The purpose of this study was to examine the utility of small bowel capsule endoscopy (SBCE) in the diagnostic pathway of patients that had elevated fecal calprotectin (FC) and normal colonoscopy.
MethodsPatients with elevated FC and normal colonoscopy that underwent SBCE in the last 4 years were included. Patients were divided into 3 groups: group 1: patients with isolated small bowel Crohn’s disease (SBCD) on SBCE; group 2: patients with elevated FC but normal SBCE; and group 3: patients with isolated terminal ileitis.
ResultsThe study included 320 patients (group 1: 254 patients, group 2: 50 patients, and group 3: 16 patients). The median age was 42.5 years (IQR 26) across the three groups and 52.4% of the patients had a new diagnosis of SBCD. In group 1, active disease was identified distally in 247 patients (77.2%), proximal involvement in 90 patients (28.1%), and extensive SBCD in 68 patients (21.3%). Magnetic resonance enterography (MRE) was carried out in 229 (90.1%) patients in group 1 and was negative in 42 patients with SBCD. The diagnostic yield of SBCE was higher than that of MRE (p < 0.0001). In group 2, the final diagnoses included Helicobacter pylori infection (n = 2), NSAID use (n = 3), celiac disease (n = 2), and microscopic colitis (n = 1). The final diagnoses in group 3 were idiopathic terminal ileitis (n = 11), inflammatory bowel disease (n = 3), and infective terminal ileitis (n = 2).
ConclusionSBCE influences the patient pathway even when negative/normal. It is better at identifying early SBCD, when compared with MRE.
El propósito del presente estudio fue examinar la utilidad de la cápsula endoscópica de intestino delgado (CEID) en el abordaje diagnóstico de pacientes con calprotectina fecal (CF) elevada y endoscopía normal.
MétodosSe incluyó a pacientes con CF elevada y colonoscopía normal que se sometieron a CEID en los 4 años previos. Los pacientes fueron divididos en 3 grupos: grupo 1: pacientes con enfermedad de Crohn con afección aislada de intestino delgado (ECID) en la CEID; grupo 2: pacientes con CF elevada, pero CEID normal; y grupo 3: pacientes con ileítis terminal aislada (ITA).
ResultadosEl estudio incluyó a 320 pacientes (grupo 1: 254 pacientes, grupo 2: 50 pacientes, y grupo 3: 16 pacientes). La edad promedio de los tres grupos fue de 42.5 años (RIC 26), el 52.4% de los pacientes estaban recién diagnosticados con ECID. En el grupo 1, la enfermedad activa fue identificada con afectación distal en 247 pacientes (77.2%), afectación proximal en 90 pacientes (28.1%), y ECID extendida en 68 pacientes (21.3%). Se realizó enterografía por resonancia magnética (ERM) en 229 pacientes (90.1%) en el grupo 1 y fue negativo en 42 pacientes con ECID. Los resultados diagnósticos con CEID fueron mejores que con ERM (p < 0.0001). En el grupo 2, los diagnósticos finales incluyeron infección por Helicobacter pylori (n = 2), uso de AINE (n = 3), enfermedad celiaca (n = 2), y colitis microscópica (n = 1). Los diagnósticos finales en el grupo 3 fueron ileítis terminal idiopática (n = 11), enfermedad intestinal inflamatoria (n = 3), e ileítis terminal infecciosa (n = 2).
ConclusiónLa CEID tiene impacto en el abordaje diagnóstico de este grupo de pacientes incluso cuando es negativa/normal. La CEID es mejor que la ERM para identificar de manera temprana la ECID.
Small bowel capsule endoscopy (SBCE) is a non-invasive investigative technique used to visualize the small bowel. The capsule utilized in SBCE contains a light-emitting diode, a metal-oxide-semiconductor imaging camera, a radio transmitter, and silver oxide batteries with a battery life of eight to 12 hours. The information from the capsule is transmitted to a sensor array in an external belt through radiofrequency and can then be downloaded onto a computer for viewing at 1:8 magnification by the endoscopist. Most small bowel capsules contain one camera with an approximate 160-degree view, providing adequate visualization of the small bowel but not of the stomach.1
Fecal calprotectin (FC), an inflammatory marker of the gut, is released from the inflamed mucosa and granulocytes in the intestines during neutrophil degranulation, as part of the innate immune response. It is a calcium and zinc-binding protein that forms part of the neutrophil cytosolic protein.2,3 A recent meta-analysis showed that elevated FC (50 μg/g or higher) has 83% sensitivity (95% CI, 74-90%) and 50% specificity (95% CI, 36-64%) for small bowel inflammation.4 It is an important part of the investigative pathway of suspected small bowel Crohn’s disease (SBCD) patients.5,6 However, the literature suggests that FC is not as reliable a biomarker in small bowel disease as in the colonic involvement of Crohn’s disease (CD).7,8
The Lewis score is a scoring system that is built within the manufacturer’s software to assess the extent of small bowel inflammation during capsule reporting. The Lewis score divides the small bowel into three parts (tertiles) and assesses the size and extent of endoscopic variables in each tertile, including ulceration, stenosis, and changes in villous appearance. A score of less than 135 is indicative of normal or clinically insignificant mucosal inflammation, 135-790 denotes mild inflammation, and a score over 790 indicates moderate-to-severe mucosal inflammation.9
Patients with altered bowel habit, elevated FC, and a negative ileocolonoscopy will often require small bowel evaluation.10,11 SBCE is used as an alternative to imaging modalities for the diagnosis of SBCD.1 Magnetic resonance enterography (MRE) has good diagnostic capabilities, but studies have found SBCE to have higher diagnostic accuracy, especially when investigating the presence of proximal small bowel disease or more subtle mucosal lesions.12–14
In addition to inflammatory bowel disease (IBD), elevated FC can be due to other causes. Studies have found an association between Helicobacter pylori (H. pylori) infection and elevated FC.15–17 Both celiac disease and microscopic colitis have been identified as causes of increased FC levels,1–15 but there is conflicting data regarding this association.18–20 The long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs), which can cause enteropathy, has been found to be associated with elevated FC.21,22 Finally, even though terminal ileitis is a relatively new entity with limited published literature, it has been identified as a cause of small bowel inflammation, and thus is associated with elevated FC.23
The aim of the present study was to examine the utility of SBCE in the diagnostic pathway of patients with elevated FC and normal colonoscopy.
MethodsA retrospective, observational, case-control study was carried out at the Academic Unit of Gastroenterology of the Sheffield Teaching Hospitals (STH). The STROBE cross-sectional study checklist was used to guide the development of the present manuscript.
A database was created of SBCE reports from all capsule endoscopies undergone by patients aged 16 or over at any STH site (Northern General Hospital or Royal Hallamshire Hospital) between 2016 and 2021. Participants were then identified from this database through search terms, such as Crohn’s disease, fecal calprotectin, and terminal ileitis. Data collection was carried out using various clinical applications. All patients were screened for NSAID use, PPI therapy, and infections, before they underwent SBCE.
Inclusion criteria were specified as patients with active isolated SBCD on SBCE (group 1), patients with a normal/negative SBCE but a FC level of 50 μg/g or higher (group 2), and patients whose SBCE report or clinical notes indicated isolated terminal ileitis (ITI) (group 3). Participants were excluded if they did not have sufficient clinical data. Data were then collected on demographics, including sex and date of birth; SBCE findings, including behavior and location of capsule findings; the Lewis score, when applicable; laboratory tests, including biomarkers (FC and C-reactive protein [CRP]), vitamin B12, ferritin, hemoglobin, white cell count, and platelet count; previous tests, including MRE and colonoscopy; clinical information, including symptom duration and the Harvey Bradshaw Index (HBI); and follow-up data, such as management changes, follow-up time, and the final diagnosis. A FC level of 50 μg/g was used, based on two previous meta-analyses by Kopylov et al. and Jung et al., showing that FC levels for diagnosing SBCD were much lower than those for diagnosing colonic CD.3,4
Statistical analysisThe SPSS version 22 was employed for the statistical analysis. Descriptive statistics were reported using median and interquartile range (IQR), given that the variables were abnormally distributed. The chi-square or Fisher’s exact test was used to compare the categorical data, and the Mann-Whitney U test was used to compare the two groups of continuous variables due to their abnormal distribution. The Wilcoxon signed rank test was used to analyze matched data, and the linear regression analysis for multiple groups. The Spearman’s rank test or Pearson’s correlation was used to analyze correlations. A p value of ≤0.05 determined statistical significance.
Ethical considerationsEthical approval was obtained from the Health Research Authority (IRAS project ID: 295838) and Sheffield Teaching Hospitals (STH21615). No experiments were carried out on animals or humans. All data was anonymized during collection. No personal data that would allow the patient to be identified has been included in this study.
ResultsThe study included 320 patients: 254 in group 1 (positive SBCE and isolated SBCD), 50 in group 2 (elevated FC but normal capsule endoscopy), and 16 in group 3 (ITI). The median age was 42.5 years (IQR 26) and there was a female predominance of 60.3% (n = 193).
Of the patients with a diagnosis of SBCD, 47.6% (n = 121) had established disease and 52.4% (n = 133) received a new diagnosis following their SBCE. The median duration of disease from diagnosis to capsule endoscopy was 68 months (IQR 139), and the median duration of symptoms from presentation to SBCE was 10 months (IQR 27).
MRE was performed in 90.1% (n = 229) of patients in group 1, 81.7% (n = 187) of which were equivocal or positive for active SBCD. There were 42 patients in the group with confirmed SBCD on capsule endoscopy but a negative MRE. Our study shows that SBCE has a higher diagnostic accuracy than MRE for diagnosing active SBCD (p < 0.0001). SBCE was carried out in patients with equivocal or positive MRE, to find further evidence of active disease (as opposed to chronic disease) prior to altering therapy. In the group of patients with negative MRE, capsule endoscopy was performed because SBCD was highly suspected, based on symptoms and elevated FC.
On SBCE, 90 (35.4%) patients in group 1 had proximal findings, 232 (91.3%) patients had distal findings, and 68 (26.8%) patients had extensive disease. When compared across the three groups, group 1 had the greatest number of patients with proximal findings, distal findings, and extensive disease on SBCE (p = 0.000 in all three), with distal findings in only 15 patients (6%) in group 3.
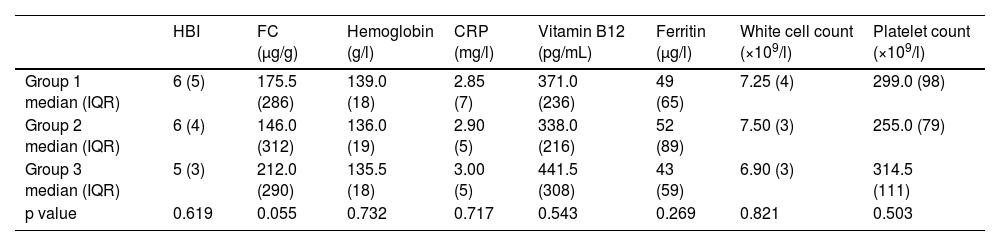
There was no statistically significant difference between blood parameters, FC, or HBI in the three groups (Table 1).
Median Harvey-Bradshaw Index, fecal calprotectin, hemoglobin, C-reactive protein, vitamin B12, ferritin, white cell count, and platelet count per group.
| HBI | FC (µg/g) | Hemoglobin (g/l) | CRP (mg/l) | Vitamin B12 (pg/mL) | Ferritin (μg/l) | White cell count (×109/l) | Platelet count (×109/l) | |
|---|---|---|---|---|---|---|---|---|
| Group 1 median (IQR) | 6 (5) | 175.5 (286) | 139.0 (18) | 2.85 (7) | 371.0 (236) | 49 (65) | 7.25 (4) | 299.0 (98) |
| Group 2 median (IQR) | 6 (4) | 146.0 (312) | 136.0 (19) | 2.90 (5) | 338.0 (216) | 52 (89) | 7.50 (3) | 255.0 (79) |
| Group 3 median (IQR) | 5 (3) | 212.0 (290) | 135.5 (18) | 3.00 (5) | 441.5 (308) | 43 (59) | 6.90 (3) | 314.5 (111) |
| p value | 0.619 | 0.055 | 0.732 | 0.717 | 0.543 | 0.269 | 0.821 | 0.503 |
CRP: C-reactive protein; FC: fecal calprotectin; HBI: Harvey-Bradshaw Index.
In a linear regression analysis of group 1, there was no correlation between the region affected on SBCE, and CRP, HBI, or FC (p = 0.560, 0.146, and 0.153 respectively).
In group 2, patients had either an entirely normal SBCE or insignificant findings. Patients with minimal inflammation/findings on SBCE were compared with those with a completely normal capsule endoscopy. No significant differences were found between FC, CRP, or HBI between those patients (Table 2). The FC level was increased in both instances, but the CRP level was clinically insignificant across the two.
Comparison of fecal calprotectin, Harvey-Bradshaw Index, and C-reactive protein in Group 2, between patients with normal small bowel capsule endoscopy and patients with minimal findings.
| Normal SBCE | Minimal inflammation on SBCE | p value | |
|---|---|---|---|
| Median FC (µg/g) | 150 (IQR 369) | 156 (IQR 1100) | 0.516 |
| Median HBI | 6 (IQR 5) | 6 (IQR 10) | 0.552 |
| Median CRP (mg/l) | 2.8 (IQR 4) | 2.4 (IQR 4) | 1.000 |
CRP: C-reactive protein; FC: fecal calprotectin; HBI: Harvey-Bradshaw Index; IQR: interquartile range; SBCE: small bowel capsule endoscopy.
When analyzing correlations, HBI did not correlate well with either FC or CRP (p = 0.196 and 0.650, respectively). However, there was a significant correlation between CRP and FC (p = 0.001).
A management change following SBCE was made in 166 (51.9%) patients. This included starting or having a dose escalation of methotrexate in 17 (10.2%) patients, biological therapy in 89 (53.6%) patients, corticosteroids in 119 (71.7%) patients, and azathioprine in 66 (39.8%) patients.
Group 1 was more likely to have a management change than groups 2 and 3 (p = 0.000), with 89.2% (148/166) of patients in group 1, 5.4% (9/166) of patients in group 2, and 5.4% (n = 9/166) of patients in group 3. When breaking this comparison down by each medication started or escalated, group 1 had a greater requirement for biological therapy (p = 0.000), corticosteroids (p = 0.000), and azathioprine (p = 0.000), but not for methotrexate (p = 0.116).
Patients with distal or extensive disease on SBCE were more likely than those with proximal disease or no findings to have a management change (p = 0.000), at 60.2% and 31.3%, compared with 3.4% and 5.1%, respectively. Additionally, patients with distal and extensive disease had a greater requirement for azathioprine (p = 0.011) and biological therapy (p = 0.002). However, there was no statistically significant difference in the starting or dose escalation of methotrexate (p = 0.569) or corticosteroids (p = 0.078), regarding the different regions affected on SBCE.
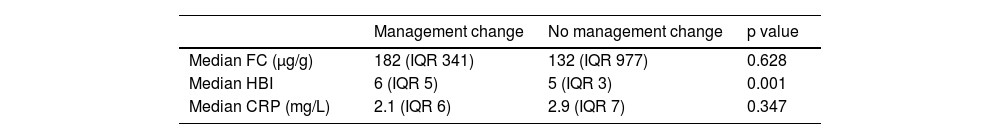
HBI predicted a change in management, but FC and CRP did not (Table 3). FC was higher in patients that had a management change following their capsule endoscopy, but it was not statistically significant.
In group 2, a cause of the increase in FC was identified in 8 patients. H. pylori infection was the cause in 2 (25%) patients, NSAID use in 3 (37%) patients, celiac disease in 2 (25%) patients, and microscopic colitis in one (12%) patient that subsequently had a repeat colonoscopy. No cause was identified in the rest of the patients in the follow-up period. In group 3, the final diagnosis was idiopathic terminal ileitis in 11 patients, IBD in 3 patients, and infective terminal ileitis in two patients.
FC is an established biomarker for detecting colonic inflammation,24 but the literature on the role of FC as a biomarker for small bowel inflammation is controversial. The present study supports the use of capsule endoscopy in patients with normal colonoscopy and elevated FC, indicating that SBCE contributes to management change and improved outcomes in this group of patients. The level of FC used for further evaluating patients with suspected SBCD is also much lower than that for colonic CD. In a meta-analysis by Kopylov et al. that included seven studies, the FC cutoff value was 50 μg/g, with a sensitivity of 0.83 and a specificity of 0.53. This has also been shown in a meta-analysis by Jung et al. that included 14 studies; with a cutoff value of 50 μg/g, sensitivity was 83% and specificity was 50%.3,4
The present study also supports previous literature showing that SBCE has a superior diagnostic yield when compared with MRE, with a higher sensitivity, specificity, positive predictive value, and negative predictive value.13,14,25 The European Society of Gastrointestinal Endoscopy (ESGE) guidelines also recommend the use of SBCE for the diagnosis of SBCD. Previous studies have shown a poor correlation between endoscopic appearance and histological diagnosis in SBCD. A study by Tun et al. has reported a histological diagnosis of only 8-15% in patients with suspected SBCD.26
There was a significant correlation between FC and CRP. This falls into the conflicting literature, with some studies suggesting that FC and CRP correlate well and perform better when combined,24,25,27,28 but other studies suggest no significant association between the biomarkers.2 Furthermore, some studies support the lack of correlation between HBI and FC or CRP.29,30 Nevertheless, other studies support a correlation between HBI and FC27 and HBI and CRP.31
Patients with distal and extensive disease on SBCE were more likely to receive a management change than patients with proximal findings or no disease. Even though the literature suggests that CD patients with proximal small bowel involvement tend to have more severe inflammation and a worse prognosis,32–34 a tendency has been found in those patients to have a milder presentation than patients with distal small bowel or colonic involvement.31 Extensive disease is a risk factor for a worse clinical course of the disease, and loss of response to therapy.35 Therefore, those patients would be expected to require more aggressive therapeutic interventions.
HBI was found to predict a change in management, given that the severity of presenting symptoms is an important consideration in the management pathway.36,37 Though not statistically significant, FC was higher in patients that received a management change. A higher FC has been associated with a worse prognosis,38,39 signifying a greater requirement for therapeutic interventions.
The final diagnoses found in group 2 were H. pylori infection, NSAID use/enteropathy, celiac disease, and microscopic colitis. Research has found an association between H. pylori infection and elevated FC,15,16 but the literature on the other diagnoses is controversial. Both celiac disease and microscopic colitis have been identified as causes of elevated FC.17 A 2012 study on celiac disease in children found that patients with untreated celiac disease had a higher FC than patients on a long-term gluten-free diet and healthy patients.40 However, other studies found no significant association between the disease and FC levels and concluded that it is an unnecessary investigation.18,19 Studies have found an association between microscopic colitis and elevated FC, which is particularly evident in lymphocytic colitis, as FC is neutrophil-based rather than lymphocyte-based.20 Another study found an association between NSAID enteropathy and elevated FC,22 albeit previous research suggests FC is usually higher in IBD.21
Inflammation/ulceration seen in the terminal ileum can be suggestive of CD, hence it is important to identify those patients early, to avoid long-term complications. Some studies have shown that only a small number of patients with ITI progress to CD.41,42 A previous study has also shown that patients with ITI have milder findings on SBCE, compared with patients diagnosed with CD, and they are more likely to improve without treatment.23 However, there is a paucity of data on how best to manage said group. It is also unclear how many of those patients eventually develop CD. In our group of patients with ITI (group 3, n = 16), only two patients had a final diagnosis of CD, which supports findings from previous literature that only a small number of patients with ITI progress to CD. In addition, corticosteroid therapy was the only treatment used in those patients, supporting the previous literature that they have milder disease. Nevertheless, longitudinal follow-up is needed to further monitor the outcomes of that group of patients.
Despite the fact that the large sample provided significant strength to the present study, the sample sizes in each individual group were fairly small, and so were a limitation. Additionally, there was a large discrepancy in numbers between the groups, introducing the possibility of skewed results.
The missing data resulting from the retrospective study design produced a considerable limitation. Patients had to be excluded due to missing data, thus creating selection bias and compromising generalizability.
Subjectivity, especially regarding the use of clinical notes, was another limitation, involving both the clinician writing the notes and the researcher interpreting them. It was minimized by the use of scoring systems to assess subjective areas, and by having strict inclusion and exclusion criteria.
ConclusionsSBCE is a useful modality to investigate patients with elevated FC. It identifies early SBCD better than MRE. SBCE influences the patient pathway, even when it is negative/normal, because alternative diagnoses can be considered. ITI is a distinct entity that requires further evaluation and FC cannot be relied upon to distinguish between terminal ileitis and SBCD. Our study adds to the literature supporting the use of SBCE for the diagnosis and management of this group of patients.
Financial disclosureNo financial support was received in relation to this study/article.