Primary tumor location has emerged as an important prognostic factor due to different biologic characteristics.
AimTo analyze the prognostic effect of tumor location in patients with colon cancer, comparing right-sided colon cancer with left-sided disease.
Materials and methodsA retrospective cohort study was conducted on patients above 18 years of age operated on for right-sided or left-sided colon cancer within the time frame of January 2016 and June 2022 at a quaternary care hospital. Sociodemographic and histopathologic variables were analyzed. Overall survival and progression-free survival were calculated.
ResultsFrom a total of 247 patients, 145 (58.7%) had right-sided colon cancer and 102 (41.2%) had left-sided disease. Mean age of the right-sided tumor patients was 70 years and mean age of the left-sided tumor patients was 64 years. The majority of the patients were women. Laparoscopic surgery was performed on 71.6% of patients and most of them presented with adenocarcinoma, 68.4% of which were well differentiated tumors. Poorly differentiated tumors were more likely in the right colon than the left, with 9.7% and 0.4%, respectively. Most of the patients did not have lymph node dissemination (N0: 54.7%), but there were more positive lymph nodes (28% vs 16.5%) and more microsatellite instability (48 vs 4 patients) in right-sided tumors. Fifty-three patients presented with metastasis, with no differences regarding laterality. Forty-four patients with right-sided cancer and 18 with left-sided cancer died. Right-sided location was the independent risk variable for overall survival (HR:1.97 [1.10–3.53]). Perineural invasion, metastasis, and disease stage were independent risk variables. Laterality was not a factor in progression-free survival.
ConclusionTumor located in the right colon was an independent risk factor impacting overall survival in colon cancer.
La localización del tumor primario ha surgido como factor pronóstico importante debido a distintas características biológicas.
ObjetivoAnalizar en pacientes con cáncer de colon el efecto pronóstico de la ubicación del tumor comparando el cáncer de colon derecho vs izquierdo.
Materiales y métodosEstudio de cohorte retrospectiva, en pacientes mayores de 18a operados por cáncer de colon derecho o izquierdo desde enero/2016 a junio/2022 en hospital de 4to nivel. Se analizaron variables sociodemográficas e histopatológicas. Se calculó supervivencia global y libre de progresión.
ResultadosTotal 247 pacientes, 145(58.7%) con cáncer de colon derecho y 102(41.2%) con cáncer de colon izquierdo. Edad promedio: Derecho 70a, izquierdo 64a. La mayoría mujeres. Cirugía laparoscópica en 71.6%. La mayoría de pacientes presentaron adenocarcinomas, 68.4% bien diferenciados. El colon derecho tuvo más probabilidad de tumores mal diferenciados: 9.7% vs 0.4% en izquierdo. La mayoría de pacientes no presentaron diseminación ganglionar N0:54.7%, aunque los tumores derechos presentaron más ganglios positivos 28% vs 16.5% y más inestabilidad de microsatélites: 48 vs 4 pacientes. Presentaron metástasis 53 pacientes, sin diferencias según lateralidad. Murieron 44 pacientes con cáncer derecho y 18 izquierdos. La localización derecha fue variable de riesgo independiente para supervivencia global HR:1,97 (1,10–3,53). La invasión perineural, las metástasis y el estadio de la enfermedad fueron variables independientes de riesgo. En supervivencia libre de progresión la lateralidad no fue factor de riesgo.
ConclusiónSe demuestra que la localización del tumor en colon derecho fue factor de riesgo independiente para la supervivencia global en cáncer de colon.
Colorectal cancer ranks fourth in cancer-related morbidity and third in cancer-related mortality worldwide, with a reported incidence rate of 19.5% and mortality rate of 9% per 100,000 inhabitants.1
In the Americas, there are more than 240,000 new colorectal cancer cases annually and approximately 112,000 deaths due to the disease. Likewise, its incidence is expected to increase 60% by 2030. In Colombia, the prevalence of colorectal cancer is 47.57 cases per 100,000 inhabitants and the mortality rate is 4.96 deaths per 100,000 inhabitants. In the past year, the number of new cases reported increased 51%, compared with that reported in 2019, and prevalence increased 23%, compared with the previous period. There were more cases in women, and the median patient age was 64 years.2 In Medellín, cancer was the second cause of death and was responsible for 25% of deaths occurring in the city between 2010 and 2014. Colorectal cancer ranks fourth in morbidity, with an incidence of 7.3% and a mortality rate of 8.1% per 100,000 inhabitants.3
The 5-year survival rate, in general, is 65% but varies from 91% for localized tumors to 73% for locoregionally advanced tumors and 14% for metastatic disease. Only one of every 3 new cases presents with localized disease at the time of diagnosis.4
Primary tumor location has emerged as an important prognostic factor due to distinct biologic characteristics, but at present, the side of the colon with cancer is not a prognostic parameter, that on its own, alters the oncologic management decision.5 Petrelli et al. and other authors have shown that right-sided colon cancer is worse than left-sided disease,5–7 in cases of localized disease as well as metastatic disease,8,9 demonstrating that they should be considered 2 heterogeneous entities,10 reporting the need for adequate specialized treatment related to location.7 Nevertheless, a Japanese study described a better prognosis for stage I and II right colon tumors, compared with left colon disease.11
The aim of this study was to analyze the prognostic effect of the anatomic location of colon cancer, comparing right-sided and left-sided disease, in a cohort of adult patients with colon cancer operated on and treated at the Instituto de Cancerología Las Américas AUNA.
Materials and methodsA retrospective, observational, case-control, analytic cohort study with a survival analysis was conducted on patients admitted to a quaternary care hospital in the city of Medellín, Colombia, within the time frame of January 2016 and June 2022. The STROBE checklist was utilized.
The data were collected from the clinical histories of the patients and the Information System for the Follow-up of Patients with Colorectal Cancer of the Instituto de Cancerología (IDC) Las Américas AUNA. The inclusion criteria were patients above 18 years of age with a histologic diagnosis of right-sided or left-sided colon cancer who underwent right hemicolectomy or left hemicolectomy, within the time frame of January 2016 and June 2022.
The sociodemographic variables were age, sex, tobacco use, and body mass index (BMI). Clinical, risk factor, and laboratory variables were analyzed and included the carcinoembryonic antigen (CEA) test result at admission, histologic subtype and grade, clinical stage (TNM 8th edition 2017), tumor location, emergency presentation with bleeding, obstruction or perforation, time between diagnosis and surgical management, number of lymph nodes examined and compromised, tumor size, circumferential resection margin status, perineural and/or lymphovascular invasion, tumor budding, tumor deposits, microsatellite instability, and the KRAS, NRAS, and BRAF somatic mutations. Dates of diagnosis, surgery, last control, recurrence or progression, and death were taken into account for the survival calculation.
Tumor anatomic location (right side vs left side) was the prognostic factor analyzed as the independent variable. Right-sided colon cancer was defined as tumor located in the cecum, ascending colon, or the hepatic angle of the colon and left-sided colon cancer was defined as tumor located in the splenic angle of the colon, descending colon, or sigmoid colon. Patients with pure transverse colon cancer, i.e., those that did not require right or left hemicolectomy due to tumor location, were not included in the study. The primary outcome variables were overall survival, defined as the period from the time of colon cancer diagnosis to the death of the patient due to any cause, and progression-free survival, defined as the period from colon cancer diagnosis to imaging evidence of local or distant disease progression. The administrative censoring data of the study were the patients that did not present with the outcome at the end of the study’s follow-up period.
Statistical analysisThe sociodemographic, clinical, and outcome variables were summarized, comparing the patients with right-sided colon cancer and those with left-sided disease. The quantitative variables were expressed as mean and standard deviation, if they met the normality assumption, and when they did not, as median and interquartile range. The qualitative variables were expressed as frequency and percentage. Statistical significance tests were carried out to identify differences between subgroups. For the quantitative variables, this was done with the Student’s t test or the Mann–Whitney U test, depending on whether the normality assumption was accepted or rejected. Regarding the qualitative variables, the chi-square test or Fisher’s exact test was used for the cases with observations within a crosstab cell less than 5. A survival analysis was carried out through survival curves, comparing patients according to tumor anatomic location (right vs left) for overall survival and progression-free survival. The curves were compared, using the log-rank statistical significance test. A multivariate analysis was carried out to estimate the association of anatomic location with overall survival and progression-free survival. The Cox proportional hazard regression test was used for determining the crude confounding variable-adjusted association, including the clinically important variables and those with a p < 0.25 in the univariate analysis, resulting in the final model. The statistical analyses were performed utilizing the SPSS-IBM version 22 (SPSS Inc., Chicago, Illinois, USA) and Rstudio software.
Ethical considerationsThe protocol was elaborated according to international ethics norms and the Colombian legislation, and the study was approved and supervised by the Independent Ethics Committee of the IDC Las Américas AUNA, which meets the Good Clinical Practice standards in all its activities.
ResultsWithin the time frame of January 2016 and June 2022, 247 patients with colon cancer were operated on. Of those patients, 145 (58.7%) had right-sided tumors and 102 (41.2%) had left-sided tumors.
The mean age of the patients with right-sided colon cancer was 70 years, which was higher than the mean age of the patients with left-sided colon cancer. Women were the predominant sex in both disease locations.
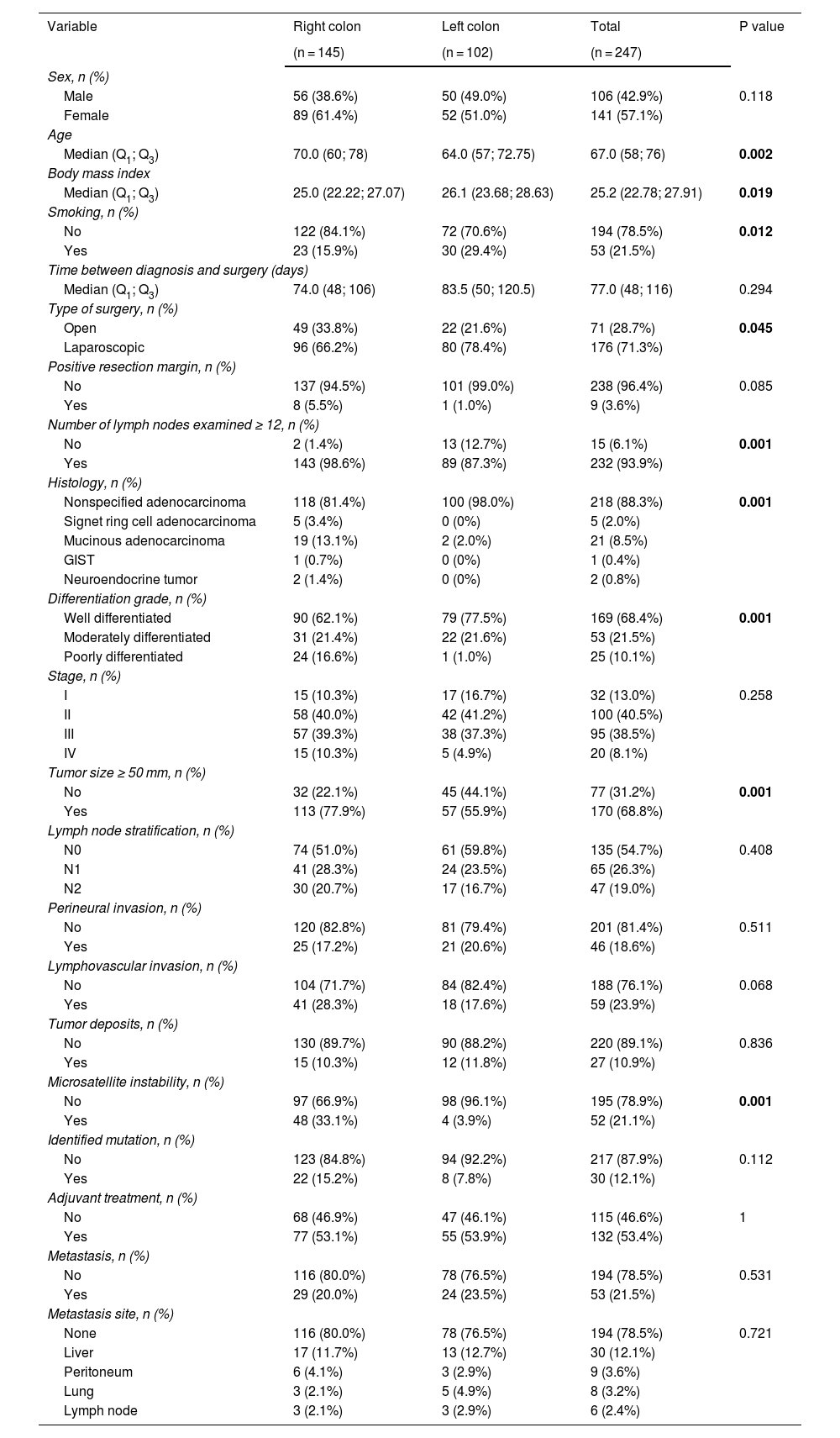
Table 1 shows the sociodemographic and clinical characteristics of the patients.
Sociodemographic and clinical characteristics.
| Variable | Right colon | Left colon | Total | P value |
|---|---|---|---|---|
| (n = 145) | (n = 102) | (n = 247) | ||
| Sex, n (%) | ||||
| Male | 56 (38.6%) | 50 (49.0%) | 106 (42.9%) | 0.118 |
| Female | 89 (61.4%) | 52 (51.0%) | 141 (57.1%) | |
| Age | ||||
| Median (Q1; Q3) | 70.0 (60; 78) | 64.0 (57; 72.75) | 67.0 (58; 76) | 0.002 |
| Body mass index | ||||
| Median (Q1; Q3) | 25.0 (22.22; 27.07) | 26.1 (23.68; 28.63) | 25.2 (22.78; 27.91) | 0.019 |
| Smoking, n (%) | ||||
| No | 122 (84.1%) | 72 (70.6%) | 194 (78.5%) | 0.012 |
| Yes | 23 (15.9%) | 30 (29.4%) | 53 (21.5%) | |
| Time between diagnosis and surgery (days) | ||||
| Median (Q1; Q3) | 74.0 (48; 106) | 83.5 (50; 120.5) | 77.0 (48; 116) | 0.294 |
| Type of surgery, n (%) | ||||
| Open | 49 (33.8%) | 22 (21.6%) | 71 (28.7%) | 0.045 |
| Laparoscopic | 96 (66.2%) | 80 (78.4%) | 176 (71.3%) | |
| Positive resection margin, n (%) | ||||
| No | 137 (94.5%) | 101 (99.0%) | 238 (96.4%) | 0.085 |
| Yes | 8 (5.5%) | 1 (1.0%) | 9 (3.6%) | |
| Number of lymph nodes examined ≥ 12, n (%) | ||||
| No | 2 (1.4%) | 13 (12.7%) | 15 (6.1%) | 0.001 |
| Yes | 143 (98.6%) | 89 (87.3%) | 232 (93.9%) | |
| Histology, n (%) | ||||
| Nonspecified adenocarcinoma | 118 (81.4%) | 100 (98.0%) | 218 (88.3%) | 0.001 |
| Signet ring cell adenocarcinoma | 5 (3.4%) | 0 (0%) | 5 (2.0%) | |
| Mucinous adenocarcinoma | 19 (13.1%) | 2 (2.0%) | 21 (8.5%) | |
| GIST | 1 (0.7%) | 0 (0%) | 1 (0.4%) | |
| Neuroendocrine tumor | 2 (1.4%) | 0 (0%) | 2 (0.8%) | |
| Differentiation grade, n (%) | ||||
| Well differentiated | 90 (62.1%) | 79 (77.5%) | 169 (68.4%) | 0.001 |
| Moderately differentiated | 31 (21.4%) | 22 (21.6%) | 53 (21.5%) | |
| Poorly differentiated | 24 (16.6%) | 1 (1.0%) | 25 (10.1%) | |
| Stage, n (%) | ||||
| I | 15 (10.3%) | 17 (16.7%) | 32 (13.0%) | 0.258 |
| II | 58 (40.0%) | 42 (41.2%) | 100 (40.5%) | |
| III | 57 (39.3%) | 38 (37.3%) | 95 (38.5%) | |
| IV | 15 (10.3%) | 5 (4.9%) | 20 (8.1%) | |
| Tumor size ≥ 50 mm, n (%) | ||||
| No | 32 (22.1%) | 45 (44.1%) | 77 (31.2%) | 0.001 |
| Yes | 113 (77.9%) | 57 (55.9%) | 170 (68.8%) | |
| Lymph node stratification, n (%) | ||||
| N0 | 74 (51.0%) | 61 (59.8%) | 135 (54.7%) | 0.408 |
| N1 | 41 (28.3%) | 24 (23.5%) | 65 (26.3%) | |
| N2 | 30 (20.7%) | 17 (16.7%) | 47 (19.0%) | |
| Perineural invasion, n (%) | ||||
| No | 120 (82.8%) | 81 (79.4%) | 201 (81.4%) | 0.511 |
| Yes | 25 (17.2%) | 21 (20.6%) | 46 (18.6%) | |
| Lymphovascular invasion, n (%) | ||||
| No | 104 (71.7%) | 84 (82.4%) | 188 (76.1%) | 0.068 |
| Yes | 41 (28.3%) | 18 (17.6%) | 59 (23.9%) | |
| Tumor deposits, n (%) | ||||
| No | 130 (89.7%) | 90 (88.2%) | 220 (89.1%) | 0.836 |
| Yes | 15 (10.3%) | 12 (11.8%) | 27 (10.9%) | |
| Microsatellite instability, n (%) | ||||
| No | 97 (66.9%) | 98 (96.1%) | 195 (78.9%) | 0.001 |
| Yes | 48 (33.1%) | 4 (3.9%) | 52 (21.1%) | |
| Identified mutation, n (%) | ||||
| No | 123 (84.8%) | 94 (92.2%) | 217 (87.9%) | 0.112 |
| Yes | 22 (15.2%) | 8 (7.8%) | 30 (12.1%) | |
| Adjuvant treatment, n (%) | ||||
| No | 68 (46.9%) | 47 (46.1%) | 115 (46.6%) | 1 |
| Yes | 77 (53.1%) | 55 (53.9%) | 132 (53.4%) | |
| Metastasis, n (%) | ||||
| No | 116 (80.0%) | 78 (76.5%) | 194 (78.5%) | 0.531 |
| Yes | 29 (20.0%) | 24 (23.5%) | 53 (21.5%) | |
| Metastasis site, n (%) | ||||
| None | 116 (80.0%) | 78 (76.5%) | 194 (78.5%) | 0.721 |
| Liver | 17 (11.7%) | 13 (12.7%) | 30 (12.1%) | |
| Peritoneum | 6 (4.1%) | 3 (2.9%) | 9 (3.6%) | |
| Lung | 3 (2.1%) | 5 (4.9%) | 8 (3.2%) | |
| Lymph node | 3 (2.1%) | 3 (2.9%) | 6 (2.4%) | |
GIST: Gastrointestinal stromal tumor.
The p values in bold print are the statistically significant values.
Less than 20% of the patients operated on presented with obstruction, perforation, or bleeding prior to surgical management, with no difference regarding tumor laterality. A total of 71.6% of the surgeries were laparoscopic. Of the patients that underwent right hemicolectomy, 34% presented with some type of complication, compared with 30% of the patients that underwent left hemicolectomy.
Regarding the pathology results, the histologic type in more than 98% of the patients with left-sided colon cancer was unspecified adenocarcinoma. In the patients with right-sided colon cancer, signet ring cell adenocarcinoma was identified in 5 patients and mucinous adenocarcinoma in 19 patients. Only one patient presented with GIST and 2 patients with neuroendocrine tumors, and all 3 of those tumors were on the right side.
Most of the study patients (68.4%) had well differentiated tumors, 36.4% of which were right-sided and 31.9% left-sided. The patients with right-sided colon cancer were more likely to have poorly differentiated tumors, at 9.7%, compared with the patients with left-sided colon cancer, at 0.4%, and the difference was statistically significant.
Of the patients with right-sided colon cancer, 78% had tumors larger than 50 mm, compared with 57% of the patients with left-sided colon cancer. Ninety-nine percent of the patients with right-sided colon cancer had more than 12 lymph nodes in the pathology report, compared with 87% of the patients with left-sided colon cancer.
The majority of cases did not have dissemination to the lymph nodes; 135 of the 247 patients were N0 (54.6%). The patients with right-sided colon cancer presented with a higher number of positive lymph nodes (n = 71; 28%), compared with the patients with left-sided colon cancer (n = 41; 16.5%).
There were no significant differences in tumor stage at the time of surgery. More than half of the patients were in stage II or III, according to the 8th classification of the American Joint Committee on Cancer (AJCC). Twenty patients had metastatic disease at the time of surgery, 15 of whom had right-sided colon cancer, and the main reason for surgery at that stage was intestinal obstruction. Six percent of the patients with right-sided colon cancer had positive resection margins, as did 1% of the patients with left-sided colon cancer. Perineural invasion was more frequent in patients with left-sided colon cancer (21%), and less frequent in patients with right-sided disease (17%), with no statistical significance. In contrast, lymphovascular invasion was more frequent in the right colon (28%), compared with the left colon (18%), and the difference was statistically significant (p = 0.03). Forty-eight patients with right-sided colon cancer and 4 patients with left-sided colon cancer presented with microsatellite instability, with a statistically significant difference (p = 0.001). K-RAS, N-RAS, and BRAF mutations were reported in only 30 patients, and tumor laterality was not identified.
Thirty-three patients presented with metastasis during follow-up and the most common site was the liver. Other common sites of metastasis were the peritoneum and lung. There were no statistically significant differences with respect to tumor laterality.
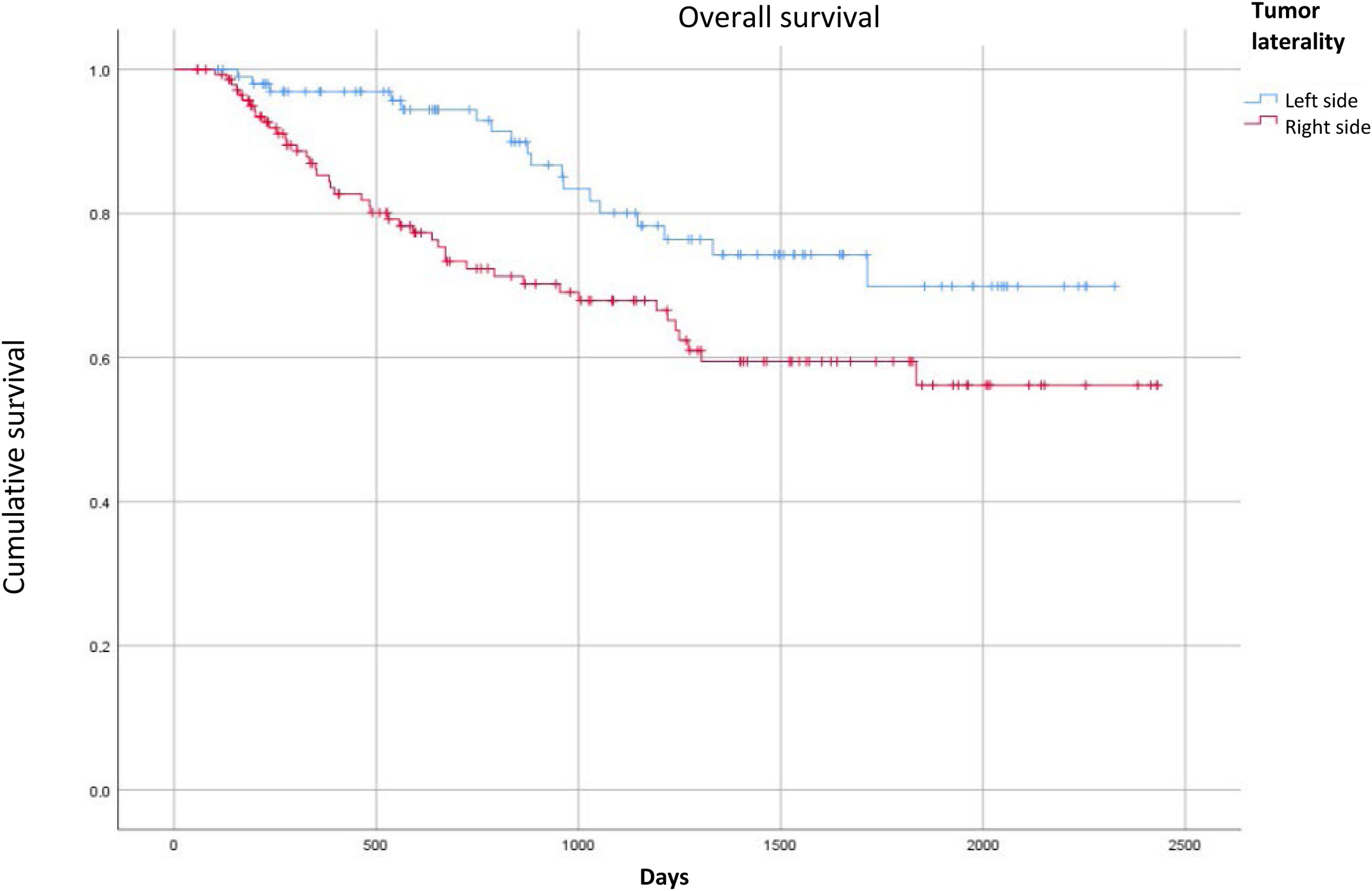
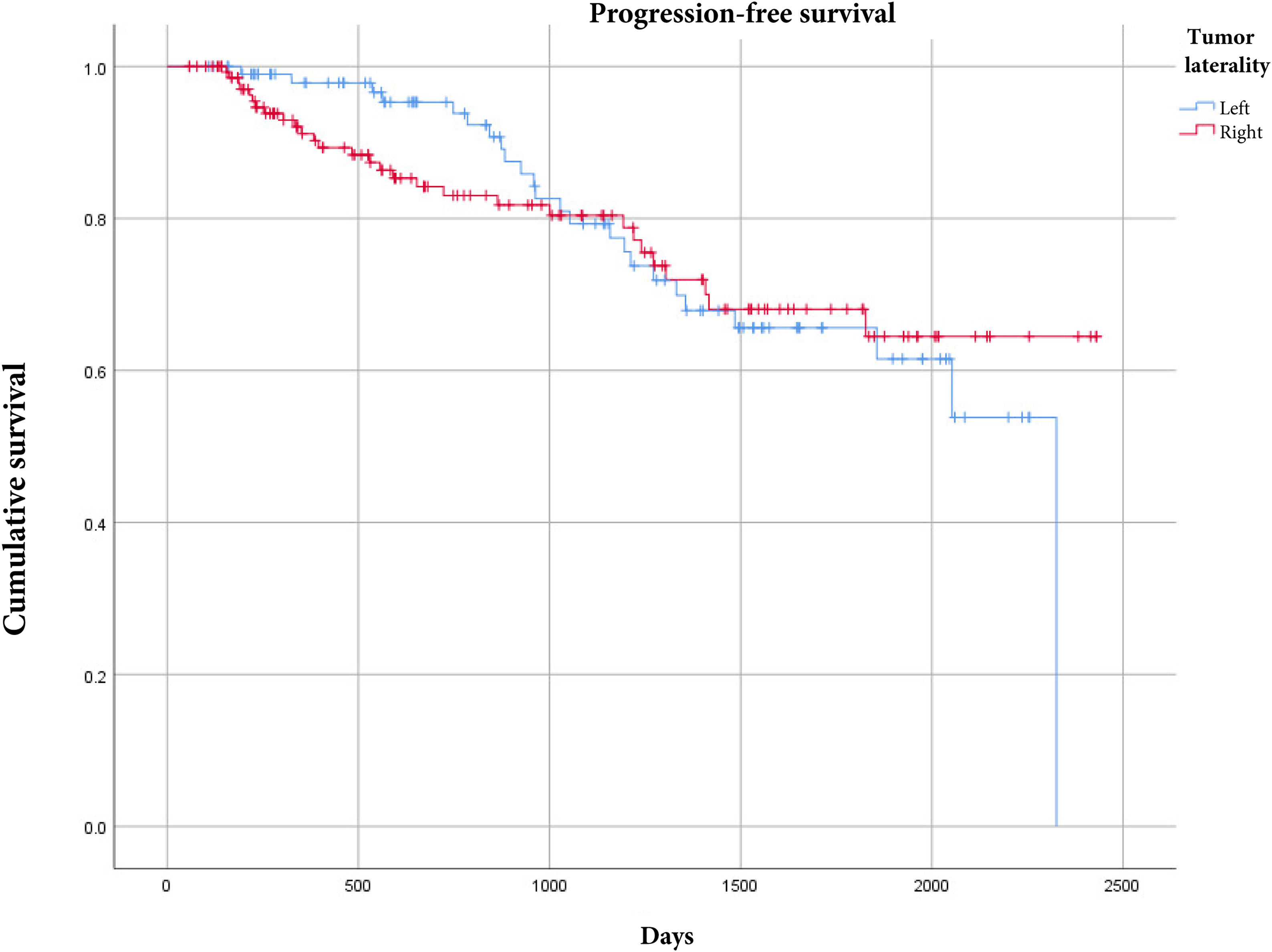
There were 44 deaths (30%) in the patients with right-sided colon cancer and 18 deaths (12%) in the patients with left-sided colon cancer. Upon comparing overall survival according to tumor laterality, the patients with right-sided colon cancer had worse overall survival with a statistically significant p value (Fig. 1). For progression-free survival, there were no differences, according to tumor laterality (Fig. 2).
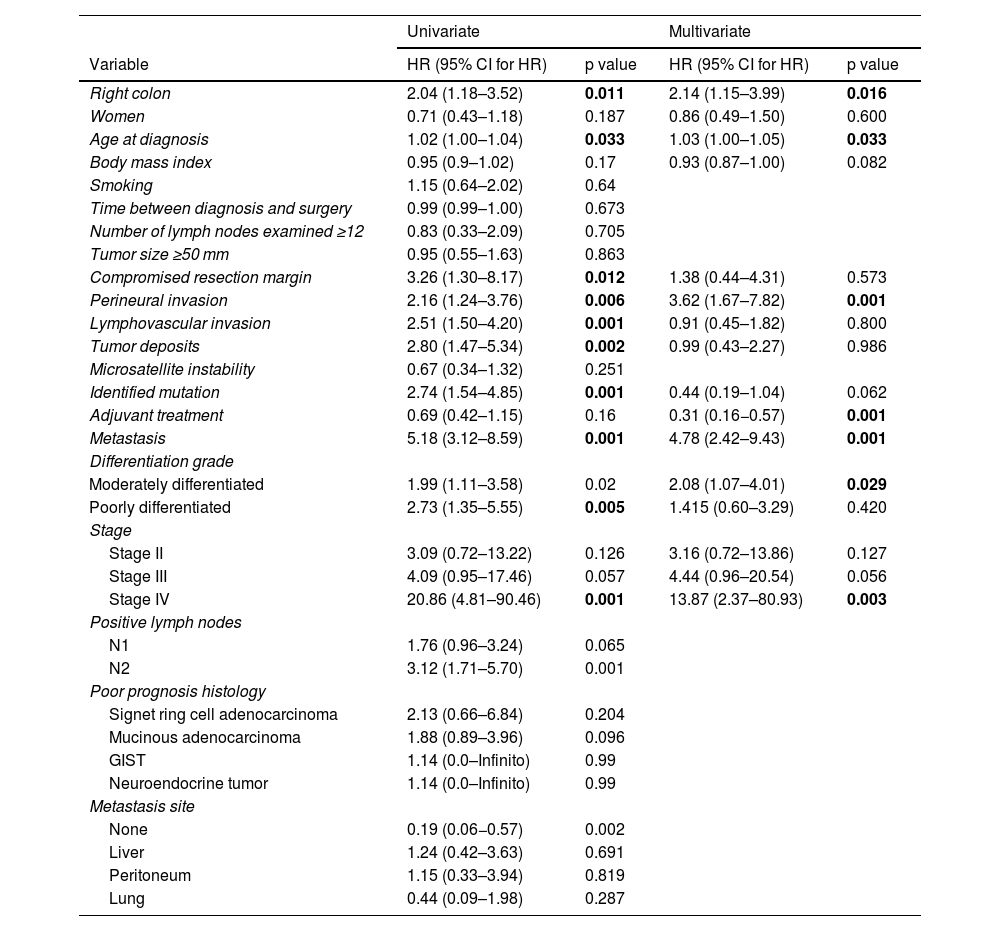
Tables 2 and 3 show the results of the univariate and multivariate analyses by outcome.
Univariate and multivariate analyses of independent risk factors for overall survival.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | HR (95% CI for HR) | p value | HR (95% CI for HR) | p value |
| Right colon | 2.04 (1.18–3.52) | 0.011 | 2.14 (1.15–3.99) | 0.016 |
| Women | 0.71 (0.43–1.18) | 0.187 | 0.86 (0.49–1.50) | 0.600 |
| Age at diagnosis | 1.02 (1.00–1.04) | 0.033 | 1.03 (1.00–1.05) | 0.033 |
| Body mass index | 0.95 (0.9–1.02) | 0.17 | 0.93 (0.87–1.00) | 0.082 |
| Smoking | 1.15 (0.64–2.02) | 0.64 | ||
| Time between diagnosis and surgery | 0.99 (0.99–1.00) | 0.673 | ||
| Number of lymph nodes examined ≥12 | 0.83 (0.33–2.09) | 0.705 | ||
| Tumor size ≥50 mm | 0.95 (0.55–1.63) | 0.863 | ||
| Compromised resection margin | 3.26 (1.30–8.17) | 0.012 | 1.38 (0.44–4.31) | 0.573 |
| Perineural invasion | 2.16 (1.24–3.76) | 0.006 | 3.62 (1.67–7.82) | 0.001 |
| Lymphovascular invasion | 2.51 (1.50–4.20) | 0.001 | 0.91 (0.45–1.82) | 0.800 |
| Tumor deposits | 2.80 (1.47–5.34) | 0.002 | 0.99 (0.43–2.27) | 0.986 |
| Microsatellite instability | 0.67 (0.34–1.32) | 0.251 | ||
| Identified mutation | 2.74 (1.54–4.85) | 0.001 | 0.44 (0.19–1.04) | 0.062 |
| Adjuvant treatment | 0.69 (0.42–1.15) | 0.16 | 0.31 (0.16−0.57) | 0.001 |
| Metastasis | 5.18 (3.12–8.59) | 0.001 | 4.78 (2.42–9.43) | 0.001 |
| Differentiation grade | ||||
| Moderately differentiated | 1.99 (1.11–3.58) | 0.02 | 2.08 (1.07–4.01) | 0.029 |
| Poorly differentiated | 2.73 (1.35–5.55) | 0.005 | 1.415 (0.60–3.29) | 0.420 |
| Stage | ||||
| Stage II | 3.09 (0.72–13.22) | 0.126 | 3.16 (0.72–13.86) | 0.127 |
| Stage III | 4.09 (0.95–17.46) | 0.057 | 4.44 (0.96–20.54) | 0.056 |
| Stage IV | 20.86 (4.81–90.46) | 0.001 | 13.87 (2.37–80.93) | 0.003 |
| Positive lymph nodes | ||||
| N1 | 1.76 (0.96–3.24) | 0.065 | ||
| N2 | 3.12 (1.71–5.70) | 0.001 | ||
| Poor prognosis histology | ||||
| Signet ring cell adenocarcinoma | 2.13 (0.66–6.84) | 0.204 | ||
| Mucinous adenocarcinoma | 1.88 (0.89–3.96) | 0.096 | ||
| GIST | 1.14 (0.0–Infinito) | 0.99 | ||
| Neuroendocrine tumor | 1.14 (0.0–Infinito) | 0.99 | ||
| Metastasis site | ||||
| None | 0.19 (0.06−0.57) | 0.002 | ||
| Liver | 1.24 (0.42–3.63) | 0.691 | ||
| Peritoneum | 1.15 (0.33–3.94) | 0.819 | ||
| Lung | 0.44 (0.09–1.98) | 0.287 | ||
GIST: gastrointestinal stromal tumor; HR: hazard ratio; 95% CI: 95% confidence interval.
The p values in bold print are the statistically significant values.
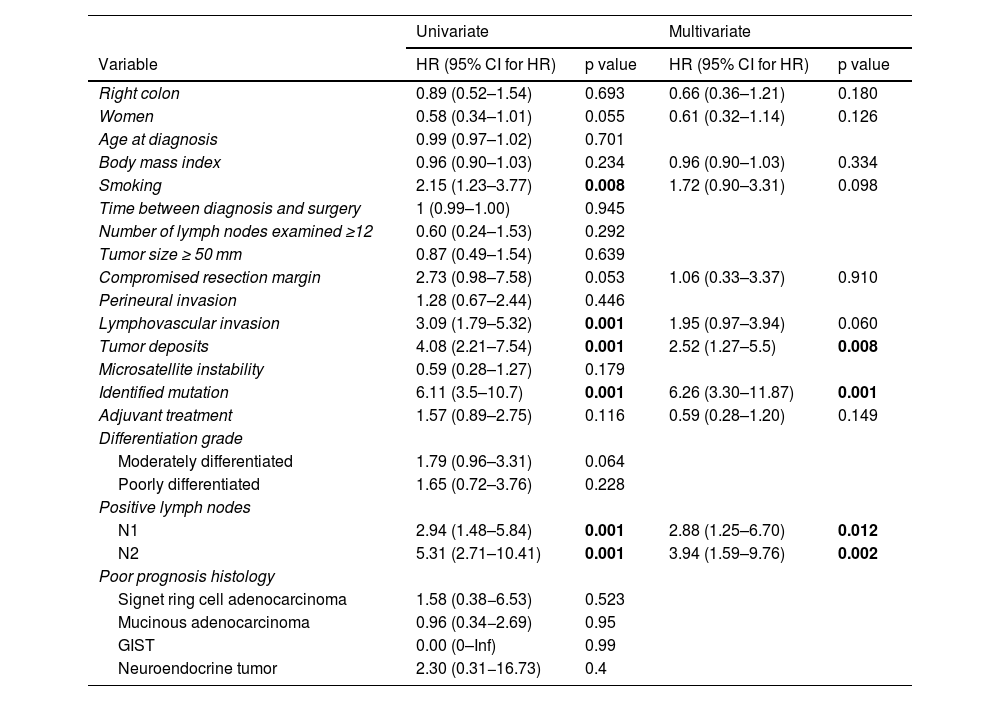
Univariate and multivariate analyses of independent risk factors for progression-free survival.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | HR (95% CI for HR) | p value | HR (95% CI for HR) | p value |
| Right colon | 0.89 (0.52–1.54) | 0.693 | 0.66 (0.36–1.21) | 0.180 |
| Women | 0.58 (0.34–1.01) | 0.055 | 0.61 (0.32–1.14) | 0.126 |
| Age at diagnosis | 0.99 (0.97–1.02) | 0.701 | ||
| Body mass index | 0.96 (0.90–1.03) | 0.234 | 0.96 (0.90–1.03) | 0.334 |
| Smoking | 2.15 (1.23–3.77) | 0.008 | 1.72 (0.90–3.31) | 0.098 |
| Time between diagnosis and surgery | 1 (0.99–1.00) | 0.945 | ||
| Number of lymph nodes examined ≥12 | 0.60 (0.24–1.53) | 0.292 | ||
| Tumor size ≥ 50 mm | 0.87 (0.49–1.54) | 0.639 | ||
| Compromised resection margin | 2.73 (0.98–7.58) | 0.053 | 1.06 (0.33–3.37) | 0.910 |
| Perineural invasion | 1.28 (0.67–2.44) | 0.446 | ||
| Lymphovascular invasion | 3.09 (1.79–5.32) | 0.001 | 1.95 (0.97–3.94) | 0.060 |
| Tumor deposits | 4.08 (2.21–7.54) | 0.001 | 2.52 (1.27–5.5) | 0.008 |
| Microsatellite instability | 0.59 (0.28–1.27) | 0.179 | ||
| Identified mutation | 6.11 (3.5–10.7) | 0.001 | 6.26 (3.30–11.87) | 0.001 |
| Adjuvant treatment | 1.57 (0.89–2.75) | 0.116 | 0.59 (0.28–1.20) | 0.149 |
| Differentiation grade | ||||
| Moderately differentiated | 1.79 (0.96–3.31) | 0.064 | ||
| Poorly differentiated | 1.65 (0.72–3.76) | 0.228 | ||
| Positive lymph nodes | ||||
| N1 | 2.94 (1.48–5.84) | 0.001 | 2.88 (1.25–6.70) | 0.012 |
| N2 | 5.31 (2.71–10.41) | 0.001 | 3.94 (1.59–9.76) | 0.002 |
| Poor prognosis histology | ||||
| Signet ring cell adenocarcinoma | 1.58 (0.38−6.53) | 0.523 | ||
| Mucinous adenocarcinoma | 0.96 (0.34−2.69) | 0.95 | ||
| GIST | 0.00 (0–Inf) | 0.99 | ||
| Neuroendocrine tumor | 2.30 (0.31−16.73) | 0.4 | ||
GIST: gastrointestinal stromal tumor; HR: hazard ratio; 95% CI: 95% confidence interval.
The p values in bold print are the statistically significant values.
Our study produced several important results. First, it showed that right-sided colon cancer was an independent risk variable for overall survival, with a HR of 1.97 (1.10–3.53). In addition, age at diagnosis, perineural invasion, metastasis, and disease stage were other independent risk variables found in the multivariate analysis. Disease stage IV was the most important risk factor, with an adjusted HR of 13.87 (2.39–80.57).
Specifically regarding progression-free survival results, in which laterality was not a risk factor, the presence of tumor deposits, positive lymph nodes, and mutations were independent risk factors. The presence of mutations was the most important risk factor, with a HR of 6.26 (3.30–11.87).
Lim et al. reported clinical and pathologic differences in right-sided colon cancer, compared with left-sided disease.7 They demonstrated the impact of tumor location on the overall survival results, with better survival rates in patients with left-sided colon cancer, compared with right-sided colon cancer, but there were no statistically significant differences regarding progression-free survival.
Among the characteristics associated with survival, advanced age at diagnosis has been shown to be a significant predictor of worse overall survival and cause of death different from tumor progression,12 mainly in patients above 65–70 years of age.13 However, there are studies in which an earlier presentation age (<50 years) was associated with advanced stages and greater recurrence, but with similar survival.14 In our study, the patients with right-sided colon cancer had a higher median age (70 years), compared with patients with left-sided disease (64 years), at the time of diagnosis, and that difference was statistically significant. The univariate analysis demonstrated a slight prognostic effect, but that association was not shown in the multivariate analysis with either overall survival or progression-free survival.
Female sex has been a factor associated with greater survival in colorectal cancer,15,16 but in our cohort, a prognostic effect related to sex was not identified. A low BMI was reported as a predictor of negative outcomes,17 even though a BMI > 35 kg/m2 emerged as a risk factor for postoperative complications and death.18,19 In our analysis, there were no differences between the 2 groups related to weight.
Smoking has also been associated with a worse prognosis, albeit with inconclusive results.20 In our cohort, there was a greater prevalence of cigarette smoking in patients with left-sided colon cancer (29%) than in patients with right-sided colon cancer (15.9%), with no prognostic effect related to smoking. There can be acute complications in colon cancer, such as bleeding, obstruction, and perforation,21 and they are associated with worse prognosis.22,23 We found no differences between our study patients who presented with said complications. The time lapse between diagnosis and surgery has been described as a factor, with reports finding that an interval of more than 4 weeks was independently related to worse overall survival.24,25 In our series, the mean time from diagnosis to surgery was over 3 months, reported for both sides of the colon, with no differences in survival, most likely attributable to the delay in performing the procedure in general.
More advanced stages of disease have a worse prognosis.26 In our study, we found no differences in stages in the patients of either group. In the univariate and multivariate analyses, presenting with stage IV importantly affected survival.
The site of metastasis has also been reported to affect prognosis. A study described a higher probability of 3-year survival only in patients with metastasis to the lungs, compared with other sites.27 Sjo et al. found a higher incidence of peritoneal carcinomatosis in right-sided colon cancer than in left-sided disease, with a mean survival of 8 months.28,29 In our study, there were no differences in metastatic sites in the 2 groups, nor was there a prognostic relation to the site of metastasis, but not presenting with metastasis was indeed related to better survival, with a statistically significant p value.
Even though elevated preoperative CEA has been described as a predictor of poor prognosis, its significance for predicting long-term outcomes is not clear.30,31 CEA was not determined in more than 50% of our study patients, and so we could not reach a conclusion about its relation to prognosis.
Regarding the histopathologic variables, more than 90% of our study patients presented with adenocarcinoma, a finding consistent with that in the national and international literature.32,33 Mucinous and signet ring cell cancers are usually considered independent factors of poor survival.34,35 In our study, there was a greater presence of those 2 cancer types in the patients with right-sided tumors (n = 24), compared with the patients with left-sided tumors (n = 2). The univariate analysis showed no prognostic relation to those two histologic types.
Histologic differentiation grade significantly affects survival and is associated with higher stage, risk of metastasis, and death.36,37 In our study, the patients with right-sided colon cancer presented with more tumors that had some degree of dedifferentiation, compared with the patients with left-sided colon cancer. In the univariate analysis, patients with moderately or poorly differentiated tumors had a worse prognosis than the patients with well differentiated tumors, but that finding was not reflected in the multivariate analysis.
In addition, the number of lymph nodes analyzed has been shown to have a positive association with better survival.38 In the present study, 99% of the patients with right-sided colon cancer had a minimum of 12 lymph nodes analyzed as the surgical quality criterion, compared with 87% of the patients with left-sided colon cancer. That difference was not associated with differences in survival.
In their publications, Dai et al. and Kornprat et al. related a higher tumor diameter to worse prognosis and survival.39,40 In our cohort, the majority of patients with right-sided colon cancer had tumors larger than 50 mm (n = 113, 78%), compared with the patients with left-sided disease (n = 57, 56%), but the difference was not related to survival.
A positive circumferential resection margin has been found to be a strong indicator of poor prognosis, regardless of stage.41,42 Malignant longitudinal resection margin involvement has been described as a predictor of local recurrence, the development of distant metastasis, and reduced disease-free survival.43 In our patients, 6% of the resected margins were compromised in the patients with right-sided colon cancer and 1% in the patients with left-sided colon cancer, with no statistically significant difference or prognostic relation between the 2 groups.
Mayo et al., Liebig et al., and Fujita et al. reported perineural invasion as a poor prognosis factor, upon its association with lower overall survival.44–46 However, there are publications in which it has not been found to be an independent predictive factor in nonmetastatic colon cancer.47,48 Similarly, lymphovascular invasion is associated with poor prognosis.47,49 In the present study, 17% of the patients with right-sided colon cancer and 21% with left-sided disease had perineural invasion, with no differences between the groups (p = 0.512) and no differences in lymphovascular invasion, which was present in 28% of the patients with right-sided colon cancer and 18% of the patients with left-sided colon cancer (p = 0.068). The univariate analysis showed that those 2 variables could have a prognostic effect, but in the multivariate analysis, only perineural invasion remained statistically significant.
An association between the presence of tumor deposits and a decrease in overall survival has been shown.50,51 In our study, there were no differences in the presence of tumor deposits between the 2 groups, nor was a prognostic relation found.
The presence of microsatellite instability that is related to defects in the mismatch repair pathway, resulting in hereditary nonpolyposis colorectal cancer syndrome, or Lynch syndrome,52 has been associated with better oncologic outcomes regarding survival time and disease-free time.53,54 Gryfe et al. reported that colorectal cancers with microsatellite instability had a 5-year survival of 74%, compared with 54% of other cancers, results that were statistically significant and maintained at all disease stages.55 In our cohort, there was a greater presence of microsatellite instability in the patients with right-sided colon cancer (33%), compared with the 4% in the patients with left-sided colon cancer. The difference between the 2 groups was statistically significant (p = 0.001), but the presence of the alteration was not related to survival.
KRAS gene mutations have been shown to be present in 20–60% of colorectal cancer cases.56 Shen et al. reported on a cohort of patients in which KRAS was more frequently mutated in patients with right-sided colon cancer.56 In other studies, the KRAS and NRAS mutations were independently correlated with worse prognosis,57–59 but that is not a unanimous finding.60–62 In our study, we found information related to those mutations in only 23% of the entire cohort, with no differences between groups or relation to survival.
Among the limitations of our study is its historic character, which may have led to nondifferential measuring bias, which could alter the quality of the results. In addition, we did not carry out multiplicity hypothesis tests, and so there is a risk of type 1 error, i.e., finding differences when there actually are none. Nevertheless, we consider said risk could have been mitigated in the multivariate analysis. Another limitation is the sample size, given that at least 14 independent variables were found in the multivariate analysis, likely making other studies with larger samples necessary for corroborating the results.
There may be a cohort effect during the 2016–2022 study period due to the COVID-19 pandemic, which could have affected the quality of healthcare and the survival prognosis.
Strengths of our study include a significant collection period, compliance with the College of American Pathologists cancer protocol, and the availability of a staff exclusively dedicated to patient records, resulting in an insignificant number of lost data in the database. In addition, a rigorous multivariate analysis was performed, determining that tumor laterality was a risk factor independent from other already known prognostic factors. In addition, because the study was conducted at a cancer referral center, the processes are standardized, with quality criteria, such as having more than 12 lymph nodes analyzed, which occurred in a large number of our study patients. Furthermore, the majority of surgical procedures were performed via laparoscopy.
In conclusion, the location of cancer in the right colon was shown to be an independent risk factor that negatively impacts overall survival in colon cancer.
Ethical considerationsThe protocol was elaborated according to international ethics norms and the Colombian legislation, and the study was approved and supervised by the Independent Ethics Committee of the IDC Las Américas AUNA, which meets the Good Clinical Practice standards in all its activities.
The authors declare this article contains no information that could identify patients, and so informed consent was not required for the publication of this article.
No experiments on humans or animals were conducted for this research.
FundingThe funding of this study was based on the authors’ own resources.
The authors declare that there are no conflicts of interest.
This work was presented as an oral communication at the 27th National Congress of the Spanish Association of Coloproctology, organized by the Spanish Association of Coloproctology, held from May 15 to 17, 2024.