There is an increase in the incidence and prevalence of eosinophilic esophagitis (EoE) worldwide and data in Latin America are scarce. The aim of our study was to describe the epidemiologic and clinical characteristics of EoE and its treatment, in adult patients in Colombia.
Patients and methodsA descriptive, cross-sectional study was conducted on patients with EoE, with the participation of 16 gastroenterologists from different Colombian cities. Demographic and clinical variables of the patients were evaluated, along with their treatment and complications. EoE severity was calculated utilizing the clinical severity index (CSI).
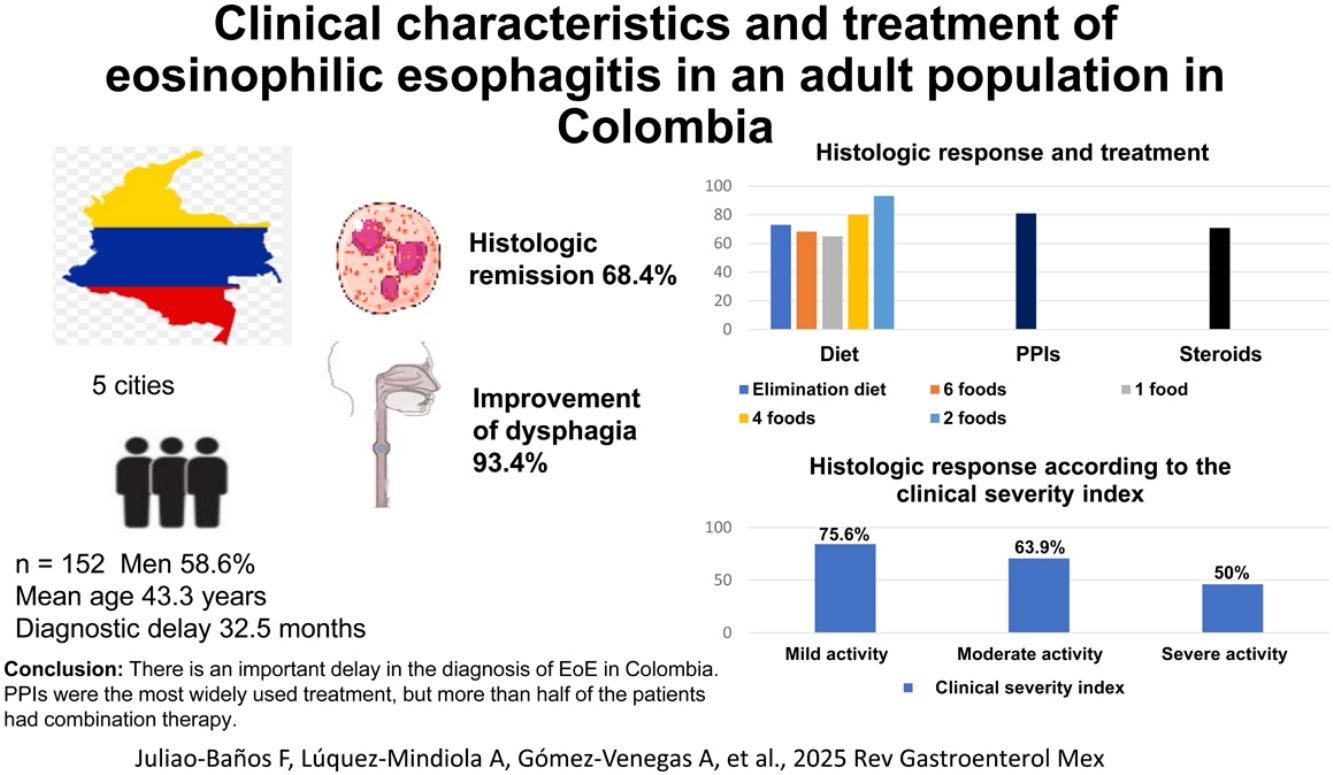
ResultsThe study included 152 patients with EoE seen within the time frame of November 2023 and July 2024. A total of 58.6% were men and the mean patient age at diagnosis was 43.3 years (range: 18−79). The time from symptom onset to diagnosis was 32.5 months (median), with an interquartile range (25−75%) of 11.0–79.8 months. A total of 134 (88.2%) patients received treatment with proton pump inhibitors (PPIs), 66 (43.4%) had dietary treatment, 42 (27.6%) received steroids, and 9 (5.9%) underwent endoscopic dilatation. A total of 57.2% of the patients received combination therapy. Histologic remission was achieved in 68.4% of the patients and dysphagia improved in 93.4% of the cases. In relation to the CSI, histologic remission was achieved in 75.6% of the patients with mild disease activity, in 63.9% with moderate activity, and in 50% with severe activity (p = 0.003).
ConclusionsOur results showed there is an important delay in the diagnosis of EoE in Colombia. The most widely used treatment was with PPIs, but more than half the patients had combination therapy. National management guidelines that consider local treatment availability need to be developed.
La esofagitis eosinofílica (EEo) presenta un incremento en la prevalencia e incidencia a nivel mundial, datos de Latinoamérica son escasos. El objetivo de este estudio es describir las características epidemiológicas, clínicas y tratamiento de pacientes adultos con EEo en Colombia.
Pacientes y métodosSe realizó un estudio multicéntrico descriptivo de corte transversal, con 16 gastroenterólogos de diferentes ciudades del país. Se evaluaron variables demográficas, clínicas, tratamiento y complicaciones. La severidad de la EEo se calculó con el índice de severidad clínico (ISC).
ResultadosDesde noviembre de 2023 hasta julio de 2024, se incluyeron 152 pacientes, 58.6% hombres, edad promedio de diagnóstico 43,3 años (18−79). El tiempo transcurrido entre el inicio de los síntomas hasta el diagnóstico fue de 32.5 meses (mediana), con un rango intercuartilar (25%–75%) de 11.0–79.8 meses. 134 (88.2%) pacientes recibieron tratamiento con inhibidores de bomba de protones (IBPs), 66 (43.4%) dieta, 42 (27.6%) esteroides y 9 (5.9%) dilatación endoscópica. 57.2% recibieron terapia combinada. Se obtuvo remisión histológica en 68.4% y mejoría de la disfagia en 93.4% de los casos. Teniendo en cuenta el ISC, se logró remisión histológica en 75.6% de pacientes con actividad leve, 63.9% actividad moderada y 50% actividad severa (p = 0.003).
ConclusionesLa EEo en Colombia presenta un retraso importante en el diagnóstico. El tratamiento más utilizado son los IBPs, aunque más de la mitad recibieron terapia combinada. Es necesario realizar guías nacionales de manejo considerando disponibilidad de tratamientos locales.
Eosinophilic esophagitis (EoE) is a chronic, progressive inflammatory disease of the esophagus that is mediated by antigens and has a Th2 cell response. Its clinical characteristics are symptoms of esophageal dysfunction, such as dysphagia, chest pain, food impaction, and a decrease in food intake. Upper gastrointestinal endoscopy reveals rings, linear furrows, whitish exudate, loss of vascularity, and luminal stricture.1 Diagnosis is made by the presence of ≥15 eosinophils per high power field (HPF), ruling out other causes of eosinophilia, such as achalasia, Crohn’s disease, celiac disease, reaction to medications, graft-versus-host disease, connective tissue disease, eosinophilic gastroenteritis, vasculitis, and infections (fungi, viruses).2 It is frequently associated with other atopic diseases, and is more frequent in men, in the White population, in the second and third decades of life, and in first-degree relatives of EoE patients. All studies show that patient quality of life notably deteriorates.3,4 EoE and gastroesophageal reflux disease (GERD) can coexist in the same person and are not mutually exclusive.5,6
In the past 2 decades, the incidence and prevalence of EoE have increased worldwide, in both children and adults. A recent systematic review with 40 studies reported a worldwide incidence of 5.31 cases per 100,000 inhabitants-year (95% CI 3.98–6.63) and prevalence of 40.04 cases per 100,000 inhabitants (95% CI 31.10–48.98), with a higher number of cases in men and in developed countries, and greater frequency in North America than in Europe. Incidence and prevalence have increased 27.2 and 9.1 times, respectively, compared with studies conducted before 2000.7
The treatment goal in EoE is the control of symptoms and inflammation, to prevent complications, such as stricture and food impaction. To achieve that, treatment with the 3 Ds (Diet, Drugs, and Dilatation) has been recommended. Dupilumab has recently been added to the treatment, making it the 4 Ds. Recently approved by the Food and Drug Administration (FDA), dupilumab is a monoclonal antibody that targets the interleukin 4 (IL-4) receptor, blocking IL-4 and IL-13 signaling, and is indicated in severe and refractory cases.8 Of those interventions, diet is the only one that treats the cause of the disease, whereas endoscopic dilatation is a treatment for alleviating the symptoms caused by stricture, but has no effects on the inflammation.9 Because it is a progressive disease, EoE, once identified, requires continuous treatment for maintaining clinical and histologic remission, thus preventing recurrences and complications. There is no justification for simply “observing” EoE patients.10
Carrying out studies on patients with EoE is not easy because symptoms may be unperceived and because there is a lack of suspicion on the part of the endoscopist when performing the endoscopy, and so esophageal biopsies are not taken to confirm the diagnosis.11 Therefore, we decided to conduct the present study to define the clinical and epidemiologic characteristics of EoE and its treatment, in an adult Colombian population, to have a better understanding of the behavior of the disease at the local level.
MethodologyA cross-sectional, analytic, multicenter study was conducted on adult patients with EoE in different Colombian cities. Gastroenterologists seeing adult patients were invited by email to take part in the study, resulting in the participation of 16 of them from the entire country, who work at different centers located in the following cities: Bogotá, Medellín, Bucaramanga, Manizales, and Cartagena. In principle, all adult patients with EoE, seen by the participating gastroenterologists at the emergency service, as hospitalized patients, or as outpatients, were included in the study.
The patients with EoE were diagnosed due to the presence of: 1) symptoms of esophageal dysfunction, such as dysphagia, chest pain, and reduced food intake, 2) histologic findings in the esophagus of ≥ 15 eosinophils/HPF or > 60 eosinophils/mm2, with this eosinophilia limited to the esophagus, 3) endoscopic findings, such as rings, furrows, mucosal edema, exudates, strictures, and crêpe-paper mucosa, that reinforce the diagnosis.5
The information was collected utilizing a database, and the clinical histories of each of the patients diagnosed with EoE or the patient consultation interviews were reviewed by the participating researchers of the study. The database was developed on a web application, facilitating data access and placement. The participating researchers received online training on the methodology utilized for the data collection, to guarantee information reliability, as well as the ethical and secure collection of the information.
The following variables were studied: age, sex, date of symptom onset, date of diagnosis, symptoms (dysphagia, decrease in food intake, chest pain, abdominal pain, heartburn, regurgitation, vomiting, food impaction in the esophagus), type of treatment: diet (elimination of 6 foods, 4 foods, 2 foods, and one food), steroids (oral or topical), type of PPI and dose (once or twice a day), esophageal dilatation (Savary, pneumatic), treatment complications, histologic remission (defined as the presence of < 15 eosinophils/HPF during follow-up), endoscopic response, and clinical response (dysphagia). EoE severity was calculated utilizing the Clinical Severity Index (CSI), which included symptom measurements (weekly, daily, several times a day), complications (impaction, perforation), inflammatory involvement (endoscopic and histologic), and fibrostenotic involvement (rings, stricture). EoE was considered inactive with a score < 1, mild at 1–6, moderate at 7–14, and severe at ≥ 15.12 Endoscopic response and dysphagia were determined, taking into account the progression of dysphagia during the follow-up and from the inflammatory and fibrostenotic endoscopic findings included in the CSI.
The STROBE checklist was utilized in this study.
Statistical analysisThe categorical variables were presented as absolute and relative frequencies and the continuous variables as mean and standard deviation (SD) or median and interquartile range (IQR) (25−75 P), according to the data distribution. Variables were compared using the chi-square test and statistical significance was set at 0.05. The SPSS® version 25 program was used.
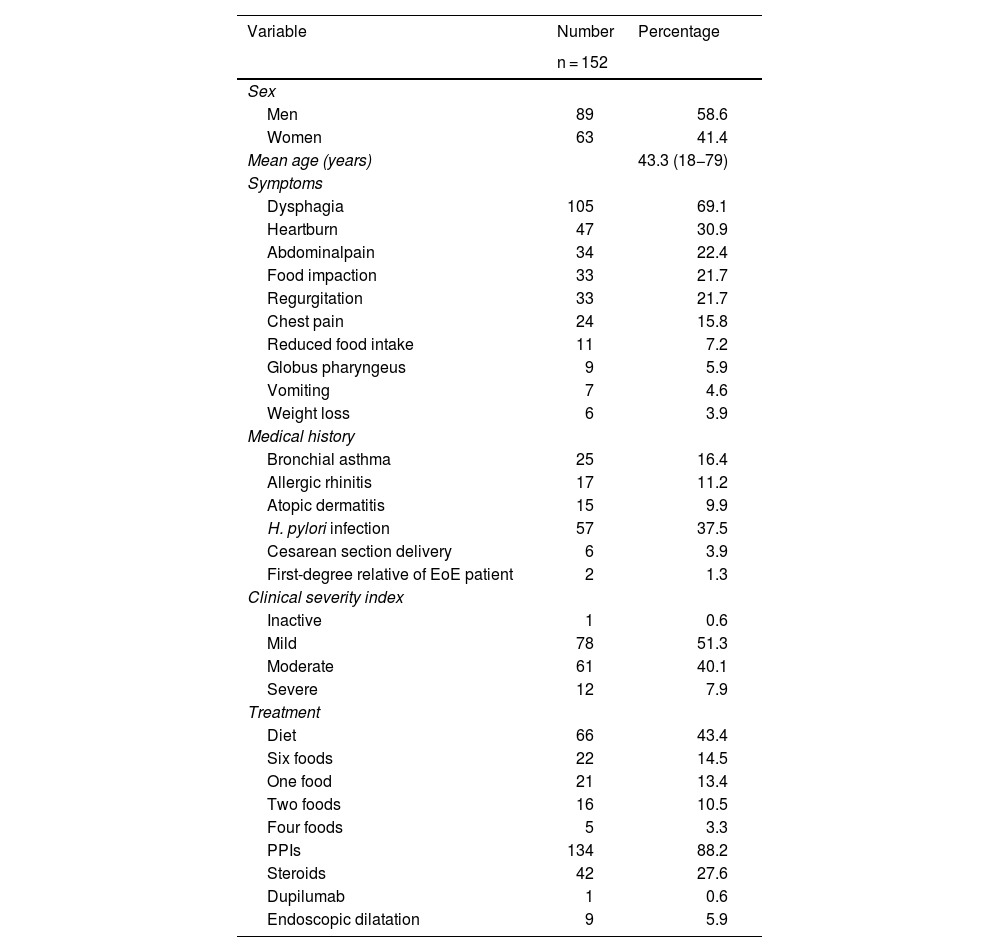
ResultsWithin the time frame of November 2023 and July 2024, 152 adult patients with EoE were included, 89 of whom were men (58.6%). Mean patient age at the time of diagnosis was 43.3 years (18−79 years), with a median age of 42.5 years and an IQR (25−75%) of 33.2–54.7 years. Seventy-eight (51.3%) patients were in the fourth and fifth decades of life. The median time from symptom onset to diagnosis was 32.5 months, with an IQR (25−75%) of 11.0–79.8 months.
Regarding the factors associated with EoE, 57 (37.5%) patients had a history of Helicobacter pylori infection, 15 (9.9%) had atopic dermatitis, 17 (11.2%) had allergic rhinitis, 25 (16.4%) presented with bronchial asthma, 6 (3.9%) patients had Cesarean section deliveries, and only 2 (1.3%) patients were first-degree relatives of an EoE patient. Of the patient total, 105 (69.1%) presented with dysphagia, 47 (30.9%) with heartburn, 34 (22.4%) with abdominal pain, 33 (21.7%) with regurgitation, 33 (21.7%) had a history of admission to the emergency service due to food impaction, 24 (15.8%) presented with chest pain, 11 (7.2%) with voluntary decrease in food intake, 9 (5.9%) with globus pharyngeus sensation, 7 (4.6%) with vomiting, 6 (3.9%) with weight loss, and 4 (2.6%) patients were asymptomatic.
Of the endoscopic findings at diagnosis, 77 (50.7%) patients presented with rings or strictures that allowed passage of the endoscope, and 9 (5.9%) patients required endoscopic dilatation because passage of the endoscope was impossible. There was one case of esophageal perforation, when attempting food disimpaction with the endoscope as an endoscopic emergency, that then required surgical management.
The CSI was calculated in all patients at diagnosis, finding that 78 (51.3%) patients presented with mild activity, 61 (40.1%) with moderate activity, and 12 (7.9%) with severe activity. One patient (0.6%) had disease inactivity. Table 1 shows the general characteristics of the study patients.
General characteristics of the patients with eosinophilic esophagitis.
| Variable | Number | Percentage |
|---|---|---|
| n = 152 | ||
| Sex | ||
| Men | 89 | 58.6 |
| Women | 63 | 41.4 |
| Mean age (years) | 43.3 (18−79) | |
| Symptoms | ||
| Dysphagia | 105 | 69.1 |
| Heartburn | 47 | 30.9 |
| Abdominalpain | 34 | 22.4 |
| Food impaction | 33 | 21.7 |
| Regurgitation | 33 | 21.7 |
| Chest pain | 24 | 15.8 |
| Reduced food intake | 11 | 7.2 |
| Globus pharyngeus | 9 | 5.9 |
| Vomiting | 7 | 4.6 |
| Weight loss | 6 | 3.9 |
| Medical history | ||
| Bronchial asthma | 25 | 16.4 |
| Allergic rhinitis | 17 | 11.2 |
| Atopic dermatitis | 15 | 9.9 |
| H. pylori infection | 57 | 37.5 |
| Cesarean section delivery | 6 | 3.9 |
| First-degree relative of EoE patient | 2 | 1.3 |
| Clinical severity index | ||
| Inactive | 1 | 0.6 |
| Mild | 78 | 51.3 |
| Moderate | 61 | 40.1 |
| Severe | 12 | 7.9 |
| Treatment | ||
| Diet | 66 | 43.4 |
| Six foods | 22 | 14.5 |
| One food | 21 | 13.4 |
| Two foods | 16 | 10.5 |
| Four foods | 5 | 3.3 |
| PPIs | 134 | 88.2 |
| Steroids | 42 | 27.6 |
| Dupilumab | 1 | 0.6 |
| Endoscopic dilatation | 9 | 5.9 |
EoE: eosinophilic esophagitis; H. pylori: Helicobacter pylori; PPIs: proton pump inhibitors; six foods: dairy, wheat, eggs, soy, nuts, and seafood; four foods: dairy, wheat, eggs, and soy; two foods: dairy and wheat; one food: dairy.
Regarding accumulated treatment of the patient total, 134 (88.2%) received treatment with PPIs, 66 (43.4%) had dietary management, 42 (27.6%) received steroids, and 9 (5.9%) underwent endoscopic dilatation. None of the dilatated patients had complications of perforation or surgery. Only one patient with EoE was treated with dupilumab due to the additional presentation of atopic dermatitis.
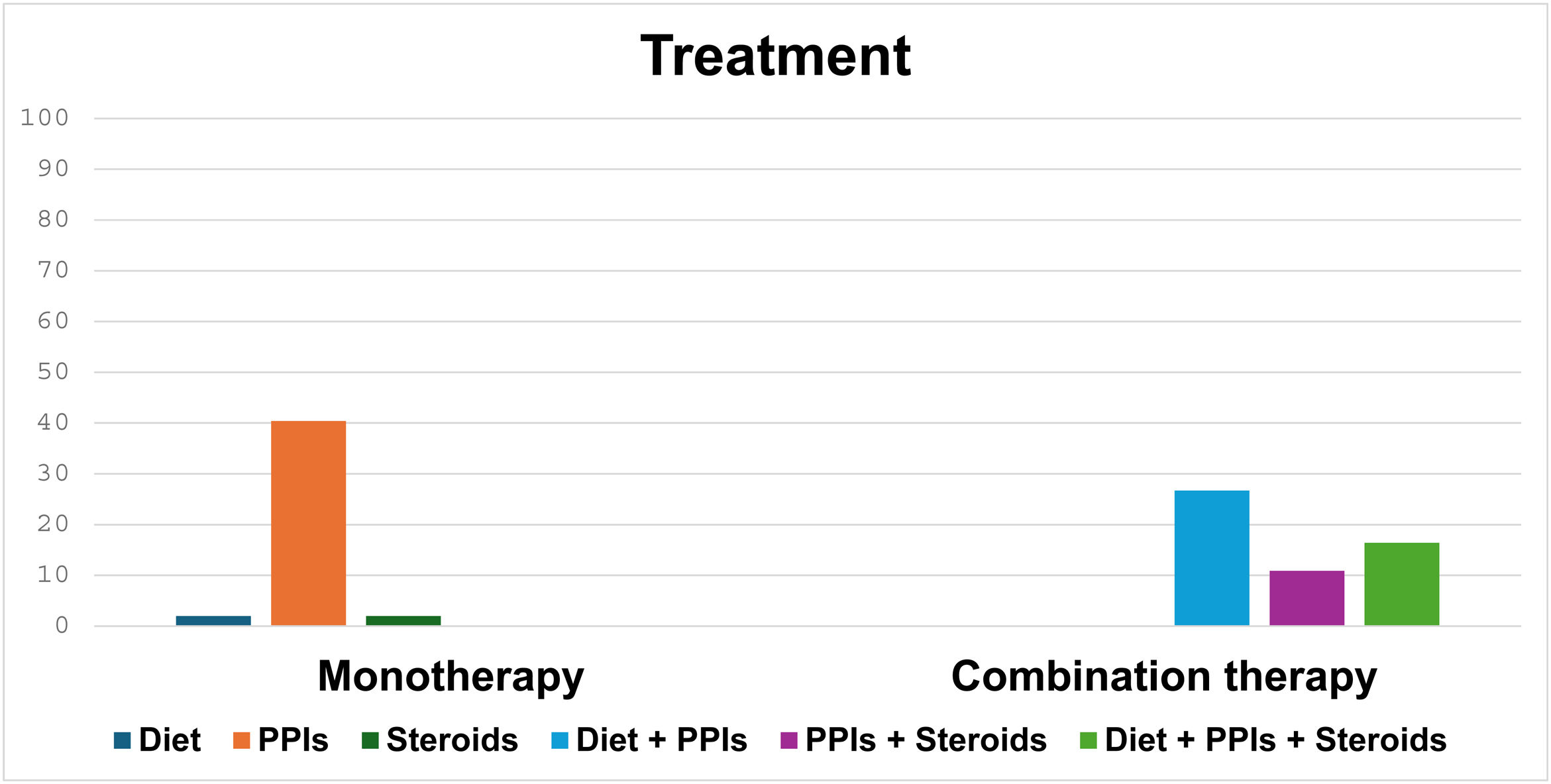
In total, 59 (38.8%), 3 (1.9%), and 3 (1.9%) patients received monotherapy with PPIs, dietary treatment, and treatment with steroids, respectively. Eighty-seven (57.2%) patients received combination treatment, of whom 43 (49.4%) were managed with diet and PPIs, 26 (29.8%) with diet, PPIs, and steroids, and 18 (20.6%) with the combination of steroids and PPIs (Fig. 1).
During the follow-up of all study patients, 68.4% had histologic remission, 68.4% had endoscopic response, and 93.4% had improved dysphagia, with the different treatments.
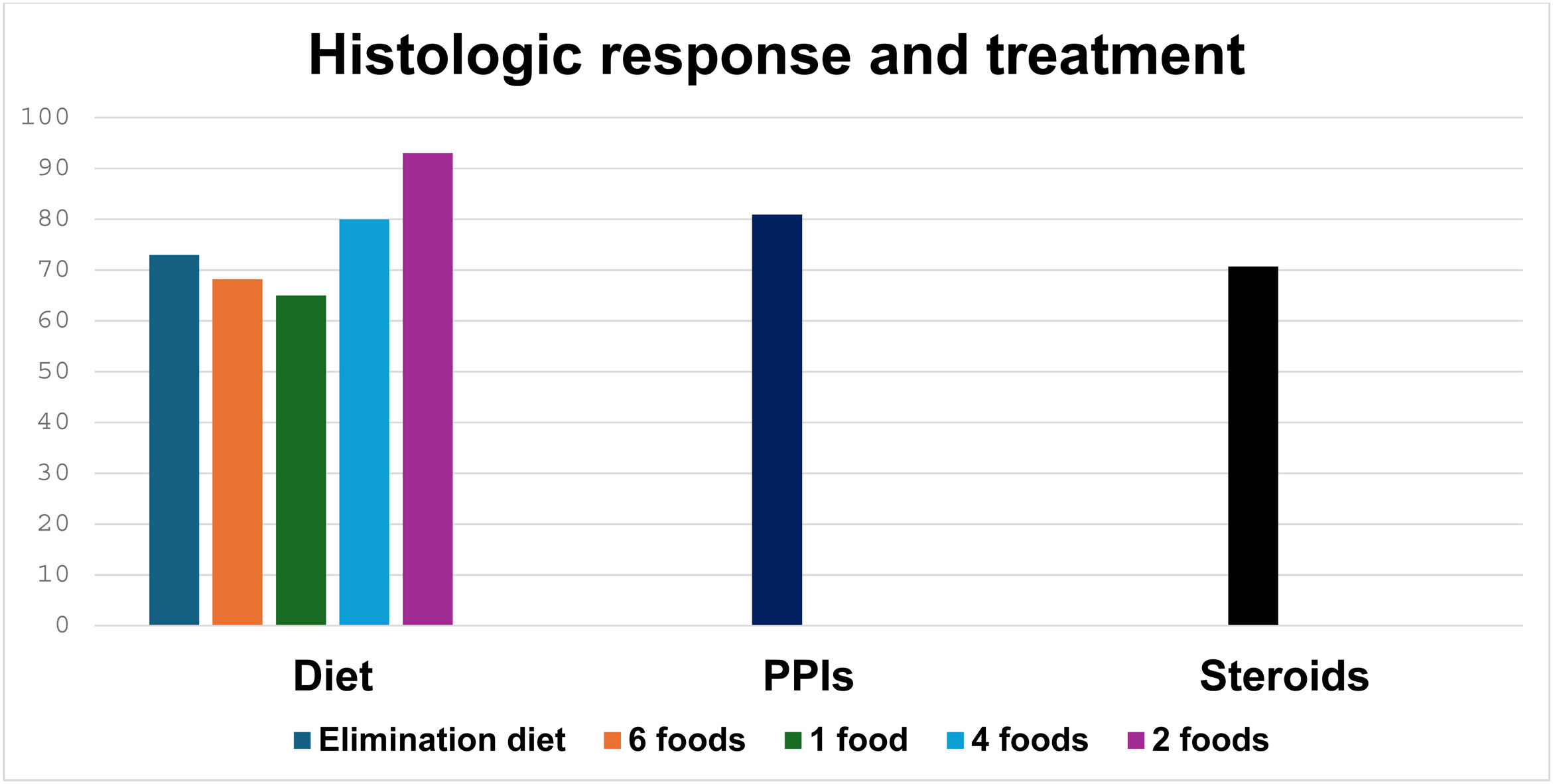
Of the 152 patients, 23 (15.1%) received a 6-food elimination diet, 22 (14.5%) a non-dairy diet, 16 (10.5%) a 2-food (dairy and wheat) elimination diet, and 5 (3.3%) a 4-food elimination diet. During follow-up, histologic response was evaluated in 66 patients that had dietary treatment, finding histologic remission in 46 (69.7%). In the patients managed with PPIs, the most widely used was esomeprazole in 107 of the 134 patients (79.9%), followed by pantoprazole (14.9%). The majority of patients (104 [77.6%]) received a double-dose PPI. During follow-up, histologic response was evaluated in the patients treated with PPIs, finding histologic remission in 85 (63.4%) patients. Regarding treatment with steroids, 42 (27.6%) patients inhaled, then swallowed the steroids, and during follow-up, 27 (64.2%) patients had histologic remission (Fig. 2).
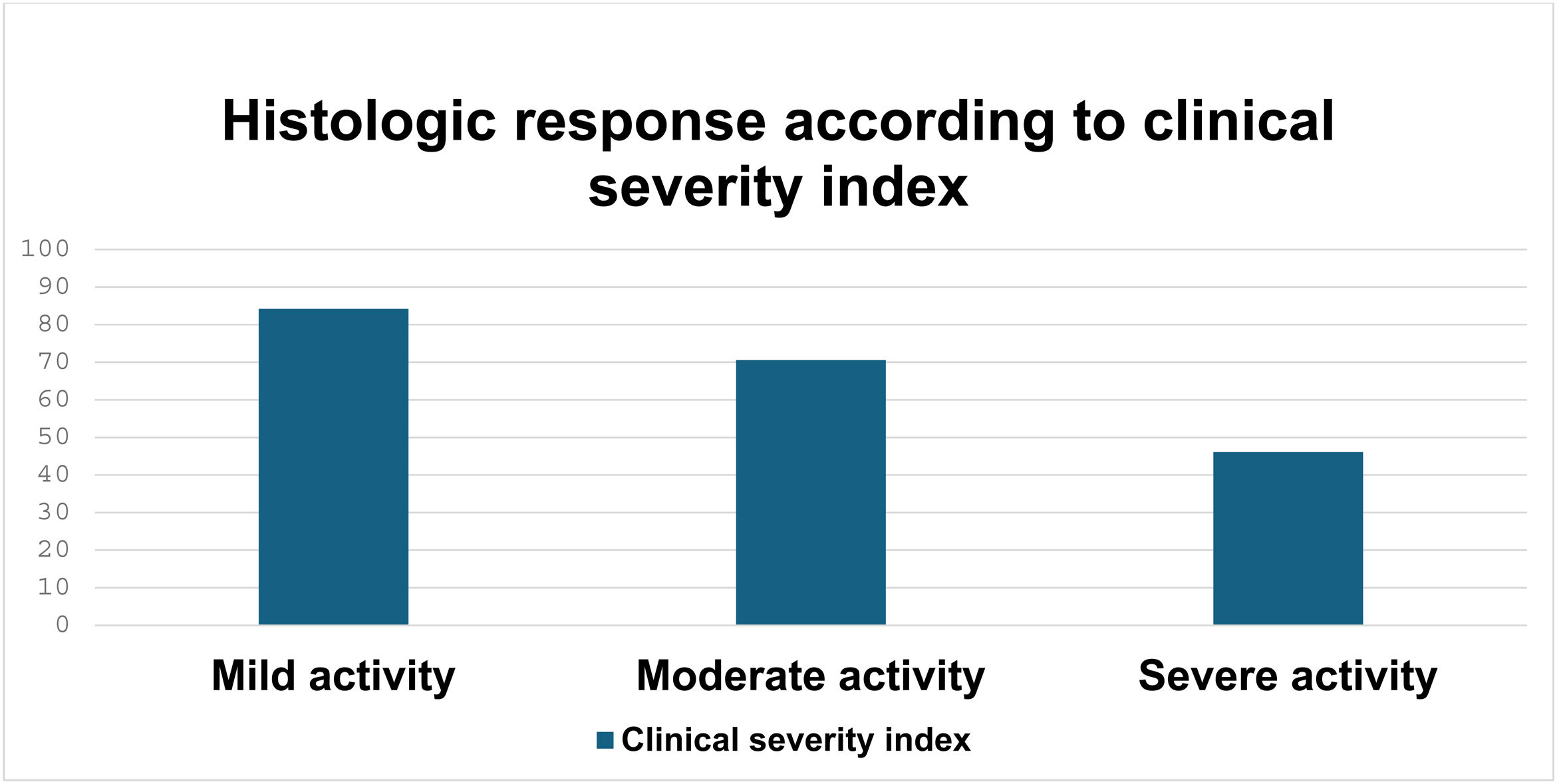
Taking the CSI into account, histologic remission was achieved in 59 of the 78 (75.6%) patients with mild activity, 39 of the 61 (63.9%) with moderate activity, and 6 of the 12 (50%) with severe activity, in whom the difference was statistically significant (p = 0.003) (Fig. 3).
DiscussionOur study on adult patients with EoE in Colombia showed their epidemiologic data, clinical characteristics, and treatment, with the highest number of patients in a Latin American study on the theme. Of the epidemiologic findings, there was a predominance of male sex (58%) in our study patients, similar to that reported in a recent systematic review of 40 studies, the large majority from the United States and Europe, that found a higher risk for presenting with EoE in men, compared with women.7 In a Chilean case series of 62 patients, risk was also predominant in the male sex (75.8%),13 and in a recently published Japanese case series, as well.14
The mean presentation age in our patients was 43.3 years, comparable to the mean 40 years reported in the literature.15 However, there was a lower mean age (33.7 years) reported in a European study.16 A retrospective study from the University of North Carolina showed an increase in the mean age at diagnosis of EoE of 22.0 years, within the time frame of 2002–2006, and of 31.8 years, within the period of 2017–2021 (p < 0.001), as well as a decrease in the number of male patients with EoE (from 80 to 68%, p = 0.002), during the same period. Those authors considered said results could be due to greater suspicion of the disease in the adult population.17
The association of EoE with allergic diseases is well-known. In our study, we found coexistence with atopic dermatitis (9.9%), allergic rhinitis (11.2%), and bronchial asthma (16.4%). In a systematic review with 21 studies, allergic rhinitis (odds ratio [OR] 5.09; 95% CI 2.91–8.90), and bronchial asthma (OR 3.01; 95% CI 1.96–4.62) were more frequent in subjects with EoE, than in the general population.18 In the previously cited Japanese study, there was a higher prevalence of bronchial asthma (OR 1.79; 95% CI 1.45–2.23, p < 0.001), allergic rhinitis (OR 1.43; 95% CI 1.16–1.77, p = 0.001), and atopic dermatitis (OR 1.66; 95% CI 1.23–2.23, p = 0 .001), in patients with EoE, compared with controls.14
In our study we found a low percentage of patients that were first-degree relatives of EoE patients (1.3%). A recent publication identified 239 first-degree relatives from 37 index EoE patients, finding 35 cases (14.6%) of EoE in those relatives, with more men than women (p = 0.027), as well as more subjects with atopic symptoms.4
The most frequent symptom in our patients was dysphagia (69.1%), and 21.7% of patients went to the emergency room due to food impaction. The latter figure could be related to the older mean age of our patients. An older age is associated with greater fibrostenotic involvement, compared with the inflammatory involvement seen in children, as has been described in recent publications.19,20
In our study, we found a median delay in the time from symptom onset to the diagnosis of EoE of 32.5 months, similar to that reported in an Italian study (median 36 months) and slightly lower than that reported in a Swiss study (median 4 years).21,22
In our case series, as initial treatment, 88.2% of patients received PPIs, 43.4% were put on an elimination diet, and 27.6% were treated with topical steroids. Our results are similar to those of the EUREOS EoE CONNECT research group, who found that, in 589 patients, PPIs were the first-line treatment in 76.4%, compared with topical steroids in 10.5% and elimination diets in 7.8%.16 That contrasts a bit with surveys applied to gastroenterologists in the United States, 54% of whom preferred topical steroids as first-line therapy in EoE.23 Another recent survey applied to 228 gastroenterologists from 18 European countries found that 82.9% of them utilized PPIs as first-line therapy, 41.6% used topical steroids, 20.6% utilized elimination diets, and 9.2% preferred combination therapy.24 In our study, 57.2% of the patients were managed with combination therapy, and of those that received monotherapy, the majority were treated with PPIs. In the abovementioned Chilean study, 40.3% of the patients received combination therapy with PPIs and steroids, and 54.8% received PPI monotherapy.13
Histologic remission was achieved in 63.4% of our patients treated with PPIs, in 69.7% treated with diet, and in 64.2% of those managed with topical steroids. In the previously mentioned multicenter “real life” European study,16 topical steroids were effective for achieving histologic remission in 67.7% of the patients, followed by elimination diets in 52.0%, and PPIs in 50.2%. In a recent systematic review with 34 studies and 1,762 patients, the histologic remission (presence of < 15 eosinophils/HPF) rate with an elimination diet was 53.8% (95% CI 48.0–59.6 %).25 In addition, a systematic review with 33 studies and 619 patients reported a histologic remission rate with PPIs in EoE of 50.5% (95% CI 42.2–58.7 %).26 Lastly, a systematic review with 8 randomized placebo-controlled studies found a histologic remission rate of 64.9% with topical steroids (95% CI 74−42%).27 Those percentages are similar to the histologic remission rates found in our study.
The combination of treatments in EoE has not been widely studied, and even criticized, due to the potential for producing additional adverse events and a negative impact on quality of life, and in cases of response, not knowing which of the treatments was efficacious.28 In our case series, more than half of the patients (57.2%) received combination treatment that included the 3 types of therapy for EoE, which could explain the higher percentage of response to different treatments. Albeit not reported much in the international literature, a recent study found that 11 of 12 patients that did not respond to monotherapy with an elimination diet or a PPI, achieved histologic remission with the combination of the two treatments.29
We found the small number of patients (5.9%) in our study that underwent endoscopic dilatation striking, taking into account the fact that 50.7% of the patients presented with rings or strictures. Even though the endoscope could be passed through the majority of them, we consider there was a certain hesitance to perform dilatations in patients with EoE, perhaps due to a fear of complications, despite the safety of the procedure described in systematic reviews30 and the recommendations of dilatation standards published by international experts, in which maintaining the permeability of the esophagus is emphasized, not only for passage of the endoscope, but also for achieving larger diameters (up to 15 mm), for improving patient symptoms, especially the presence of dysphagia.31,32 New procedures, such as the Endo-Flip (Functional Lumen Imaging Probe), enable the capacity and distensibility of the esophageal wall to be measured, which can define phenotypes, severity, and personalized treatment in patients with EoE.33
ConclusionsIn Colombia and other Latin American countries, the prevalence of EoE is unknown and may be underestimated, most likely due to a lack of diagnostic suspicion resulting from insufficient awareness of the disease on the part of gastroenterologists, the great variability in its clinical presentation, and the lack of access to endoscopic procedures in different regions of the country. All of the above may contribute to the difficulty in identifying EoE and providing its timely treatment, and as a consequence, favoring complications, such as strictures, in the long term.
Our study showed the existence of EoE in an adult population in Colombia, a predominance of the disease in males, dysphagia as the principal symptom, and its association with allergic diseases, such as allergic rhinitis and bronchial asthma. We also found there was an important delay in the diagnosis of EoE and that PPIs were the most frequent initial treatment in our environment, even though more than half of our patients (55%) received combination treatment as initial therapy, a behavior that differs from the recommendations in international consensuses and guidelines.5,27,34,35
There are barriers to the treatment of EoE in Colombia. For example, viscous steroids are not available, nor has the use of dupilumab been approved by the regulatory authorities. On the other hand, we know that there is poor adherence to elimination diets, which could explain the greater use of PPIs in the management of EoE we found. Limitations of our study include its observational design, possible measuring biases, sample size, and the fact that generalizations to other geographic regions could not be made. A weakness of our study is the scant use of the Eosinophilic Esophagitis Endoscopic Reference Score (EREFS)36 in our endoscopic reports. It is widely recommended in the international guidelines, and its lack of routine use hinders the evaluation of the endoscopic response to different treatments.
Taking all the above into account, we consider the development of Colombian guidelines on EoE, based on our national reality, a necessity. They would be essential for implementing public health strategies for the early diagnosis of EoE, its classification, and its timely treatment.
Ethical considerationsThis work meets the current norms in bioethical research and was authorized by the institutional ethics committee of the Hospital Pablo Tobón Uribe. The authors declare this article contains no personal information that could identify patients.
Financial disclosureOwn source.
The authors declare that there is no conflict of interest.