Gastric cancer is one of the most frequent neoplasias in Peru and worldwide, with surgery as the only potentially curative or palliative treatment. Laparoscopic gastrectomy is the most frequent alternative surgical technique utilized, but one of its main drawbacks is the technical difficulty involved in perigastric lymphadenectomy. The aim of the present study was to evaluate the clinical and surgical characteristics, postoperative complications, and survival rate in patients with advanced gastric cancer that underwent open gastrectomy or laparoscopic gastrectomy at the Hospital Nacional P.N.P “Luis N. Sáenz” in Lima, Peru, within the time frame of 2005 to 2014.
Materials and methodsAn analytic, longitudinal, retrospective cohort study was conducted on 482 patients that underwent surgery for gastric cancer, within the time frame of January 2005 to December 2014. The clinical, epidemiologic, and postoperative characteristics were evaluated, and a survival analysis was carried out.
ResultsOf the 475 patients included in the study, 236 underwent open gastrectomy and 239 had laparoscopic gastrectomy. Median follow-up time was 61.9 months in the open surgery group and 46.7 months in the laparoscopy group. There were fewer postoperative complications in the laparoscopy group and no statistically significant difference between the two groups in relation to the survival analysis.
ConclusionsIn our study, laparoscopic gastrectomy resulted in fewer postoperative complications, compared with the open procedure, but did not modify overall survival during the follow-up period.
El cáncer gástrico es una de las neoplasias más frecuentes en el Perú y el mundo, con la cirugía como único tratamiento con potencial curativo y paliativo en otros casos, para la cual se utiliza con mayor frecuencia la gastrectomía laparoscópica como técnica quirúrgica alternativa, siendo las dificultades técnicas en la linfadenectomía perigástrica uno de sus principales inconvenientes. El presente estudio buscó evaluar las características clínicas y quirúrgicas, las complicaciones postoperatorias y la sobrevida en pacientes con cáncer gástrico avanzado utilizando gastrectomía abierta frente a la gastrectomía laparoscópica en el Hospital Nacional P.N.P «Luis N. Sáenz», en Lima-Perú, entre los años 2005 y 2014.
MétodosSe realizó un estudio analítico, longitudinal, de tipo cohorte retrospectiva, que incluyeron 482 pacientes operados por cáncer gástrico recolectados entre enero de 2005 a diciembre de 2014, evaluándose las características clínicas, epidemiológicas, postoperatorias y de análisis de supervivencia.
ResultadosDe los 475 pacientes incluidos, se realizaron 236 gastrectomías abiertas y 239 gastrectomías laparoscópicas, con una mediana de tiempo de seguimiento de 61.9 meses en el grupo de cirugía abierta y 46.7 meses en el grupo de laparoscopias, encontrando menores complicaciones postoperatorias en el grupo de cirugías laparoscópicas y sin diferencias estadísticamente significativas en los análisis de sobrevida entre ambos grupos.
ConclusionesNuestro estudio encuentra que la cirugía laparoscópica tiene menores complicaciones postoperatorias en comparación a la gastrectomía abierta, pero sin modificar la sobrevida global durante el periodo de seguimiento.
Gastric cancer is an important health problem and is the fourth most frequent type of cancer worldwide.1 In Peru, it is the second most frequent cancer and the first in mortality, with an approximate survival period of 29 months, and incidence is 15.8 per 100,000 inhabitants. That is one of the highest rates in the world, comparable to incidence in China and Japan, but unlike in those countries, the large majority of patients are discovered at advanced stages of the disease.2,3
The treatment of gastric cancer is stage-dependent and is potentially curative in stages I to III, with the exception of stage Ia, in which endoscopic resection of the submucosa is indicated. Palliative treatment is recommended for stage IV disease and gastrectomy is the treatment of choice in the other stages.4
Laparoscopic gastrectomy is a technique that is revolutionizing gastric cancer surgery, especially in countries with a high incidence of the pathology.5 Numerous studies have confirmed its value at early disease stages. The Korean management guidelines include it as acceptable treatment for early gastric cancer,6 and due to its minimally invasive nature, its use is increasing across the world.7
There is a lack of solid evidence on the safety and long-term results of laparoscopic gastrectomy. Therefore, at present, it cannot replace open gastrectomy as the procedure of choice8 due to the technical difficulties of resection, insufficient data on adequate oncologic procedure,5 and recurrence patterns that are different from those seen with open gastrectomy, such as peritoneal recurrence and metastasis at port insertion sites.9 However, thanks to improved technology, great advances have been made in laparoscopy as treatment for gastric cancer, including the complete removal of the perigastric lymph nodes.10 Nevertheless, studies are still needed that show equal survival rates between the laparoscopic technique and the open procedure in patients with advanced cancers.
In Peru, and Latin America in general, there is not much evidence on gastric cancer and its treatment. Therefore, the aim of our study was to compare open gastrectomy with laparoscopic gastrectomy in cases of advanced cancer, with respect to postoperative complications and overall survival.
MethodsType of study. An analytic, longitudinal, retrospective cohort study was conducted on patients that underwent surgery for gastric cancer at the Hospital Nacional PNP “Luis N. Saenz”, within the time frame of January 2005 to December 2014, and that were followed up to February 2017, when the study ended.
Population and sample. The study included patients that were diagnosed with advanced gastric cancer through clinical, imaging, and anatomopathologic studies. Two hundred and thirty-six of those patients underwent open gastrectomy and 239 underwent laparoscopic gastrectomy. The inclusion criteria were patients above 14 years of age that underwent either open or laparoscopic gastrectomy, were operated on at the Hospital Nacional PNP “Luis N. Saenz”, and whose clinical histories were complete. The exclusion criteria were patients with incomplete anatomopathologic results and patients that could not be located for postoperative evaluation.
Sample size was calculated through the survival data technique, in which data from previous studies were used, showing that the probability of 5-year survival for open gastrectomy was 8%, and was 15% for laparoscopic gastrectomy. The exposed/nonexposed risk ratio was 1, power was 80%, the alpha value was 0.05%, and the rate of patients lost to follow-up was 10%.4
The data were obtained from the clinical histories that were selected through non-probability convenience sampling. A unique code was assigned to each collection card. The patients were followed after surgery by means of consultations and telephone calls to determine their survival status, up to February 2017.
Surgical technique. The decision to perform open gastrectomy or laparoscopic gastrectomy was individually made by each surgeon based on the location and type of the neoplasia, the surgeon's level of skill, and patient preference. Preoperative data included upper endoscopy, tomography of the brain, chest, and abdomen, biopsies, and later tumor staging.
Open gastrectomy and laparoscopic gastrectomy were performed through the conventional technique described in previous studies.11 In the patients that presented with lesions in the antrum, subtotal gastrectomy was performed, and total gastrectomy was carried out in the patients with tumors in the gastric fundus and body. Lymph node dissection was performed according to that described for the treatment of gastric cancer, classifying lymph node groups 1, 2, 3, 4d, 4sa, 4sb, 5, 6, and 7 as D1 and the added groups of 8a, 9, 10, 11p, 11d, 12a, 19, 20, 110, and 111 as D2. Reconstruction was carried out through Roux-en-Y anastomosis. Some laparoscopic surgeries were video-assisted when the gastrectomy and reconstruction were completed by widening the entrance port of the midline trocar (T1) by approximately 5cm.
Statistical analysis. Descriptive and inferential (bivariate and multivariate) analyses were carried out and the overall survival analysis was performed through the Kaplan-Meier estimate (statistical significance with the log-rank test). The Cox regression model was employed for the multivariate analysis and statistical significance was set at a p < 0.05.
Ethical disclosures. The present study met the ethical recommendations of the Declaration of Helsinki of the World Medical Association and was approved by the ethics committees of the Hospital Nacional PNP “Luis N. Saenz” and the Universidad Peruana Cayetano Heredia.
We, the authors, declare that no experiments on humans or animals were performed in the present study. We also declare that patient data have been handled confidentially and anonymously, following the protocols of our work center and that the patients gave their informed consent.
ResultsClinical and epidemiologic characteristics (Table 1).
Clinical characteristics of the patients with gastric cancer, comparing the two surgical techniques, at the Hospital Nacional P.N.P “Luis N. Sáenz”, 2005 - 2014 (n = 475).
| Characteristics | Frequency | Percentage |
|---|---|---|
| Sex | ||
| Men | 346 | 72.8 |
| Women | 129 | 27.2 |
| Age (years) | ||
| X ± SD | 66.7 ± 8.1 | |
| Type of gastrectomy | ||
| Subtotal gastrectomy | 394 | 82.9 |
| Total gastrectomy | 81 | 17.1 |
| Lymph node dissection | ||
| D1 | 160 | 33.7 |
| D2 | 315 | 66.3 |
| Follow-up time (months) | ||
| Median ± IQI | 53.8 ± 33.5 | |
| Hemoglobin level (g/dl) | ||
| Median ± IQI | 12 ± 1.6 | |
| Albumin level (g/dl) | ||
| Median ± IQI | 3.4 ± 0.6 | |
| Histologic type | ||
| Carcinoma | 451 | 95.0 |
| Epithelial tumors | 12 | 2.5 |
| Non-epithelial tumors | 12 | 2.5 |
| Anatomopathologic clinical stage | ||
| Stage IIA | 33 | 6.9 |
| Stage IIB | 80 | 16.9 |
| Stage IIIA | 85 | 17.9 |
| Stage IIIBc | 114 | 24.0 |
| Stage IIIC | 145 | 30.5 |
| Stage IV | 18 | 3.8 |
| Surgery duration (minutes) | ||
| Median ± IQI | 400 ± 150 | |
| Surgical reintervention | ||
| Yes | 31 | 6.5 |
| No | 444 | 93.5 |
| Oral diet resumed (days) | ||
| Median ± IQI | 7 ± 2.0 | |
| Metastasis | ||
| Yes | 19 | 4 |
| No | 456 | 96 |
| Ascites | ||
| Yes | 141 | 29.7 |
| No | 334 | 70.3 |
| Comorbidities | 144 | 30.2 |
| Diabetes | 47 | 9.9 |
| Cardiovascular disease | 52 | 10.9 |
| Respiratory disease | 45 | 9.5 |
| Post-operative complications | 143 | 30.1 |
| Pneumonia | 77 | 16.2 |
| Anastomosis fistula | 22 | 4.6 |
| Bleeding | 5 | 1.1 |
| Pancreatic fistula | 30 | 6.3 |
| Abscess | 11 | 2.3 |
| Relapse | 106 | 22.3 |
IQI: interquartile interval.
Within the time frame of January 1, 2005 to December 31, 2014, 482 patients were included in the study. Seven of those patients were excluded due to the postoperative diagnosis of gastric lymphoma, leaving a final total of 475 study patients.
Two hundred and thirty-six open gastrectomies and 239 laparoscopic gastrectomies were performed, with a median follow-up of 53.8 months.
Of the total number of procedures performed, 394 were subtotal gastrectomies and 81 were total gastrectomies, carrying out 160 D1 lymph node dissections and 315 D2 lymph node dissections. A total of 106 patients (22.3%) presented with relapse.
Inferential analysis (Table 2)
Inferential analysis comparing the two surgical techniques in patients with gastric cancer at the Hospital Nacional P.N.P “Luis N. Sáenz”, 2005 - 2014 (n = 475).
| Characteristics | Open surgery n = 236 | Laparoscopic surgery n = 239 | p | OR | 95%CI | |||
|---|---|---|---|---|---|---|---|---|
| N° | % | N | % | Inferior | Superior | |||
| Sex | ||||||||
| Women | 70 | 54.3 | 59 | 45.7 | 0.222 | 1.29 | 0.84 | 1.97 |
| Men | 166 | 48.0 | 180 | 52.0 | ||||
| Age (years) | ||||||||
| Median ± IQI | 68 ± 11.5 | 66 ± 10 | 0.026a | |||||
| Type of gastrectomy | ||||||||
| Total gastrectomy | 58 | 71.6 | 23 | 28.4 | <0.001 | 3.06 | 1.77 | 5.40 |
| Subtotal gastrectomy | 178 | 45.2 | 216 | 54.8 | ||||
| Lymph node dissection | ||||||||
| D2 | 131 | 41.6 | 184 | 58.4 | <0.001 | 0.37 | 0.25 | 0.56 |
| D1 | 105 | 65.6 | 55 | 34.4 | ||||
| Follow-up time (months) | ||||||||
| Median ± IQI | 61.9 ± 45.7 | 46.7 ± 26.3 | <0.001a | |||||
| Hemoglobin level (g/dl) | ||||||||
| Median ± IQI | 11.8 ± 1.8 | 12.4 ± 1.6 | <0.001a | |||||
| Albumin level (g/dl) | ||||||||
| Median ± IQI | 3.2 ±0.6 | 3.5 ± 0.4 | <0.001a | |||||
| Histologic type | ||||||||
| Carcinoma | 219 | 48.6 | 232 | 51.4 | 0.013b | |||
| Epithelial tumors | 11 | 91.7 | 1 | 8.3 | ||||
| Non-epithelial tumors | 6 | 50.0 | 6 | 50.0 | ||||
| Anatomopathologic clinical stage | ||||||||
| Stage IIA | 3 | 9.1 | 30 | 90.9 | <0.001b | |||
| Stage IIB | 32 | 40.0 | 48 | 60.0 | ||||
| Stage IIIA | 39 | 45.9 | 46 | 54.1 | ||||
| Stage IIIB | 55 | 48.3 | 59 | 51.7 | ||||
| Stage IIIC | 92 | 63.4 | 53 | 33.6 | ||||
| Stage IV | 15 | 83.3 | 3 | 16.7 | ||||
| Surgery duration (minutes) | ||||||||
| Median ± IQI | 280.0 ± 40.0 | 430.0 ± 40.0 | <0.001a | |||||
| Surgical reintervention | ||||||||
| Yes | 14 | 45.2 | 17 | 54.8 | 0.602 | 0.82 | 0.37 | 1.82 |
| No | 222 | 50.0 | 222 | 50.0 | ||||
| Oral diet resumed (days) | ||||||||
| Median ± IQI | 7.0 ± 2.0 | 5.0 ± 0.0 | <0.001a | |||||
| Metastasis | ||||||||
| Yes | 16 | 84.2 | 3 | 15.8 | 0.002b | 5.72 | 1.60 | 30.96 |
| No | 220 | 48.3 | 236 | 51.7 | ||||
| Ascites | ||||||||
| Yes | 108 | 76.6 | 33 | 23.4 | <0.001 | 5.27 | 3.30 | 8.50 |
| No | 128 | 38.3 | 206 | 61.7 | ||||
| Comorbidities | ||||||||
| Yes | 98 | 68.1 | 46 | 31.9 | <0.001 | 2.98 | 1.93 | 4.61 |
| No | 138 | 41.7 | 193 | 58.3 | ||||
| Diabetes comorbidity | ||||||||
| Yes | 26 | 55.3 | 21 | 44.7 | 0.416 | 1.27 | 0.67 | 2.48 |
| No | 210 | 49.1 | 218 | 50.9 | ||||
| Cardiovascular disease comorbidity | ||||||||
| Yes | 37 | 71.2 | 15 | 28.8 | 0.001 | 2.78 | 1.43 | 5.61 |
| No | 199 | 47.1 | 224 | 52.9 | ||||
| Respiratory comorbidity | ||||||||
| Yes | 35 | 77.8 | 10 | 22.2 | <0.001 | 3.99 | 1.87 | 9.24 |
| No | 201 | 46.7 | 229 | 53.3 | ||||
| Postoperative complications | ||||||||
| Yes | 103 | 72.0 | 40 | 28.0 | <0.001 | 3.85 | 2.47 | 6.06 |
| No | 133 | 40.1 | 199 | 59.9 | ||||
| Postoperative complication (Pneumonia) | ||||||||
| Yes | 66 | 85.7 | 11 | 14.3 | <0.001 | 8.05 | 4.05 | 17.36 |
| No | 170 | 42.7 | 228 | 57.3 | ||||
| Postoperative complication (Anastomosis fistula) | ||||||||
| Yes | 10 | 45.5 | 12 | 54.5 | 0.685 | 0.84 | 0.32 | 2.16 |
| No | 226 | 49.9 | 227 | 50.1 | ||||
| Postoperative complication (Bleeding) | ||||||||
| Yes | 2 | 40.0 | 3 | 60.0 | 0.660 | 0.67 | 0.06 | 5.93 |
| No | 234 | 49.8 | 236 | 50.2 | ||||
| Postoperative complications (Pancreatic fistula) | ||||||||
| Yes | 18 | 60.0 | 12 | 40.0 | 0.243 | 1.56 | 0.69 | 3.64 |
| No | 218 | 49.0 | 227 | 51.0 | ||||
| Postoperative complication (Abscess) | ||||||||
| Yes | 8 | 72.7 | 3 | 27.3 | 0.122 | 2.76 | 0.65 | 16.3 |
| No | 228 | 49.1 | 236 | 50.9 | ||||
| Relapse | ||||||||
| Yes | 78 | 73.6 | 28 | 26.4 | <0.001 | 3.72 | 2.26 | 6.23 |
| No | 158 | 42.8 | 211 | 57.2 | ||||
IQI: interquartile interval; SD: standard deviation.
Statistically significant differences were found in relation to the type of gastrectomy, type of lymph node dissection, hemoglobin level, albumin level, histologic type, preoperative, postoperative, and anatomopathologic clinical stages, surgery duration, oral diet resumption, metastasis, ascites, comorbidities, postoperative complications, and relapse.
Survival analysis (Table 3).
Survival analysis with variables related to gastric cancer at the Hospital Nacional P.N.P “Luis N. Sáenz”, 2005 - 2014 (n = 475).
| Characteristics | X2a | p |
|---|---|---|
| Type of surgery | 5.42 | 0.020 |
| Sex | 0.02 | 0.888 |
| Age (years) | 223.3 | <0.001 |
| Type of gastrectomy | 0.67 | 0.413 |
| Lymph node dissection | 29.14 | <0.001 |
| Follow-up time (months) | 2016.8 | <0.001 |
| Hemoglobin level (g/dl) | 163.3 | <0.001 |
| Albumin level (g/dl) | 593.2 | <0.001 |
| Histologic type | 0.92 | 0.6299 |
| Anatomopathologic clinical stage | 239.7 | <0.001 |
| Surgery duration (minutes) | 25.7 | 0.578 |
| Surgical reintervention | 8.36 | 0.004 |
| Oral diet resumed (days) | 519.8 | <0.001 |
| Metastasis | 66.6 | <0.001 |
| Ascites | 108.5 | <0.001 |
| Comorbidities | 15.6 | <0.001 |
| Diabetes comorbidity | 1.01 | 0.3150 |
| Cardiovascular disease comorbidity | 8.67 | 0.003 |
| Respiratory comorbidity | 3.76 | 0.052 |
| Postoperative complications | 49.35 | <0.001 |
| Postoperative complication (Pneumonia) | 24.26 | <0.001 |
| Postoperative complication (Anastomosis fistula) | 12.81 | <0.001 |
| Postoperative complication (Bleeding) | 0.58 | 0.446 |
| Postoperative complication (Pancreatic fistula) | 7.55 | 0.006 |
| Postoperative complication (Abscess) | 1.77 | 0.183 |
| Relapse | 43.75 | <0.001 |
During the follow-up period of the 475 patients, there were 170 deaths, accounting for a mortality rate of 35.8%. The follow-up period was 61.9 months in the open gastrectomy group and 46.7 months in the laparoscopic gastrectomy group.
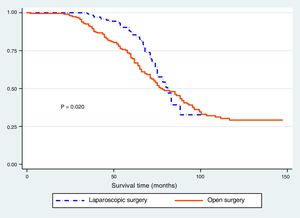
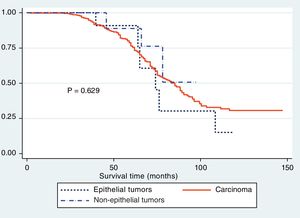
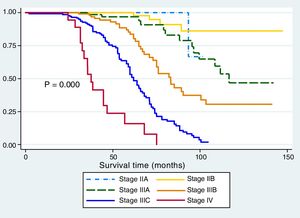
The Kaplan-Meier curves showed a statistically significant longer survival period in the patients that underwent laparoscopic surgery (fig. 1). The differences between histologic type and survival were not statistically significant (p > 0.05) (fig. 2), but Figure 3 showed statistical significance with respect to anatomopathologic stage (p < 0.05).
The multivariate Cox regression analysis produced no statistically significant difference in the type of surgery when adjustments for anatomopathologic stage, complications (fistula), lymph node dissection, age, and sex were made (Table 4).
Cox model comparing the two surgical techniques in patients with gastric cancer at the Hospital Nacional P.N.P “Luis N. Sáenz”, 2005 - 2014 (n = 475).
| Characteristics | Hazard Ratio | p | 95%CI | |
|---|---|---|---|---|
| Inferior | Superior | |||
| Type of surgery | 1.07 | 0.750 | 0.72 | 1.58 |
| Anatomopathologic stage IIIB | 2.83 | 0.001 | 1.54 | 5.20 |
| Anatomopathologic stage IIIC | 8.99 | 0.000 | 5.10 | 15.88 |
| Anatomopathologic stage IV | 33.66 | 0.000 | 16.14 | 70.20 |
| Complication (Anastomosis fistula) | 2.93 | 0.003 | 1.35 | 4.64 |
| Lymph node dissection | 0.61 | 0.005 | 0.44 | 0.86 |
| Age | 1.04 | 0.000 | 1.02 | 1.07 |
| Sex | 0.65 | 0.021 | 0.45 | 0.94 |
In recent years, laparoscopic surgery has developed into one of the most important and alternative procedures in many interventions in which open surgery had previously been the only indication,5 and gastric surgeries are no exception. Since Kitano's description12 of the use of laparoscopic surgeries in early gastric cancers, its performance has been increasing, together with improvements in laparoscopic surgical instruments and the perfecting of the surgical technique.9
Subtotal gastrectomy was the most frequently performed procedure in our study and its use was significantly higher in the laparoscopic surgeries. Kim et al.13 reported similar results that were statistically significant, with 133 subtotal surgeries out of 146 laparoscopic gastrectomies. Hao et al.14 and Hu et al.15 also described a more frequent use of subtotal gastrectomy, albeit without statistical significance. However, in the study by Tu et al. there was a higher number of total surgeries in both groups.16
In relation to lymph node dissection in our study, we found that D2 dissection was carried out more frequently in the laparoscopic surgery group, reaching statistical significance. Similarly, Lee et al. reported that D2 dissection was carried out in all the cases of both groups9 and Hao et al. described the performance of D2 dissection in more than 90% of the cases in the two groups.14 However, the differences were not statistically significant in the two studies. Those findings indicate that an important percentage of D1 lymph node dissection continues to be carried out in Peru, despite the current suggestions in the international guidelines. That situation should be changed, because radical surgery, which is sought in advanced gastric neoplasias, is not being performed.17
The mean levels of hemoglobin and albumin were significantly higher in the laparoscopic gastrectomy group. Said difference could be explained by the fact that patients in better condition were operated on with the laparoscopic procedure, due to selection bias, given that more stable patients tend to be selected for laparoscopic surgeries. However, it is striking that very few survival studies mention the values of those very important parameters and the differences between them. Moisan et al. found hematocrit was higher in the open surgery group, compared with laparoscopic surgeries,11 whereas Huscher et al.18 reported that preoperative hemoglobin was 11.9g/dl in the open gastrectomy group and 12.9g/dl in the laparoscopic gastrectomy group. As in our study, Moisan et al.11 and Shida et al.19 found that albumin values were higher in the laparoscopic gastrectomy groups. None of the cases showed statistical significance.
We found statistically significant differences between the groups in relation to anatomopathologic stages. The laparoscopic gastrectomy group presented with earlier disease stages and the open gastrectomy group with advanced-stage disease, which can be explained by the fact that the decision to perform laparoscopic surgery is based on higher patient outcome expectations and the selection of more stable patients. That was corroborated by Shida et al.19 and Kim et al.,13 who reported that laparoscopic gastrectomy was performed on a majority of patients with stage I and stage II disease, and open gastrectomy was performed on a majority of patients with stage III tumors. Their results were statistically significant and were the same when the stages were separated into T and N extension stages.
Lu et al.20 indicated that surgery duration above 240min was a risk factor for morbidity in patients undergoing gastrectomy, and it is a widely studied variable. We found a significantly longer operation time in the laparoscopic group, concurring with the majority of studies that report statistically significant longer laparoscopic surgery duration.5,7,10,11,13–15,19,21–27 Other studies show no differences between the two groups.28–30 Nevertheless, some studies have reported a statistically significant lower surgery duration in laparoscopic gastrectomies.16,31,32 A shorter operating time has also been observed thanks not only to technologic improvement and the development of better instruments, but also when the procedure is performed by an experienced surgeon, in which duration can be the same as in open surgery.20
We found that the time in which oral diet could be resumed was significantly shorter in the patients that underwent laparoscopic gastrectomy, as a result of less injury to adjacent organs and better postoperative recovery, which are characteristic of laparoscopy. The same was reported in the majority of studies with statistically significant results in favor of laparoscopic gastrectomy.21
In the present study, comorbidities were greater in the open gastrectomy group, with statistical significance in the respiratory and cardiovascular comorbidities. That was not surprising, given that it is much more frequent, as mentioned above, for the more stable patients to be placed in a laparoscopy group and those with a problem to be placed in an open gastrectomy group. Most studies only describe the presence or absence of comorbidities15,22,23 or the number of comorbidities,20,23 but not their specific types, and with no differences between groups in any of the results.
There was a greater number of postoperative complications in the open surgeries in our study. In their study, Lu et al.24 found a statistically significant higher number of postoperative complications in the open surgery group, within the first 30 postoperative days. Hao et al.14 reported that postoperative morbidity was statistically lower in the laparoscopic group, but there was no significant difference in complications between the two groups. Likewise, Lin et al.30 reported that the postoperative complications were significantly fewer in the laparoscopic group. The mortality rate was also lower, but with no statistical significance. In the study by Strong et al., they found greater early and late complications in the open gastrectomy group, reaching statistical significance. In contrast, other authors reported no gastrectomy group predilection and no differences in their results.26
Pneumonia presented in 16.6% of our cases, with a statistically significant predominance in the open surgery group in the bivariate analysis. The complications that followed in frequency were pancreatic fistula and anastomosis fistula, with no differences between groups in the bivariate analysis. Similarly, pneumonia was the main complication in the study by Lu et al.24 The most frequent complication reported by Kim et al.13 was intra-abdominal abscess. Intra-abdominal abscess and anastomosis leakage were the most frequent complications in the studies by Shida et al.19 and Inokuchi et al.25 In addition, Hao et al. reported that duodenal stump leakage, bowel obstruction, and incisional wound infection were observed with greater frequency.14
In relation to histologic type, adenocarcinoma was the most frequent in our patients, correlating with that described in the epidemiology of gastric cancer, and was followed by non-epithelial tumors. Strong et al.26 reported that laparoscopic surgery was the procedure of choice in stromal gastric tumors, given that node dissection is not as important as in adenocarcinomas. Chen et al.33 reported that, regarding stromal tumors, there were more complications in the open surgery group, reaching statistical significance.
Upon evaluating survival in relation to each of the study variables we found that age in years, type of lymph node dissection performed, hemoglobin and albumin levels, clinical anatomopathologic stage, comorbidities, and postoperative complications were the variables that affected survival in patients with gastric cancer. Different studies have also evaluated survival in relation to other variables in addition to type of surgery. Kashihara et al.32 showed that the presence of tumor differentiation, tumor invasion, metastasis to lymph nodes, staging, and venous and lymphatic invasion were statistically significant risk factors, and that in the multivariate analysis, tumor invasion and lymph node metastasis were survival risk factors. In their multivariate analysis, Chen et al.7 found that pathologic stage T4a, N+, and lack of adjuvant chemotherapy were prognostic factors for poor survival.
Finally, the overall survival in our two study groups was 59.9%, and in the Kaplan-Meier curve comparison of the two types of surgery, initial survival was better in the laparoscopic gastrectomy group, reaching statistical significance. However, in the multivariate analysis (Cox regression) that significance was lost. Our results coincide with the recent studies we reviewed throughout the bibliographic material, showing that the main advantage of laparoscopic gastrectomy is not its better survival profile, but rather its possible benefits in relation to faster recovery, shorter oral diet resumption time, and fewer postoperative complications, resulting in better postoperative recovery.
The main limitation of the present study was the difficulty in group homogeneity, making future studies comparing more homogeneous groups (for example, in disease severity) necessary.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Panduro-Correa V, Dámaso-Mata B, Loza-Munárriz C, Herrera-Matta JJ, Arteaga-Livias K. Comparación de gastrectomía abierta frente a laparoscópica en cáncer gástrico avanzado. Revista de Gastroenterología de México. 2020;85:32–41.