of 3.9:1 (Fig. 2). There was a predominance of women over men in UC (1.1:1), as well as in CD (1.4:1). Mean age at CD diagnosis was 45.7 years (range: 9-90) and 42.7 years (range: 6-93) at UC diagnosis, with a statistically significant difference (p=0.033). Table 1 shows the demographic and clinical characteristics of the patients.
Demographic and clinical characteristics.
| IBD (n): 2,291 | UC | CD | p |
|---|---|---|---|
| n: 1,813 (79.1%) | n: 456 (19.9%) | 0.000 | |
| Mean age at diagnosis: years (SD) | 42.7 (16.2) | 45.7 (17.5) | 0.033 |
| Ratio by sex (women:men) | 1.1: 1 | 1.4: 1 | 0.053 |
| Hospitalization | 689 (38.2%) | 269 (59.3%) | 0.000 |
| Surgery | 121 (6.7%) | 126 (27.6%) | 0.000 |
| Mortality | 10 (0.7%) | 13 (2.2%) | 0.015 |
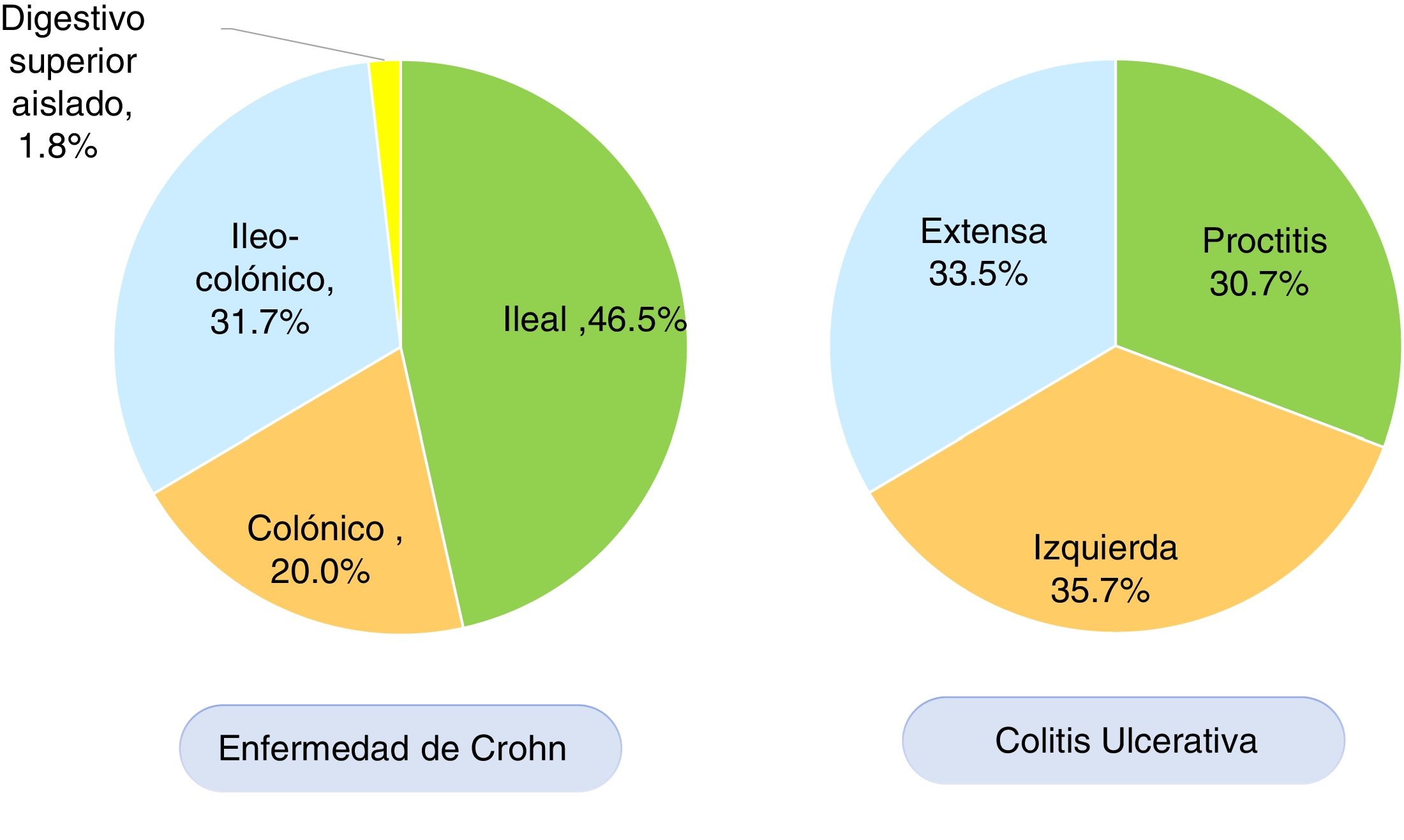
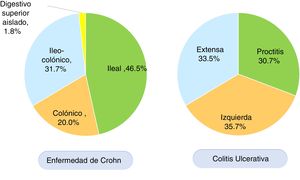
According to the Montreal classification, endoscopic UC distribution was as follows: 557 patients (30.7%) presented with proctitis, 648 (35.7%) with left colitis, and 608 (33.5%) with extensive colitis (Fig. 3). Regarding UC activity, 306 (16.8%) patients were in remission (S0) during follow-up at the respective hospital center, 480 (26.5%) had mild activity (S1), 473 (26.1%) had moderate activity (S2), and 554 (30.6%) had severe activity (S3). Patients with UC that were < 40 years of age had more severe colitis than patients ≥ 40 years of age (OR: 1.34, 95% CI: 1.04-1.73, p=0.024) and the difference was statistically significant. There was no significant difference in the comparison of UC extension in patients under or above 40 years of age.
In CD, the anatomic location was ileal in 212 (46.5%) patients, colonic in 91 (20.0%), ileocolonic in 145 (31.7%), and isolated to the upper digestive tract in eight (1.8%) patients (Fig. 3). The behavior of CD, according to the Montreal classification, was inflammatory in 201 (44.0%) patients, stricturing in 155 (34.0%), and penetrating in 55 (12.1%) patients. Forty-five (9.8%) patients with CD presented with perianal fistulizing involvement. There was no significant difference in the comparison of CD location in patients under or above 40 years of age.
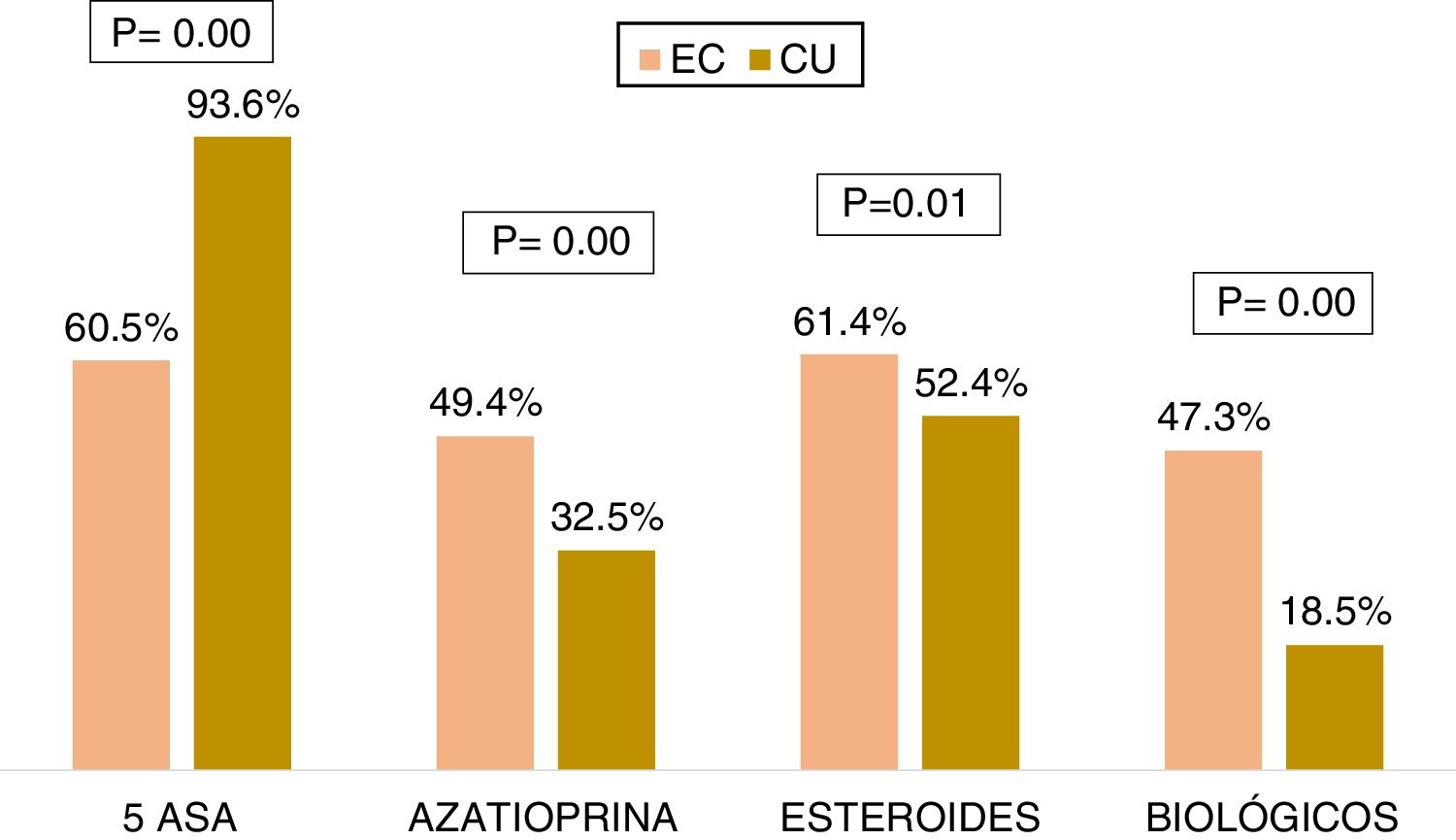
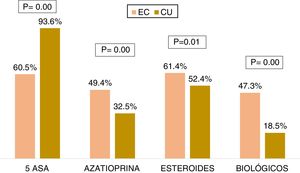
Medical treatmentRegarding the accumulated medical treatment in UC, 93.6% of the patients were treated with 5-ASAs, 32.5% with azathioprine, 52.4% with steroids, and 18.5% with biologic therapy. The most widely used first-line biologic drug in UC was infliximab (54.8%). In CD, 60.5% of the patients received 5-ASAs, 49.4% azathioprine, 61.4% steroids, and 47.3% of the patients underwent biologic therapy. The most widely used first-line biologic drug in CD was adalimumab (56.6%). The patients with CD received more steroids (OR: 1.44, 95% CI: 1.15-1.80, p=0.001), azathioprine (OR: 2.12, 95% CI: 1.71-2.63, p=0.000), and biologic therapy (OR: 3.93, 95% CI: 3.14-4.93, p=0.000) than the patients with UC, and the differences were statistically significant (Fig. 4).
Patients < 40 years of age with UC (OR: 1.69, 95% CI: 1.29-2.21, p=0.000) or CD (OR: 2.19, 95% CI: 1.38-3.44, p=0.001) had greater use of biologic therapy than those ≥ 40 years of age, with statistically significant differences.
Patients with extensive UC had greater use of biologic therapy (OR: 2.78, 95% CI: 2.10-3.65, p=0.000) than patients with non-extensive UC (left colitis and proctitis). Patients whose UC activity was severe had statistically significant greater use of biologic therapy (OR: 5.04, 95% CI: 3.75-6.78, p=0.000) than patients with moderate or mild UC.
On the other hand, patients with inflammatory CD (B1) had statistically significant less use of biologic therapy (OR: 0.26, 95% CI: 0.17-0.41, p=0.000) than the patients with non-inflammatory CD (B2 and B3). No significant difference was found between biologic therapy use and CD location (L1, L2, L3).
Surgical treatmentSurgical treatment was required in 126 (27.6%) patients with CD and 121 (6.7%) patients with UC, and the difference was significant (OR: 5.30, 95% CI: 3.99-7.03, p=0.000). UC patients < 40 years of age had a lower colectomy rate than patients ≥ 40 years of age (OR: 0.50, 95% CI: 0.31-0.82, p=0.005). Patients with extensive UC (OR: 5.40, 95% CI: 3.5-8.3, p=0.000) and severe activity (OR: 8.64, 95% CI: 5.4-13.78, p=0.000) had a higher colectomy rate than patients with non-extensive colitis and no severe activity, and the differences were significant. On the other hand, patients with inflammatory CD (B1) had a lower surgery rate (OR: 0.08, 95% CI: 0.043-0.15, p=0.000), compared with the patients with non-inflammatory CD. No significant differences were found between the frequency of surgery and CD location (L1, L2, L3).
HospitalizationHospitalization was necessary in 269 (59.3%) patients with CD and 689 (38.2%) patients with UC, and the difference was statistically significant (OR:2.36, 95% CI: 1.91-2.91, p=0.005). Hospitalization was more frequent in UC patients < 40 years of age (OR: 1.27, 95% CI: 1.02-1.56, p=0.030) and CD patients < 40 years of age (OR: 1.77, 95% CI: 1.13-2.78, p=0.001).
Hospitalization was more frequent in patients with extensive UC (OR: 4.34, 95% CI: 3.47-5.44, p=0.000) and severe activity (OR: 28.45, 95% CI: 19.9-40.7, p=0.000), compared with patients with non-extensive UC and activity that was not severe, and the differences were significant. Hospitalization was less frequent in patients with inflammatory CD (B1) (OR: 0.12, 95% CI: 0.07-0.19, p=0.000) than in the patients with non-inflammatory CD. No significant difference was found between hospitalization frequency and CD location (L1, L2, L3).
MortalityDeath was documented in ten patients with CD (2.19%) and thirteen patients with UC (0.71%), with a statistically significant difference (OR: 3.07, 95% CI: 1.34-7.05, p=0.015). Of the ten patients with CD, five died from postoperative complications, three from causes not related to CD, and cause of death could not be determined from the data in two patients. In UC, three patients died from postoperative complications, two from toxic megacolon, four from causes other than UC, and the cause of death could not be determined from the clinical history in four patients.
DiscussionThe present national register was achieved, thanks to the work and motivation of the different hospital centers in Colombia interested in the diagnosis and management of patients with IBD, and is a giant step toward understanding the epidemiology, clinical behavior, and treatment of Colombian patients with IBD. In addition, it is the national register with the largest number of patients in Latin America. Compared with previous Colombian studies, (8-10) the UC:CD ratio in the present register of 3.9:1 indicates that a greater number of CD cases than UC cases have been detected over the past decade. The predominance of UC over CD is similar to that reported in other Asian and Latin American countries, with the exception of Brazil, where there is a predominance of CD over UC.6,7 A study on 716 patients with IBD in Chile found 71% of cases with UC, 27% with CD, and 2% with IBD-U, for a UC:CD ratio of 2.6:1.21 Other studies in regions with a higher prevalence of IBD, such as the analysis by the Norwegian IBSEN group, reported 61.5% of patients with UC, 28.1% with CD, and 10.5% with IBD-U.16,19 A multicenter European register (EC-IBD) found 62.6% cases with UC, 32.0% with CD, and 7.4% with IBD-U.22,23 A Dutch study described a lower number of UC cases (53.0%), compared with CD (40.1%), and 6.8% of cases with IBD-U.24 A recent article from Hong Kong, with a register of 2,575 patients with IBD, within the time frame of 1981-2014, reported 59.8% of patients with UC, 38.2% with CD, and 2.0% with IBD-U, finding a decrease from the UC:CD ratio of 8.9:1 in the 1980s to 1.03:1, in the past 30 years.25 The same phenomenon is occurring in Colombia, and can be explained by a higher diagnostic suspicion of CD, better ileal intubation rates in patients with chronic diarrhea and anemia, and greater access to diagnostic methods for the study of small bowel diseases in our medical environment, such as capsule endoscopy and magnetic resonance enterography (MRE).
In the present study, in patients with UC, anatomic location was proctitis in 30.7% of the cases, left colitis in 35.7%, and extensive colitis in 33.5%. Our data were similar to those of other case series, in which the average locations were distributed in thirds. The Chilean study on 508 patients with UC mentioned above, described proctitis in 28% of cases, left colitis in 22%, and extensive colitis in 50%.21 A Hungarian study on 220 patients with UC reported proctitis in 26.8% of patients, left colitis in 50.9%, and extensive colitis in 22.3%.26 The Norwegian study by the IBSEN group found proctitis in 32.9% of cases, left colitis in 35%, and extensive colitis in 32.1%.16 The Asian study conducted in Hong Kong reported proctitis in 34.5% of cases, left colitis in 32.0%, and extensive colitis in 33.5%.25
Anatomic location in CD in the present register (ileal 46.5%, colonic 20.0%, ileocolonic 22.0%) showed a high percentage of patients with ileal involvement, compared with other case series. The Hungarian study26 reported ileal (20.2%), colonic (35.6%), and ileocolonic (44.2%) location. The Hong Kong register reported location in the ileum (24.5%), colon (32.3%), and ileum and colon (43.1%).25 In the Chilean study, ileal (27%), colonic (44%), and ileocolonic (28%) location was found.21 Another Dutch study described involvement of the terminal ileum in 31%, the colon in 27%, and the ileum and colon in 31%.24 The Norwegian IBSEN group reported 27.0% of patients had location in the terminal ileum, 48.5% exclusively in the colon, and 22.7% in the ileum and colon.19
CD behavior in our environment was found to be inflammatory in 53.9% of patients, stricturing in 34.0%, and penetrating in 12.1%. A total of 9.8% of our patients had perianal fistulizing involvement. Those results are somewhat different from findings in developed countries. The Norwegian IBSEN group determined that, at diagnosis, behavior in 62.0% of their patients was inflammatory, stricturing in 27.0%, and penetrating in 11%.19 In the Dutch study, 76% of patients presented with inflammatory behavior, 14% with stricturing disease, and only 7% with penetrating behavior at diagnosis.24 In the Hong Kong study, behavior was inflammatory in 65.2% of patients, stricturing in 25.1%, penetrating in 16.1%, and perianal in 24.5%.25 The abovementioned Hungarian study described inflammatory behavior in 64.4% of patients, stricturing disease in 17.8%, penetrating disease in 17.8%, and perianal behavior in 11.1% at diagnosis.26 In the Chilean case series on 196 CD patients, 80% presented with inflammatory disease, 10% with stricturing behavior, 9% with penetrating behavior, and 28% with perianal involvement.21 Those results could be explained by a delay in CD diagnosis due to the prolonged period of time between symptom onset and diagnosis, favoring disease progression, as has been shown in previous local studies.10,12
The large majority of patients with UC received 5-ASAs (93.6%), but 60.5% of the patients with CD received accumulated treatment with 5-ASAs, despite little clinical evidence for their use in that entity, according to international recommendations.15 A Swiss study found that 59% of CD patients were treated with 5-ASAs.27 A survey among German gastroenterologists showed that 10-36% of them prescribed 5-ASAs as monotherapy for treating CD.28 Thus, the continued provision of medical education at all levels is important for the adequate treatment of patients with CD.
In the present national register, 18.5% of our patients with UC and 47.3% with CD received biologic therapy. Those percentages are high compared with figures from other hospital centers. In the Hong Kong study mentioned above, 15.3% of CD patients received biologic therapy, compared with 1.3% of UC patients.25 The Dutch COIN study reported the use of biologic therapy in 22.7% of patients with CD and 4% of patients with UC.29 In a study based on a national register in Hungary,30 stratified by pediatric, adult, and older adult populations, biologic therapy was used in 15%, 9%, and 2% of the patients with CD, respectively, and in 4%, 3%, and 1% of the patients with UC, respectively. In the previously described Chilean study, biologic therapy was used in 34% of CD patients and 7% of UC patients.21 In a Danish study on 48,967 patients with IBD diagnosed between 1979 and 2011, in the first 9 years, anti-tumor necrosis factor (anti-TNF) agents were used in 23% of patients with CD and in 9% of patients with UC.31 A recent study on a French national register of 201,001 IBD patients found that the probability of anti-TNF therapy use at five years in CD patients was 33.8%, as monotherapy, and 18% as therapy combined with an immunosuppressant. In UC patients, those figures were 12.9% and 7.4%, respectively.32 A possible explanation for the high percentage of biologic therapy use in our patients is that the majority of hospitals that participated in the national register and provided the highest number of patients are both local and national referral centers that receive conventional management-refractory patients with complicated UC and CD (with strictures and fistulas), whose next treatment option is often biologic therapy. Therefore, as stated above, the percentage of individuals with CD with inflammatory behavior (B1) was lower, compared with other case series.
The percentage of patients with CD that required surgical management in our study was 27.6%, and 6.7% of our UC patients underwent colectomy. The patients with extensive and severe UC were at greater risk for colectomy and individuals with inflammatory CD (B1) had a lower risk for surgery. Similar results were reported by the IBSEN group that found a 19% colectomy rate in UC patients with extensive colitis, compared with 8% for left colitis and 5% for proctitis, at follow-up year ten.16 In the Hungarian study, the probability of colectomy at five years in UC patients was 5%. In CD patients, the probability of surgery was 9.8%, 18.5%, and 21.3%, after one, three, and five years of follow-up.26 The multicenter European register (EC-IBD) reported an accumulated surgery rate of 31.6% in CD at ten years, 22 and the Norwegian IBSEN group found the surgery rates in CD of 13.6%, 27.0%, and 37.9% at years one, five, and ten of follow-up. Stricturing and penetrating behaviors were independent risk factors for surgery, similar to that found in our national register.19 A systematic review based on population studies reported that the risk for surgery in CD was 16.3%, 33.3%, and 46.6% at years one, five, and ten of follow-up, respectively. In UC, the risk for colectomy was 4.9%, 11.6%, and 15.6% at years one, five, and ten of follow-up, respectively.33
In UC, 38.2% of the patients were hospitalized, and a majority of those patients had extensive UC, were < 40 years of age, and presented with severe activity. A recent systematic review with 20 studies carried out to determine the risk factors for colectomy in UC found that extensive colitis (OR 3.68, 95% CI: 2.39-5.69) and a history of hospitalization (OR 4.13, 95% CI: 3.23- 5.27) were risk factors.34 For patients with CD, 59.3% were hospitalized, of whom a larger number were < 40 years of age and presented with noninflammatory disease (B2, B3). A review of cohorts of patients with CD reported hospitalization rates of 31.9% and 61.8%, at years one and ten of follow-up, respectively.35 Hospitalization frequency in the Chilean study was similar to that of our register, reporting 35% in UC and 55% in CD.21 Regarding IBD mortality in our environment, 0.71% of the patients with UC and 2.19% of the patients with CD died, which are low rates. The mortality rates in the Hong Kong study25 were 1.2% in UC and 0.7% in CD. In the Dutch study, the mortality rates were 4% for CD and 7% for UC during the follow-up.24
ConclusionsIn conclusion, the present national register has the highest number of patients with IBD in Latin America and shows a predominance of UC over CD (3.9:1) in Colombia, as occurs in other Latin American countries.7 The clinical behavior of our patients with CD was more severe, compared with results from other hospital centers worldwide. The patients with CD had a higher surgery rate and mortality rate, compared with the UC patients. Despite the clinical evidence against it, there is still a high percentage of 5-ASA use in CD (60.5%) in Colombia. Biologic therapy is very widely used in Colombia, predominantly using anti-TNF drugs. In UC, patients < 40 years of age, with extensive and severe disease, have a poor prognosis. In CD, patients < 40 years of age, with noninflammatory disease (B2, B3, perianal), have a worse prognosis. Among the limitations of the present study was its retrospective design, which can produce selection bias in relation to the data collection, as well as the fact that it was conducted on patients from referral hospitals, which are centers that treat the more complex cases of IBD.
Author contributionsJuliao-Baños F: Study design, patient recruitment, data collection, document drafting. Puentes F: Patient recruitment and data collection. López R: Patient recruitment and data collection. Saffon MA: Patient recruitment and data collection. Reyes G: Patient recruitment and data collection. Parra V: Patient recruitment and data collection.
Galiano MT: Patient recruitment and data collection. Barraza M: Patient recruitment and data collection. Molano J: Patient recruitment and data collection. Álvarez E: Patient recruitment and data collection. Corrales R: Patient recruitment and data collection.
Vargas LE: Patient recruitment and data collection. Gil F: Patient recruitment and data collection. Álvarez P: Patient recruitment and data collection. Limas L: Patient recruitment and data collection. Prieto R: Patient recruitment and data collection. Yance P: Patient recruitment and data collection. Díaz F: Patient recruitment and data collection. Bareño J: Statistical analysis and document review.
Financial disclosureThe authors declare there was no financial support in relation to the development of the present study.
Conflict of interestThe authors declare that there is no conflict of interest.
Fabián Juliao-Baños1, Mateo Arrubla1, Joselyn Camargo1, Fabián Puentes2, Lázaro Arango2, Rocío López3, Rafael García3, Belén Mendoza3, María A. Saffon4, Luis F. Roldan4, Julio Zuleta4, Gustavo Reyes5, Viviana Parra6, Cristian Flórez6, Edilberto Nuñez6, María T. Galiano7, Marcos Barraza8, Isabel C. Sanchez8, Jenny L. Molano9, Jorge I. Lizarazo9, Iván Cuellar9, Eligio Álvarez10, Rubén Corrales11, Fabio Gil5, Luz E. Vargas12, Patricia Álvarez13, Luis M. Limas14, Robín Prieto 15, Hernán Ballén15, Lidsay Delgado15, Paola Yance16, Felha Díaz17.
Affiliations:
1. Hospital Pablo Tobón Uribe. Medellín. 2. Cirujanos Unidos. Manizales. 3. Fundación Santa Fe. Bogotá. 4. Instituto Gastroclínico. Medellín. 5. Clínica Colombia. Bogotá. 6. Gastroadvanced. Bogotá-Medellín. 7. MTG Servimed SAS. Bogotá. 8. Dr. Endodigestivos. Pereira. 9. Emdiagnóstica SAS. Bogotá. 10. IMAT. Montería. 11. Clínica Intermedios. Montería. 12. Clínica La Misericordia. Barranquilla. 13. Clínica La Carolina. Bogotá. 14. LIMEQ. Tunja. 15. Hospital Central de la Policía. Bogotá. 16. Gastrosalud. Santa Marta. 17. Hospital Universitario del Caribe. Cartagena.
Please cite this article as: Juliao-Baños F, Puentes F, López R, Saffon MA, Reyes G, Parra V, et al. Caracterización de la enfermedad inflamatoria intestinal en Colombia: resultados de un registro nacional. Revista de Gastroenterología de México. 2021;86:153–162.