The Milan criteria have been the subject of discussion in recent years due to their restrictive nature. Expansion of the criteria and the use of locoregional therapies to downstage patients and increase the number of transplant candidates have been proposed. Our study analyzed the results of patients that underwent transplant due to hepatocellular carcinoma, comparing those that met the Milan criteria and those that exceeded them.
Materials and methodsA retrospective, observational, single-center study was conducted on liver transplantations due to hepatocellular carcinoma, within the time frame of 2010−2021. Demographic and clinical variables, overall survival, and disease-free survival were analyzed. The Student’s t test or Mann–Whitney U test were applied for the quantitative variables and the Fisher’s exact test for the categorical variables. The survival function was estimated through the Kaplan–Meier method and the log-rank test was applied for comparing the groups.
ResultsOf the 96 transplanted patients, 78 met the Milan criteria and 18 exceeded them. Patients that did not meet the Milan criteria had a higher number of nodules (1.6 vs. 3.5 nodules; p = 0.000), larger main lesions (24.38 vs. 38.55 mm; p = 0.000), a higher bilobar hepatocellular carcinoma rate (21.79% vs. 72.22%, p = 0.000), and higher tumor burden. There were no significant differences regarding overall survival, but there was a lower rate of disease-free survival in the group exceeding the criteria.
ConclusionDownstaged patients that received locoregional therapies had lower disease-free survival rates than patients that met the Milan criteria, but there were no significant differences regarding overall survival.
Los criterios de Milán han sido discutidos debido a su carácter restrictivo. Se ha propuesto su ampliación, y el empleo de terapias locorregionales que infraestadifiquen y aumenten los candidatos a trasplante. Nuestro estudio analiza los resultados de los pacientes trasplantados por carcinoma hepatocelular que cumplían criterios de Milán y aquellos que los excedían.
Materiales y métodosEstudio observacional, retrospectivo y unicéntrico sobre trasplantados hepáticos por carcinoma hepatocelular entre el 2010−2021. Se analizaron variables demográficas, clínicas y supervivencia global y libre de enfermedad. Se aplicó la prueba t-Student o la de U de Mann-Whitney para variables cuantitativas; para categóricas el test exacto de Fisher. Se estimó la función de supervivencia mediante el método de Kaplan-Meier y se aplicó el log rank para comparar entre grupos.
ResultadosDe los pacientes 96 trasplantados, 78 cumplían criterios de Milán y 18 los excedían. Los pacientes que no cumplían los criterios de Milán presentaban mayor número de nódulos (1,6 vs. 3,5 nódulos (p = 0,000)), mayor tamaño de las lesiones principales (24,38 vs. 38,55 mm, p = 0,000) y mayor tasa de carcinoma hepatocelular bilobular (21,79% vs. 72,22%, p = 0,000), así como mayor carga tumoral. No hubo diferencias significativas en cuanto a la supervivencia global, aunque sí en cuanto a la supervivencia libre de enfermedad, siendo menor en el grupo que excedía los criterios.
ConclusiónLos pacientes infraestadiados con terapias locorregionales presentan tasas de supervivencia libre de enfermedad menores a los pacientes que cumplen los criterios de Milán, sin diferencias significativas en cuanto a la supervivencia global.
Primary liver tumors are the third cause of cancer deaths worldwide, with a global incidence in 2020 above 900,000 cases and a total of 830,000 deaths, the most frequent of which is hepatocellular carcinoma (HCC).1
There are several treatment modalities for HCC that include liver resection, locoregional therapies, and liver transplantation. The decision to perform one alternative or another depends on a thorough overall evaluation of tumor burden, liver function, the future remnant, comorbidities, and the patient’s functional status.2
Liver transplantation has been considered the treatment of choice with curative intent for selected patients, with 5-year survival rates above 70%.3–6 However, despite the selection criteria of the patients that are transplant candidates for the treatment of HCC, disease recurrence rates vary from 8 to 20%.7,8
In 1996, the first successful case series on cirrhotic patients that underwent liver transplantation due to unresectable HCC was conducted by Mazzaferro et al., establishing some of the internationally accepted criteria, known as the Milan criteria.9 Considered the reference standard for the selection of patients with HCC that are candidates for transplantation,10 they have overall survival and disease-free rates at 4 years close to 75% and 92%, respectively.9 Despite their success, the Milan criteria have been the subject of discussion in recent years, due to their restrictive nature regarding indications for transplant.
Therefore, different groups have evaluated expanding the criteria, developing new, more flexible models that enable a higher number of patients to opt for transplantation as treatment. Among them are the University of California San Francisco criteria, promoted by Yao et al., with one and five-year survival rates similar to those of patients that meet the Milan criteria.11 The Kyoto criteria and the new, expanded criteria developed by the Milan working group, known as the Up-to-Seven criteria, have five-year survival rates of 70–80%.6,12
Locoregional treatments have also been developed as bridging therapy prior to transplantation, to increase the number of HCC patients that are candidates for transplant. This therapy has two goals: to prevent tumor progression in patients on the waitlist for liver transplantation,13,14 and to reduce the tumor burden in patients that do not meet the transplant criteria, downstaging them.13,15 The locoregional treatments most widely used as bridging therapy are radiofrequency (RF) and transarterial chemoembolization (TACE).15–18
The aim of the present study was to carry out a comprehensive analysis of the demographic, clinical, and biologic characteristics of patients that underwent transplantation due to HCC, evaluating their survival and tumor recurrence, and comparing those variables between patients that met the Milan criteria and those that exceeded them.
Materials and methodsA retrospective, observational, single-center cohort study was conducted on patients that underwent transplantation due to HCC at the Hospital Universitario Virgen de las Nieves, Granada, Spain, within the time frame of January 1, 2010, and December 31, 2021. The study was approved by the hospital’s ethics committee.
The inclusion criterion was the indication for liver transplant due to HCC. The exclusion criteria were duplicate clinical histories, diagnosis of another type of tumor in the anatomopathologic report, and death during the intraoperative or immediate postoperative periods.
The diagnosis of HCC was confirmed through computed tomography (CT) and/or magnetic resonance imaging (MRI). The patients that exceeded the Milan criteria and the patients that met the criteria, but were estimated to have a prolonged time on the waitlist, underwent bridging therapies. The treatments employed were TACE and RF, indicating the latter for treating single nodules smaller than 15 mm, repeating the sessions in patients with suspected viable tumor in the control tests, whether through ultrasound, MRI, or CT. The Liver Imaging Reporting and Data System (LI-RADS) criteria were used in patients with treated lesions (LR-TR) to evaluate radiologic response, establishing three categories: LR-TR nonviable, LR-TR viable, and LR-TR equivocal.
The post-transplant follow-up period was a minimum of 18 months.
For the analysis by groups, the Milan criteria group (MC group) was defined as patients that presented with a single HCC lesion under 5 cm, or 2 or 3 lesions under 3 cm in the pretransplant imaging tests. The non-Milan criteria group (NMC group) were patients that exceeded the Milan criteria, but not the Up-to-Seven criteria.
The variables analyzed included demographic, clinical, biochemical, pathologic, and follow-up progress data. The tumor burden score (TBS) of the patients was collected, calculated using the equation proposed by Sasaki et al., and the patients were divided by risk category.16
Statistical analysisA descriptive statistical analysis was carried out, in which the quantitative variables were expressed as mean and standard deviation or median and interquartile range, according to their distribution. The Shapiro–Wilk test was employed to evaluate the normality of the continuous variables. The categorical variables were expressed through frequency and percentage tables.
The results of the quantitative variables of the MC group and NMC group were compared, using the corresponding Student’s t test or Mann–Whitney U test. The categorical variables were compared, using the chi-square test or Fisher’s exact test. On the other hand, the function of overall survival and disease-free survival was estimated through the Kaplan-Meier method, both generally and stratified by the MC/NMC group inclusion criteria. Lastly, the log-rank test was applied to compare the MC and NMC groups.
The calculations were made, utilizing the STATA version 14 statistics program.
Ethical considerationsIn the present study, we followed the protocols of our work center on the publication of patient data, preserving data anonymity. Informed consent was not requested for the publication because our study reveals no personal data that could identify patients. The work meets the current bioethical research norms and was approved by the ethics committee of the Hospital Universitario Virgen de las Nieves.
ResultsOf the total number of transplants performed (397), 104 cases were due to HCC. Eight of those cases were excluded from the study: one because of a duplicate clinical history, another because it corresponded to cholangiocarcinoma in the explant, a third due to intraoperative death from hemodynamic failure, and 5 because of death in the immediate postoperative period (two cases due to primary graft non-function, two to acute thrombosis of the hepatic artery, and one to abdominal sepsis). Finally, 96 patients were analyzed, 78 of whom (81.2%) met the Milan criteria for liver transplantation (the MC group) vs. 18 patients (18.8%) that exceeded the criteria (the NMC group).
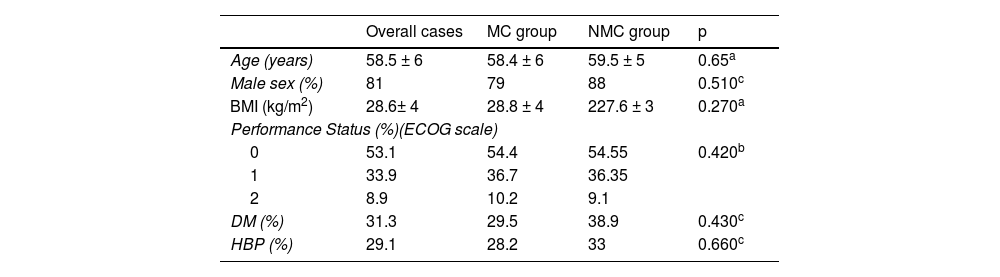
Table 1 describes the demographic characteristics analyzed in an overall manner and by group. The predominant sex was male (81%), and the mean patient age was 58.5 ± 6 years.
Demographic characteristics of transplanted patients due to hepatocellular carcinoma.
| Overall cases | MC group | NMC group | p | |
|---|---|---|---|---|
| Age (years) | 58.5 ± 6 | 58.4 ± 6 | 59.5 ± 5 | 0.65a |
| Male sex (%) | 81 | 79 | 88 | 0.510c |
| BMI (kg/m2) | 28.6± 4 | 28.8 ± 4 | 227.6 ± 3 | 0.270a |
| Performance Status (%)(ECOG scale) | ||||
| 0 | 53.1 | 54.4 | 54.55 | 0.420b |
| 1 | 33.9 | 36.7 | 36.35 | |
| 2 | 8.9 | 10.2 | 9.1 | |
| DM (%) | 31.3 | 29.5 | 38.9 | 0.430c |
| HBP (%) | 29.1 | 28.2 | 33 | 0.660c |
BMI: body mass index; DM: diabetes mellitus; ECOG: Eastern Cooperative Oncology Group; HBP: high blood pressure; MC: Milan criteria; NMC: non-Milan criteria.
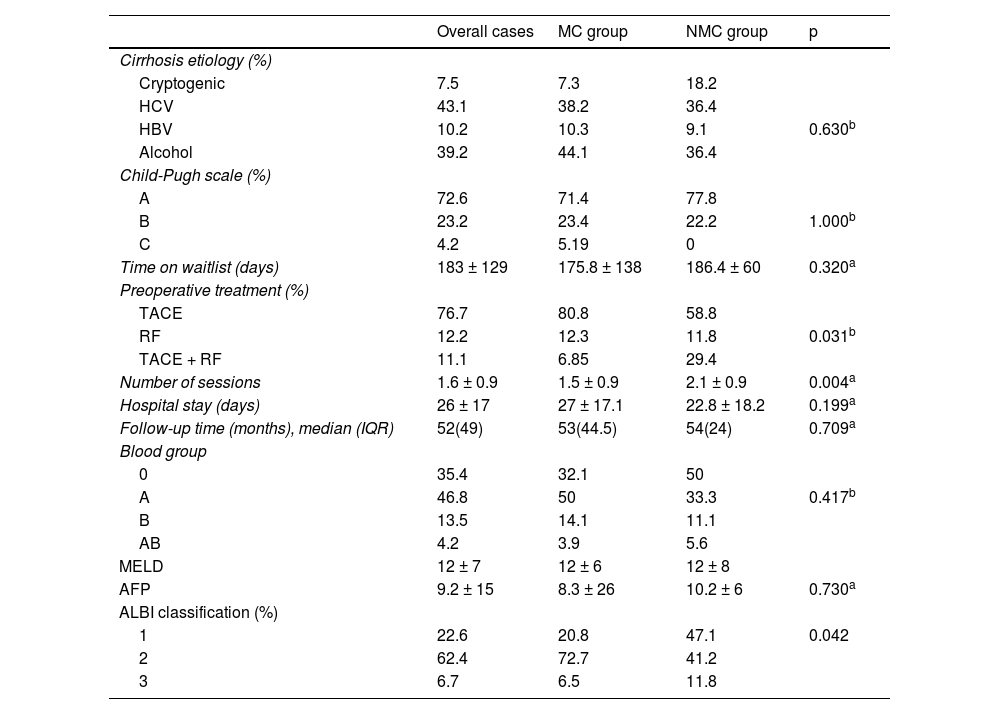
Table 2 summarizes the analysis of the clinical and biochemical variables. Bridging therapies were offered to 100% of the patients that exceeded the Milan criteria, repeating sessions until reaching downstaging, compared with 92.4% of patients that met the criteria at the baseline. The most frequent treatment was TACE, performed in 87.5% of patients. The mean number of sessions was 1.6 in the overall sample, albeit the NMC group had a higher number of sessions than the MC group (2.1 vs. 1.5; p = 0.0045).
Clinical and biochemical characteristics.
| Overall cases | MC group | NMC group | p | |
|---|---|---|---|---|
| Cirrhosis etiology (%) | ||||
| Cryptogenic | 7.5 | 7.3 | 18.2 | |
| HCV | 43.1 | 38.2 | 36.4 | |
| HBV | 10.2 | 10.3 | 9.1 | 0.630b |
| Alcohol | 39.2 | 44.1 | 36.4 | |
| Child-Pugh scale (%) | ||||
| A | 72.6 | 71.4 | 77.8 | |
| B | 23.2 | 23.4 | 22.2 | 1.000b |
| C | 4.2 | 5.19 | 0 | |
| Time on waitlist (days) | 183 ± 129 | 175.8 ± 138 | 186.4 ± 60 | 0.320a |
| Preoperative treatment (%) | ||||
| TACE | 76.7 | 80.8 | 58.8 | |
| RF | 12.2 | 12.3 | 11.8 | 0.031b |
| TACE + RF | 11.1 | 6.85 | 29.4 | |
| Number of sessions | 1.6 ± 0.9 | 1.5 ± 0.9 | 2.1 ± 0.9 | 0.004a |
| Hospital stay (days) | 26 ± 17 | 27 ± 17.1 | 22.8 ± 18.2 | 0.199a |
| Follow-up time (months), median (IQR) | 52(49) | 53(44.5) | 54(24) | 0.709a |
| Blood group | ||||
| 0 | 35.4 | 32.1 | 50 | |
| A | 46.8 | 50 | 33.3 | 0.417b |
| B | 13.5 | 14.1 | 11.1 | |
| AB | 4.2 | 3.9 | 5.6 | |
| MELD | 12 ± 7 | 12 ± 6 | 12 ± 8 | |
| AFP | 9.2 ± 15 | 8.3 ± 26 | 10.2 ± 6 | 0.730a |
| ALBI classification (%) | ||||
| 1 | 22.6 | 20.8 | 47.1 | 0.042 |
| 2 | 62.4 | 72.7 | 41.2 | |
| 3 | 6.7 | 6.5 | 11.8 | |
AFP: alpha-fetoprotein; ALBI: albumin-bilirubin; HBV: hepatitis B virus; HCV: hepatitis C virus; IQR: interquartile range; MC: Milan criteria; MELD: Model for End-Stage Liver Disease; NMC: non-Milan criteria; RF: radiofrequency; TACE: transarterial chemoembolization.
Of the 78 patients that received locoregional treatment as bridging therapy to transplantation, re-evaluation imaging tests were carried out on 71 patients (90.5%): 8 (8.2%) through ultrasound, 68 (70.1%) through MRI, and 12 (12.4%) through CT. Of those patients, 59% were catalogued as LR-TR nonviable, 32.5% as LR-TR viable, and the remaining cases (8.5%) as LR-TR equivocal.
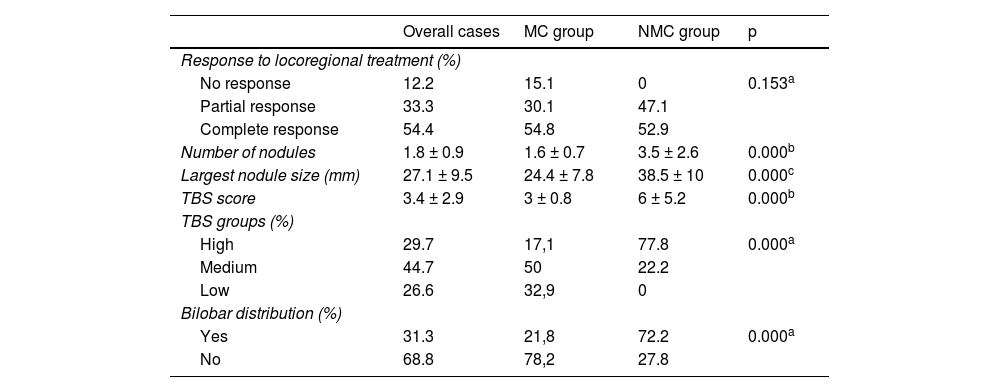
Regarding the clinical-pathologic variables (Table 3), of the 78 patients treated through TACE or RF prior to liver transplant, tumor necrosis was present in 87.73% in the histopathologic analysis of the liver explant. It was complete in 54.44% of the cases and partial in 33.33% of the nodules treated, with no significant differences between the groups analyzed (p = 0.153).
Pathologic characteristics.
| Overall cases | MC group | NMC group | p | |
|---|---|---|---|---|
| Response to locoregional treatment (%) | ||||
| No response | 12.2 | 15.1 | 0 | 0.153a |
| Partial response | 33.3 | 30.1 | 47.1 | |
| Complete response | 54.4 | 54.8 | 52.9 | |
| Number of nodules | 1.8 ± 0.9 | 1.6 ± 0.7 | 3.5 ± 2.6 | 0.000b |
| Largest nodule size (mm) | 27.1 ± 9.5 | 24.4 ± 7.8 | 38.5 ± 10 | 0.000c |
| TBS score | 3.4 ± 2.9 | 3 ± 0.8 | 6 ± 5.2 | 0.000b |
| TBS groups (%) | ||||
| High | 29.7 | 17,1 | 77.8 | 0.000a |
| Medium | 44.7 | 50 | 22.2 | |
| Low | 26.6 | 32,9 | 0 | |
| Bilobar distribution (%) | ||||
| Yes | 31.3 | 21,8 | 72.2 | 0.000a |
| No | 68.8 | 78,2 | 27.8 | |
MC: Milan criteria; NMC: non-Milan criteria; TBS: tumor burden score.
Regarding the correlation between the grade of tumor necrosis and the posttreatment LR-TR category, 37.1% of the patients evaluated as LR-TR viable had “total response” vs. 62.9% that had “total non-response”, 59.2% of the LR-TR nonviable patients had “total response” vs. 40.8% that had “total non-response”, and 62.5% of the LR-TR equivocal patients had “complete response” vs. 37.5% con “complete non-response”; the differences were not significant (p = 0.123). There were no statistically significant differences in overall survival and disease-free survival between the LR-TR viable, nonviable, and equivocal groups (p = 0.3484 and p = 0.4152, respectively).
Mean time on the transplant waitlist was 183 ± 129 days.
With respect to the biochemical variables analyzed, the patients were most frequently classified as albumin-bilirubin (ALBI) grades 1 and 2; 47% of the NMC patients were classified as grade 1 vs. 20.78% of the MC patients (p = 0.042). The mean overall preoperative alpha-fetoprotein (AFP) level was 9.2 ± 15 ng/mL; the MC group had 8.3 ± 26 ng/mL vs. 10.2 ± 6 ng/mL in the NMC group, and the difference was not statistically significant (p = 0.730).
The patients that were initially beyond the Milan criteria had a higher number of nodules (1.6 vs. 3.5 nodules; p = 0.000), a higher number of main lesions (24.38 vs. 38.55 mm; p = 0.000), a higher rate of bilobar HCC (21.79 vs. 72.22%, p = 0.000), and a higher tumor burden in absolute values (3.02 ± 0.8 vs. 6 ± 5.2; p = 0.00), as well as in the stratification by risk group, resulting in 77.8% of the patients in the NMC group being catalogued as a high TBS group vs. 17.1% of the patients in the MC group (p = 0.000).
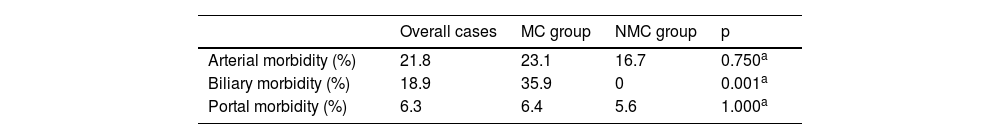
Regarding postoperative complications, there was a greater percentage of bile duct complications (in the form of ischemic cholangiopathy) in the MC group vs. the NMC group (18.9% vs. 0%; p = 0.001) (Table 4).
Thirty-one patients (32.3%) died during the follow-up period; 22 in the MC group and 9 in the NMC group. A total of 52.1% patients died within the first 12 posttransplant months and there were no deaths due to HCC recurrence.
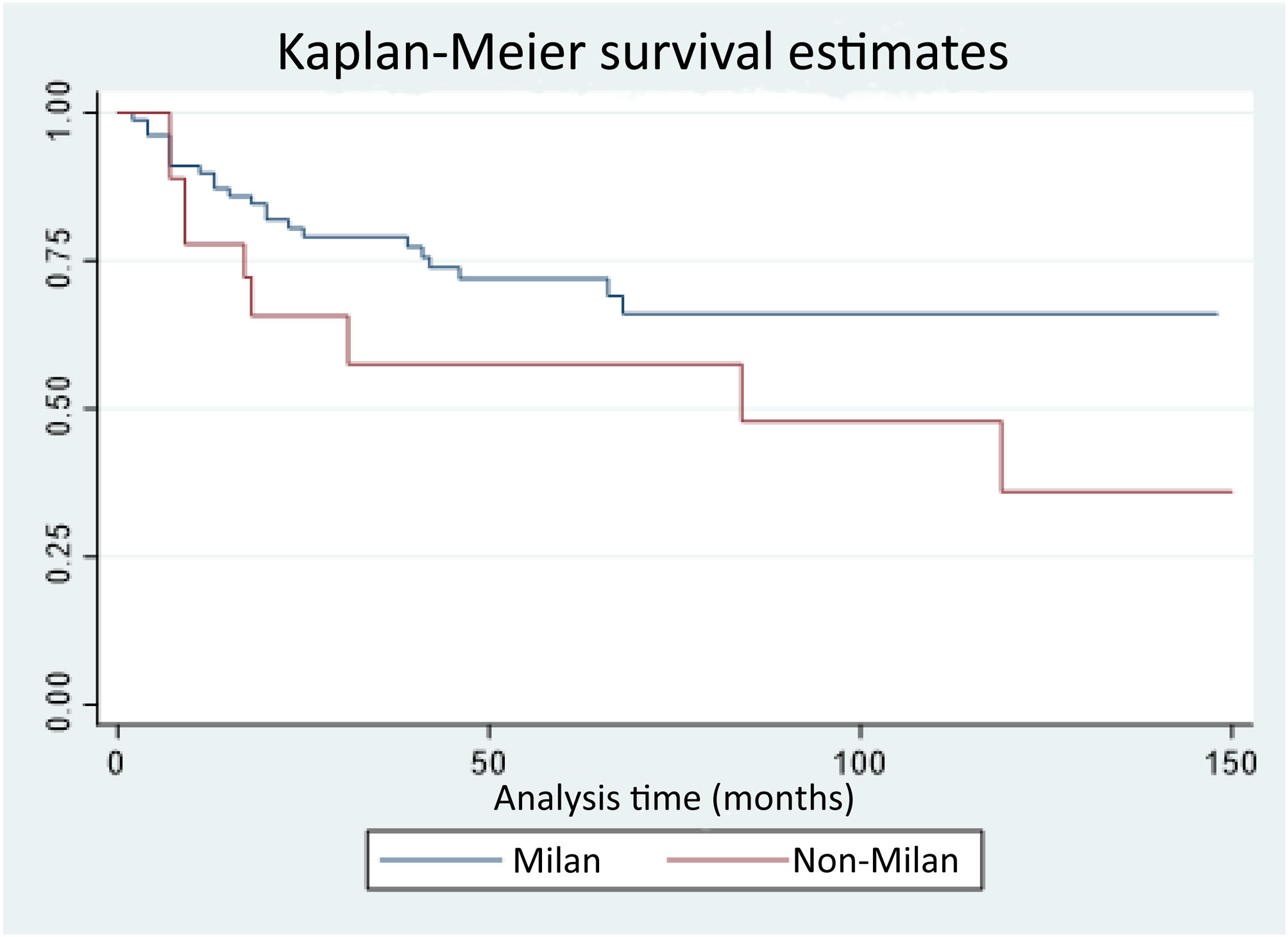
Overall survival rates at three, six, 12, 24, and 48 months were 98%, 96%, 87%, 77%, and 69%, respectively. In the survival analysis by group, there was a higher survival rate at 12, 24, and 48 months in the patients in the MC group compared with the NMC group (89.7%, 80.4%, and 71.9% vs. 77.7%, 65.6%, and 57.4%, respectively), with no significant differences (p = 0.09) (Fig. 1).
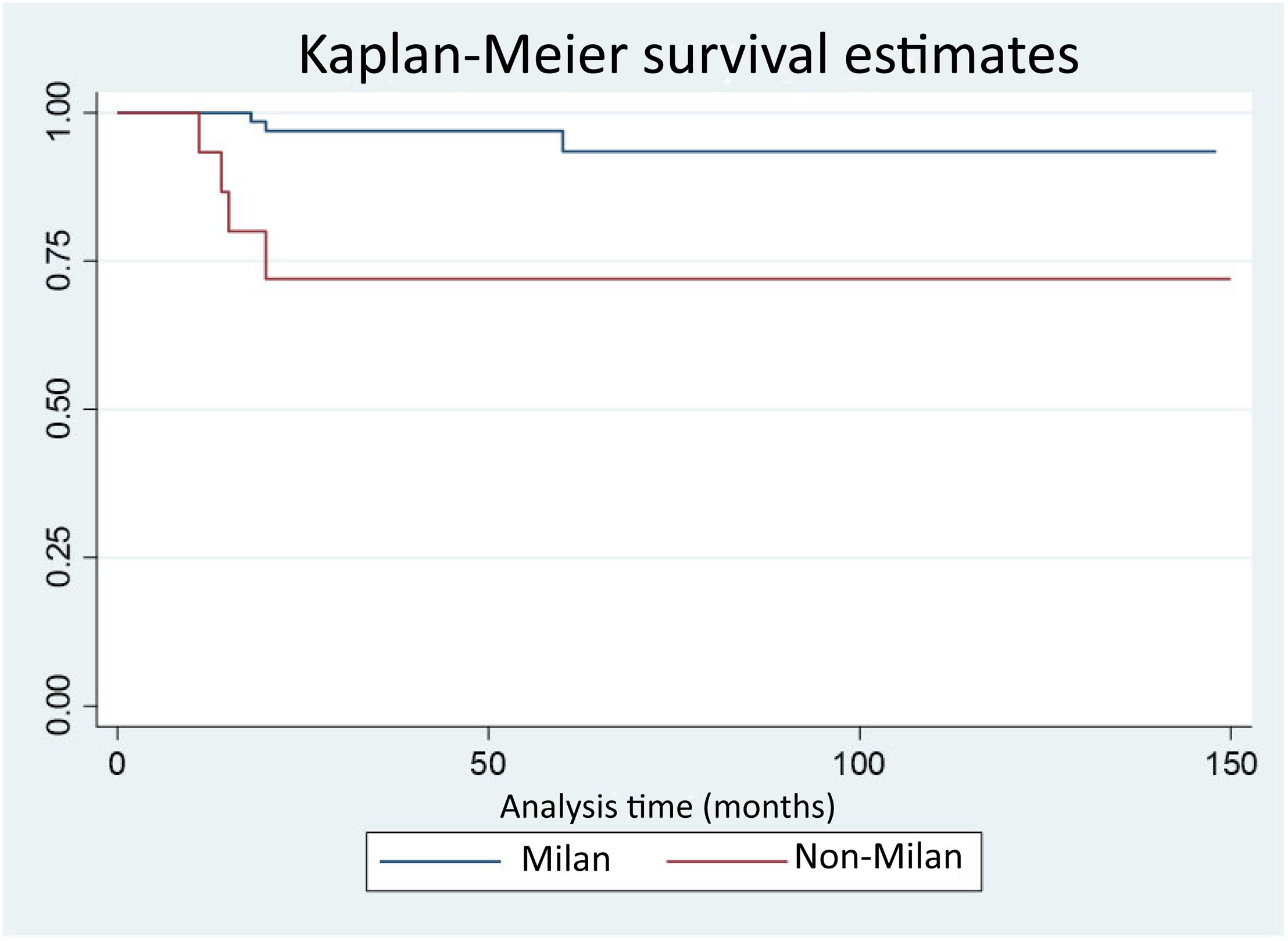
Seven (7.3%) patients presented with HCC recurrence; three in the MC group and four in the NMC group. Disease-free survival at 12, 24, and 48 months was 100%, 96.9%, and 96.9% in the MC group vs. 93.3%, 72%, and 72% in the NMC group, with statistically significant differences (p = 0.0027). Eighty percent of the cases of recurrence presented within the first 18 posttransplantation months (Fig. 2). All the patients that experienced recurrence had extrahepatic disease and the most frequent locations were peritoneal and bone metastases.
DiscussionIn our analysis, we obtained an overall recurrence rate of 7.3%, slightly lower than the rates in other published works that vary from 8 to 20%.7,8,13,15,19 Our results show there are differences with respect to disease-free survival after liver transplant in transplanted patients within the Milan criteria vs. patients beyond the Milan criteria, with no significant difference in overall survival between the two groups. Kardashian et al. carried out a review of a United States register, with a large cohort (3,570 patients with tumors within the Milan criteria and 789 that exceeded the Milan criteria, of whom 465 underwent downstaging). Those authors determined that posttransplant overall survival and disease-free survival were significantly lower in the tumors beyond the Milan criteria; in the patients that met the Milan criteria, there was 11% recurrence, and the percentage increased to 16% in the group that underwent downstaging and to 32% in the group that did not undergo downstaging.20 Those data correspond with ours, with lower recurrence rates in our study.
Locoregional treatments have been performed at our center in patients with HCC within the Milan criteria as bridging therapy until transplantation in the majority of cases, given that the mean time on the waitlist is greater than six months. This concurs with the recommendation issued in 2015 by the United Network for Organ Sharing, the European Association for the Study of the Liver, and the European Organisation for Research and Treatment of Cancer, which suggests the use of locoregional therapies in patients on waitlists for more than six months due to the risk of disease progression and patient dropout.21,22 The primary goal of these therapies is to delay tumor progression until an adequate organ becomes available to the patient, thus minimizing the possibility of exclusion from the transplant due to HCC progression, with rates that can reach up to 10–20%.19–21 In addition, these treatments appear to provide oncologic benefits. In several studies, patients that presented with a higher tumor response after locoregional treatment had better results after transplantation.22–24 At transplant units with shorter waitlist times, the role of locoregional therapies in patients within the Milan criteria is not clearly defined.25 Despite the large number of studies on the expansion of the criteria for indicating liver transplant in HCC and the increasingly extended use of therapies for downstaging patients, a management protocol has yet to be established and the results after downstaging are not well-defined. In addition, the success rates of the locoregional therapies utilized in downstaging are extremely variable (24–90%), mainly due to the retrospective design of the published studies and the fact that there is no clear definition of treatment modality, tumor volume to treat, or tumor response. In 2015 a systematic review was published that concluded there was important heterogeneity among the studies, regarding the definition of tumor burden, time on the waitlist, the protocols utilized for downstaging, and the evaluation of treatment response.17
There are increasingly fewer doubts that morphologic criteria centered only on tumor size and number are insufficient for selecting patients for liver transplantation, and so biologic, inflammatory, radiologic, pathologic, and genetic markers that predict the biologic behavior of the tumor should be combined. Several studies have shown that preoperative AFP levels above 400 ng/mL, unfavorable tumor differentiation grades (3 and 4), positron emission tomography positivity, multilobar tumors, or the presence of microscopic or macroscopic vascular invasion predict the possibility of recurrence more accurately than the isolated use of the Milan criteria.26–29 Liang et al. analyzed patients that were transplanted due to HCC with the expanded criteria and that presented with disease recurrence. They found that the patients with recurrence had a higher total tumor and main tumor nodule size than the patients with no recurrence, enabling them to establish new, broader indications. Thus, in tumors with a maximum size of 6 cm and a total tumor sum of 10 cm, they obtained five-year overall and disease-free survival rates of 77.7% and 20.5%, respectively, which are comparable to the rates obtained when meeting the Milan criteria. This signifies a benefit of up to 35% more patients that could be candidates for transplantation.30 These results are in line with ours but given our small sample size and low number of cases of recurrence, we have not been able to establish which variables are associated with recurrence.
The present study has a series of limitations. First, it is a retrospective study with a non-randomized design. Second, the sample size is small for carrying out a multivariant analysis that would identify independent predictive disease recurrence parameters and provide solid conclusions. And third, genetic or molecular markers that could condition greater or lesser tumor aggressivity and the tendency to recur have not been evaluated.
In conclusion, according to our study, downstaging is a safe option in patients presenting with HCC that are beyond the Milan criteria, allowing them to become candidates and opt for transplantation, with lower disease-free survival rates than transplanted patients that meet the Milan criteria, albeit with no significant differences in overall survival.
Prospective studies with a larger sample size are needed to confirm our results, as well as to analyze the variables associated with recurrence and standardize downstaging strategies, the type of locoregional treatment, a more adequate response evaluation, and the subsequent follow-up, for improving results.
Financial disclosureNo financial support was received in relation to this study/article.