Complementary feeding (CF) is defined as the feeding of infants that complements breastfeeding, or alternatively, feeding with a breast milk substitute, and is a process that is more than simply a guide as to what and how to introduce foods. The information provided by healthcare professionals must be up-to-date and evidence-based. Most of the recommendations that appear in the different international guidelines and position papers are widely applicable, but some must be regionalized or adapted to fit the conditions and reality of each geographic zone. The Nutrition Working Group of the Latin American Society for Pediatric Gastroenterology, Hepatology and Nutrition (LASPGHAN) summoned a group of experts from each of the society’s member countries, to develop a consensus on CF, incorporating, whenever possible, local information adapted to the reality of the region. The aim of the present document is to show the results of that endeavor. Utilizing the Delphi method, a total of 34 statements on relevant aspects of CF were evaluated, discussed, and voted upon.
La alimentación complementaria (AC) se define como la alimentación de los lactantes que complementa a la lactancia humana o en su defecto, a la lactancia con un sucedáneo de la leche humana, y es un proceso que va más allá de simplemente una guía sobre qué y cómo introducir los alimentos. La información brindada por parte de los profesionales de la salud debe ser actualizada y basada en evidencia. Existen diferentes guías o documentos de posición a nivel internacional, que, aunque la mayoría de las recomendaciones pueden ser aplicables, hay algunas otras que requieren una regionalización o adecuación a las condiciones y realidad de cada zona. El grupo de trabajo de Nutrición de la Sociedad Latinoamericana de Gastroenterología, Hepatología y Nutrición Pediátrica convocó a un grupo de expertos, representantes de cada uno de los países que conforman la sociedad, con el objetivo de desarrollar un consenso sobre AC, que incorporó cuando así fue posible, información local que se adapte a la realidad de la región. El objetivo de este documento es mostrar los resultados de dicho trabajo. A través de metodología Delphi, se evaluaron, discutieron y votaron un total de 34 declaraciones o enunciados con respecto a aspectos relevantes de la AC.
Complementary feeding (CF), defined as the feeding of infants that complements breastfeeding, or alternatively, feeding with a breast milk substitute, is a process that is more than simply a guide as to what foods to introduce and how to do so. It is a process that encompasses different aspects, such as the correct time to introduce foods, the favoring of a responsive CF style (creating a proper atmosphere, considering sensory aspects, interpreting hunger and satiety cues), cultural aspects, and parent and/or caregiver perception. It also implies a progressive change of textures to promote the movements of the tongue, lips, and jaw, for ensuring the correct development of the organs involved in chewing, speaking, and pronunciation. In addition, it is a very important period for establishing food preferences that will be lasting in later stages.1 It is important to analyze each of the factors to carry out a feeding process that is adequate and satisfactory for each infant and his/her parents and/or caregivers. Information provided by healthcare professionals must be up-to-date and science-based. Most of the recommendations that appear in the different international guidelines and position papers are widely applicable, but some must be regionalized or adapted to fit the conditions and reality of each geographic zone.2,3 A recent survey of a group of Latin American healthcare professionals revealed that knowledge about CF was still incomplete and insufficient.4 Thus, the Nutrition Working Group of the Latin American Society for Pediatric Gastroenterology, Hepatology and Nutrition (LASPGHAN) convened a group of experts from each of the society’s member countries, to develop a consensus on CF that incorporated, when possible, local information adapted to the reality of the geographic region. The aim of the present document is to show the results of that endeavor.
Materials and methodsThe Nutrition Working Group of the LASPGHAN summoned a group of expert specialists from each of the member countries, including Spain and Portugal. Five working subgroups were formed to cover the different topics of CF, and they were coordinated by members of the Nutrition Working Group of the LASPGHAN (RVF, LLM, MCBM, VHR, and EO), who acted as facilitators. Each of the participants was randomly assigned to one of the 5 working subgroups. The facilitators formulated a series of statements, according to the different topics, and then searched for evidence that supported the statements. An initial information search was conducted in the following databases: The Cochrane Central Register of Controlled Trials (CENTRAL), PubMed (MEDLINE), and Ovid (EMBASE), encompassing the period from January 1, 1990 to October 31, 2019. The keywords for the bibliographic search were the following MeSH terms: “breastfeeding”, “bottle feeding”, “complementary feeding”, “dietary sucrose”, “dietary sugars”, “feeding behaviors”, “feeding methods”, “honey”, “immune tolerance”, “infant feeding”, “infant food”, “infant formula”, “infant nutrition”, “meals”, “micronutrients”, “responsive feeding”, “sugar-sweetened beverages”, “toddler feeding”, “water requirements”, “weaning” and their Spanish equivalents. All publications in English and Spanish were identified (original articles, consensuses, guidelines, systematic reviews, and meta-analyses), as well as publications the coordinators considered relevant, and they were shared with the entire group. The information corresponding to each subgroup was analyzed and the different statements were adapted and perfected. The initial face-to-face work meeting was conducted at the LASPGHAN congress in November 2019, where the statements were first presented. An external advisor, who is an expert on the subject (BK), participated. Each subgroup extracted the information that supported the different statements, through the creation of evidence tables, when possible. A second virtual meeting was then carried out, at which the final statements were presented and the support for each one was demonstrated. All the attending participants had the opportunity to make comments on and ask questions about the different statements. Each of the statements was then evaluated through a Delphi process of anonymous electronic voting (with the possibility of writing comments) to determine the level of agreement on the statements. Each statement was evaluated on a 3-point Likert scale: a) in agreement, b) in disagreement, and c) abstained. Only the representatives of each country participated in the voting; the coordinators/facilitators did not. After the first voting round, the results were presented in a virtual work meeting, at which the participants commented on the corresponding statements. The statements reaching consensus (≥ 75% in agreement) were accepted; those that did not (< 75% in agreement) were reevaluated for either their elimination or their reformulation by the members of the subgroup that had worked on them, undergoing a second anonymous voting round, and so on successively, as many times as needed. The coordinators/facilitators of each subgroup analytically and synthetically carried out the corresponding part of the manuscript.
ResultsTwenty-one representatives of the member countries of the LASPGHAN participated: Argentina, Bolivia, Brazil, Chile, Colombia, Costa Rica, Cuba, the Dominican Republic, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Portugal, Spain, Uruguay, and Venezuela. Nineteen (90.5%) of the participants were physicians, half of whom specialized in pediatric gastroenterology and nutrition and the remaining participants were nutritionists (with a degree in nutrition as their core training) trained in pediatric nutrition. A total of 34 statements were formulated. After a single voting round and discussion, 33 statements with a consensus above 75% were included in the final document presented herein.
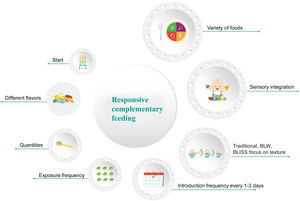
The CF process is more than the simple selection of the time for starting said feeding or choosing the first food. Despite the fact that there are new trends or focuses on the introduction of textures, they are only one of all the important aspects to be taken into account. Fig. 1 highlights the most important elements in the entire CF process. Statement 1. Exclusive breastfeeding for the first 6 months of life is recommended for healthy infants in the regions of Ibero-America (in agreement: 100%)
Breast milk is the best feeding option because of its short-term and long-term benefits.5,6 It positively impacts infant survival, not only due to its supply of energy and nutrients, but also because of protective immunologic factors.7 In a systematic review, having been breastfed for a given amount of time, was shown to reduce gastroenteritis by 65% and sudden death syndrome by 36%. Exclusively breastfeeding for 3 months reduced otitis media by 50% and atopic dermatitis by 40%. Likewise, breastfeeding for at least 3 months reduced the odds of developing asthma by 40% and obesity and diabetes by 20%. Breastfeeding for 4 months reduced respiratory diseases by 70% and breastfeeding for 6 months reduced the possibility of developing acute leukemia by 20%.8 Exclusive breastfeeding influences key aspects, such as physical growth, neurologic development, taste acceptance, allergy risk reduction, dental malocclusion, and bonding.9 Statement 2. Complementary feeding should be promoted and explained based on a responsive feeding style (in agreement: 100%)
Different articles and studies highlight the reciprocity between child and caregiver that the feeding process involves.10 Responsive CF is based on hunger and satiety cues from the child, recognition of said cues, a correct response by the caregiver, and finally, the predictable reaction of the child. It is important for caregivers to understand that the gastric capacity of infants is limited, and so they need to be fed with portions and volumes that are appropriate for their age and developmental stage and with the frequency necessary for satisfying their nutritional needs.11 Statement 3. In healthy Ibero-American infants that are exclusively breastfed, complementary feeding should be started at 6 months of age (in agreement: 100%)
When exclusively breastfed infants reach 6 months of age, it is difficult to meet their nutritional requirements, especially those of energy, iron, and zinc.12 In addition, the majority of infants have developed sufficiently to be able to receive other foods. In geographic regions with unfavorable environmental conditions, breastfeeding during that period helps reduce exposure to infectious diseases or foodborne illnesses.2,13 Premature interruption of breastfeeding or a low supply of breast milk can contribute to an insufficient supply of nutrients and energy, leading to an elevated risk for malnutrition, if foods with low nutritional quality, and in inadequate quantities, are given too soon.14,15 A systematic review16 showed that educational interventions improved CF practices, with respect to the duration of exclusive breastfeeding, the appropriate age for introducing complementary foods, and associated hygiene habits. Statement 4. The establishment of complementary feeding does not imply the suspension of breastfeeding, which should be maintained at least until two years of age (in agreement: 90.5%, abstained: 9.5%)
Breast milk can supply half or more of the energy requirements in the 6-to-12-month-old infant and one-third of the energy requirements and those of other nutrients in the 12-to-24-month-old infant.13,17 Breast milk continues to supply better quality nutrients than those of complementary foods, as well as providing protective factors. Breast milk is a key source for obtaining energy and nutrients during illness and reduces mortality in malnourished infants.18 Likewise, during the period of 6 to 24 months of age, the majority of food habits, preferences, and aversions that largely condition the type of future eating are established.19,20 Statement 5. In partially or totally formula-fed infants, complementary feeding can be started at 4 months of age (in agreement: 85.7%, in disagreement: 4.7%, abstained: 9.5%)
Currently, age is the only parameter for deciding when to start CF in the infant that has no safety problem related to swallowing. The window of time for starting is from 4 to 6 months of age.2,3 Contrary to what was previously referred to, and recently reaffirmed by the European Feeding Safety Authority (EFSA), neither supposed gastrointestinal, renal, dental immaturity, etc., nor the developmental milestone of sitting up without help, are limitations for deciding to start CF, given the lack of confirmatory evidence.21 The risk of early introduction of complementary foods, before 4 months of age, can include the possibility of choking, an increase in acute gastroenteritis and upper respiratory tract infections, interference with the bioavailability of iron and zinc from breast milk, and the substitution of milk by other less nutritious foods (inadequate breast milk substitutes).22 Statement 6. The weight of an infant should not be an indicator for starting or delaying complementary feeding (in agreement: 95.2%, abstained: 4.7%)
The introduction of CF should not be different from the recommendations for the healthy infant, with respect to low weight infants or infants with excessive weight gain, albeit there is very little or no scientific information on the subject. There is no consistent evidence of an association of the age at which CF is introduced with a later risk for overweight or obesity. Therefore, there is no need to delay starting CF, to have a protective effect.23 In our social context, the introduction of complementary foods is recommended at 6 months of age, following the recommendations of the World Health Organization (WHO) for the promotion of exclusive breastfeeding for the first 6 months of life. Statement 7. Complementary feeding in the preterm infant can be started between 4 and 6 months corrected age (in agreement: 90.5%, in disagreement: 4.7%, abstained: 4.7%)
Some guidelines indicate that the preterm infant is prepared for starting the CF process when he/she has lost the extrusion reflex, accepts the spoon (oral hypersensitivity is reduced), and his/her feeding already meets the specific requirements.24 Delaying the introduction of CF can affect growth and neurodevelopment and early CF introduction can increase the risk for infection and hospital admission.25,26 Based on the limited evidence available, the recommendation is to wait until 6 months corrected age. Introducing CF before 4 months corrected age is not recommended.27,28 There is no evidence on the effects of introducing complementary foods at 4 or 6 months corrected age on weight, height, and head circumference.21 Statement 8. Complementary feeding in exclusively breastfed infants should be started and maintained with foods that have high iron, zinc, calcium, vitamin A, and folate bioavailability, such as red meat, viscera and/or fortified infant cereals with no added sugar (in agreement: 100%)
Different studies have evaluated the effects of utilizing meat and fortified infant cereals as complementary foods on biochemical, anthropometric, and developmental parameters in exclusively breastfed infants.29–32 The recommendation is to supply from 0.9 to 1.3mg/kg/day of iron, first with source foods, and if not possible, with fortified foods, before starting supplementation, especially for infants between 6 and 12 months of age that have been exclusively breastfed up to 6 months of age. In places where there is a scarcity of foods of animal origin that are sources of iron, zinc, calcium, vitamin A, and folate, iron-enriched complementary foods should be offered,13 given that it is difficult to meet the iron requirements if such foods are not used.2 Statement 9. In the preterm infant, complementary feeding should include all food groups, giving preference to foods with higher energy and protein density and with sufficient iron supply (in agreement: 100%)
The WHO describes a good complementary food as one that is a satisfactory source of energy, protein, and micronutrients, such as iron, zinc, calcium, vitamin A, and folate. Likewise, it states the need to include foods of animal origin that are sources of iron and zinc and to use fortified foods as an alternative for filling critical gaps. Foods that are sources of iron and those that are iron-enriched should be given preference before using iron supplementation.13 Preterm infants have greater energy, protein, and iron requirements, compared with full-term infants.33,34 Thus, in addition to offering CF that is varied, as recommended for all infants, the greater requirements of energy and those nutrients must be met.33 Statement 10. A new food can be introduced every day, not delaying the introduction of new foods for more than every 3 days (in agreement: 95.2%, abstained: 4.7%)
The American Academy of Pediatrics (AAP) states that new foods should be introduced alone and for several days, to identify adverse reactions.3 However, adverse reactions can present immediately (hours, days) or be delayed (weeks),35 and so a new food can be introduced daily or every 2 or 3 days, but ideally not longer than that, because the number of new foods reached during the first month of CF may be insufficient for achieving a diverse diet, and as a result, provide minimally acceptable nutrition.36 Statement 11. Complementary feeding should be varied, and from the start, include all the food groups, as well as the 5 basic tastes. Four weeks after starting complementary feeding, at least 2 foods from each food group should be incorporated (in agreement: 100%)
The WHO has established the minimally acceptable diet indicator for children from 6 to 23 months of age. It consists of daily meal frequency and daily food diversity, making reference to receiving a minimum of 5 of the 8 food groups: 1) breast milk; 2) grains, roots, and tubers; 3) vitamin A-rich fruits and vegetables; 4) meat, fish, and poultry; 5) legumes, nuts and seeds; 6) eggs; 7) other fruits and vegetables; and 8) dairy products.36 In accordance as well with that stipulated by the AAP, the progression of foods from 4 groups: grains, meat, fruits, and vegetables, can be reasonably reached in the first month of CF.3
The number of foods depends on the number of exposures to each food. If the child is to be exposed to each food for 3 consecutive days, as indicated by the AAP,37 and at least one different food from each group is introduced during the first 2 weeks of CF, four food groups will be recognized. Thus, toward 6 and ½ months of age, the infant could have all the food groups on his/her plate, as indicted by Harvard University’s Kids Healthy Eating Plate, which includes 4 main food groups: vegetables, fruits, whole grains, and protein.38 At one month of having started CF, at least 2 or 3 foods from each group will have been incorporated, thus guaranteeing a healthy diet.13
Programming taste or food preferences starts in pregnancy, through the diet of the mother and the placental transport to the fetus,39 then by means of breast milk and the subsequent introduction of complementary foods, through which direct exposure to foods with different tastes takes place: sweet, salty, sour, bitter, and umami.40 To maintain the characteristics of a good complementary food, according to the WHO, the infant should be exposed to those tastes during the first two weeks of CF.13 For example, vegetables: broccoli (a predominantly bitter taste) and tomato (a predominantly umami taste); fruits: strawberries and mangoes (a predominantly sweet taste and bitter taste, respectively, depending in their ripeness); cereals: fortified infant rice and wheat cereals (mainly a neutral or salty taste); proteins; chicken or beef liver (bitter) and egg (mainly salty or neutral); and fats: avocado (neutral taste) and nuts (neutral or salty taste). Statement 12. In healthy formula-fed infants, complementary feeding can be started with any of the food groups, but at 2 weeks from having started, at least one food from each group should be offered (in agreement: 100%)
While the need in exclusively breastfed infants is to fill the iron and zinc gap,13 those nutrients are almost completely covered in formula-fed infants.41 In addition, there is no scientific evidence supporting one food or food group as the best option for starting CF; it depends on cultural and religious aspects and the socioeconomic situation of the family.3 Statement 13. From the start of complementary feeding, three mealtimes can be offered, after which one or two snacks can then be added (in agreement: 95.2%, abstained: 4.7%)
The transition regarding the number of meals is gradual during this period of growth.13 The appropriate number of meals to offer will depend on the appetite of the child, the quantity of foods eaten in each meal, the energy requirement, and the energy density, suggested to be a minimum of 0.8kcal/g, assuming a gastric capacity of 30g/kg of body weight/day. Four meals are recommended if the density of the foods is 0.8kcal/g and 3 meals if the density is 1kcal/g. Energy density is reached through greater food diversity.12,13,42 Snacks are defined as food eaten between main meals.3 If the child receives few meals or meals that are below the recommended density, he/she will not receive the sufficient quantity of food to meet his/her energy needs.13 Statement 14. Daily exposure to fruits and vegetables should be promoted, given that it results in their greater long-term acceptance (in agreement: 100%)
The first flavors the infant experiences are through the mother’s diet, by means of the amniotic fluid and then through breast milk. Formula-fed infants often receive a single type of flavor, limiting their experiencing different ones. Despite the lack of variety of flavors in formulas, their taste varies depending on the type, brand, composition, and processing. Infants have been shown to develop flavor preferences that reflect the type of formula they were fed.43,44
Even though children are born with a biologic predisposition to prefer sweet flavors and avoid bitter ones, such as those of dark green leafy vegetables, there are opportunities for repeated and varied exposures before starting CF to learn to enjoy the flavors of the foods they will receive during this process.43 Nevertheless, the development of food preferences begins mainly when infants discover the first solids. Those experiences aid in forming the brain connections involved in the pleasure of foods and the control in their consumption. Those learning processes likely have a long-term impact, and so it is imperative to establish preferences for fruits and vegetables when the infants are learning to eat.45 Sensory characteristics (texture, taste, and smell) and a variety of fruits and vegetables are important factors for their acceptance, due to the limited oral abilities typical of the infant’s age.45 Therefore, a frequent, opportune, and varied introduction of complementary foods is recommended, with respect to tastes and textures, for better acceptance of fruits and vegetables.43,46,47 Statement 15. Healthy breastfed infants require around 10 exposures to a food (particularly vegetables) to have a positive reaction and long-term acceptance. Formula-fed infants or infants that are more sensitive to tastes and textures may require 10 to 15 exposures to fruits and vegetables for their short-term or long-term acceptance (in agreement: 100%)
Repeated exposure to foods is one of the main determining factors for their acceptance, given that different stimuli are utilized (auditory, visual, olfactory, and tactile), increasing familiarity with them and reducing neophobic reactions. The effect of repeated exposure (8 to 10 exposures) is sufficiently strong for increasing the acceptance of foods that were previously rejected; even 10 to 15 exposures may be needed to increase the infant’s liking them.43,45,47,48 Several studies have found that daily exposures to vegetables during the CF period increase intake, liking, and pace of eating,49 as well as greater acceptance of new foods in infants that receive a wide variety of vegetables upon starting CF, including vegetables offered during the following month, increasing intake and liking of them for up to 6 years of age.50,51 Despite the efficacy of exposure to foods to achieve their acceptance, parents often offer a limited number of repetitions (<5), before deciding that the infant does not like a food. The mechanism of repeated exposure, at different times, and with the original taste, is completely effective for increasing the acceptance and liking of foods.43,48,51 Statement 16. The quantity of foods per meal during the complementary feeding period is between 3 and 4 spoonfuls for infants from 6 to 8 months of age, between 4 and 8 spoonfuls for infants from 9 to 11 months of age, and between 8 and 12 spoonfuls for infants between 12 and 23 months of age (in agreement: 95.2%, abstained: 4.7%)
With the understanding that a spoonful is equivalent to approximately 15g, the quantity of foods required gradually increases month to month, as the child becomes older and milk intake is reduced. Said quantity depends on the energy density (kcal/mL or g) of the food offered. Complementary foods should provide an energy density of 0.8 to 1kcal/g.13,52 In practice, the persons that feed the children do not measure the energy density of the foods given, and so the quantity of food offered is recommended to be based on the principles of responsive CF, paying attention to hunger and satiety cues.42 Statement 17. Starting with purees/mashed foods and progressing to lumpy textures and soft solids is recommended before 10 months of age, to reduce the risk for aversion to textures (in agreement: 100%)
A critical window of time is suggested to be before 10 months of age for introducing lumpy textures,12 because infants introduced to lumps after 10 months have been seen to be more selective at later stages and can present with greater eating problems, as well as a lower intake of fruits and vegetables.2,46,52,53 Infants introduced to foods with a lumpy texture after 9 months of age significantly presented with greater eating problems at 7 years of age. Thus, it is of the greatest importance for health professionals to encourage the progression from purees or mashed foods to foods with a lumpy texture before 10 months of age,53 due to the fact that offering foods with the correct texture is essential for development and for nutritional reasons.2 Statement 18. The focuses of Baby-Led Weaning or Baby-Led Introduction to SolidS (BLISS) should be assessed by a nutritionist or pediatrician with training in the subject. Parents should be completely aware of the risks they may involve (in agreement: 95.2%, abstained: 4.7%)
Said focus should be advised by a medical or nutrition professional trained in the subject to provide individualized recommendations regarding macronutrient and micronutrient intake, because the professional must address the possible concerns about iron status, choking periods, and lack of growth due to inadequate energy intake.54–56 Statement 19. Once complementary feeding is started, all foods should be introduced, including those considered potentially allergenic, such as eggs, fish, wheat, peanuts, peanut butter, soy, corn, seafood, and dairy products, regardless of a family atopic history (in agreement: 85.7%, in disagreement: 4.5%, abstained: 9.5%) Statement 20. Boiled eggs (with no need to separate the yolk and the white), fish, and peanut butter can be introduced at 4 months of age, in infants considered ready for starting complementary feeding (in agreement: 76.2%, in disagreement, 14.3%, abstained 9.5%) Statement 21. Exposure to foods considered potentially allergenic should not only be done opportunely, but also frequently, at least twice a week, to induce and maintain immunologic tolerance (in agreement: 85.7%, abstained: 14.3%) Statement 22. In infants with an allergy, the introduction of foods considered potentially allergenic should not be delayed (in agreement: 76.2%, in disagreement: 9.5%, abstained: 14.3%)
There is no convincing evidence that delaying the introduction of foods protects against the development of food allergies, including foods considered highly allergenic: fish, eggs, etc.2,57–60 Recent studies suggest that oral exposure to food allergens, between 3 and 6 months of age, reduces the risk for food allergy.61 Nevertheless, there is still little evidence, and the majority is of low-to-moderate certainty of effect. What is known is that there is no increase in the risk for food allergy, and so the introduction of those foods can be considered in that period. None of the studies have been conducted on Latin American populations. The goal of protecting breastfeeding, and in turn, the recommendation for maintaining it exclusively the first 6 months of life, continues to be reasonable.62,63 In addition, the introduction of CF does not necessarily promote weaning, as shown in the EAT study, which found that the introduction of foods between 3 and 6 months of age did not affect breastfeeding at 6 and 9 months of age.64 Regarding dairy products, they should be considered equivalent to other foods of animal origin and in no way should be compared with and/or replace breast milk, or alternatively, infant formula. Statement 23. Vegan, raw vegan, and macrobiotic complementary feeding practices are not recommended (in agreement: 100%)
Starting CF with a vegan, raw vegan, or macrobiotic system is not recommended, basically because those regimens may be deficient in iron, zinc, calcium, vitamin B2, vitamin B12, vitamin D, vitamin A, omega 3, and proteins.3,65,66 Studies on the safety of raw vegan, macrobiotic, or fruitarian diets were not identified, and so those practices are not recommended in the infant. There are few data on the medium-term and long-term impact of eliminating animal products from the diet of children, especially of the youngest. However, in recent years, tools have been published (food interchange tables, supplementation recommendations) that facilitate carrying out a vegan/vegetarian diet, reducing the risk for deficiencies.67 If a parent chooses a vegan diet for his/her child, it should be done with regular medical and expert dietary supervision and mothers should receive and follow the nutritional advice. Infants that cannot have breast milk should receive a soy-based formula. Statement 24. When caregivers request advice for implementing a vegetarian complementary feeding regimen, it should be carried out under strict supervision by a medical professional trained in nutrition (in agreement: 100%)
Vegan diets, with the appropriate supplements, translate into adequate growth and development. Periodic medical and dietary supervision must be a priority. Infants should be supplied with vitamin B12, vitamin D, iron, zinc, folic acid, omega-3 long-chain polyunsaturated fatty acids, proteins, and calcium. The risks of not following the advice are grave and include irreversible cognitive damage due to vitamin B deficiency, as well as death.3,67–69 Statement 25. Drinking water intake may be considered from the start of complementary feeding (in agreement: 100%)
Adequate water intake for infants from 6 to 12 months of age is 800ml/day and is calculated in relation to the supply of water from breast milk, at a mean volume of 600ml/day, in addition to the water present in complementary foods.70 Thus, infants that are adequately fed with breast milk do not require an additional supply of water. However, its introduction could be beneficial in relation to the forming of habits, without having a negative impact on the infant’s nutritional status.71
In non-breastfeeding infants, the renal solute load is greater, thus contributing to greater urine loss. According to mathematical calculations of the potential renal solute load of complementary foods, water should be included as part of an adequate dietary pattern in non-breastfed infants, to take care of the status of hydration and renal homeostasis.30 Statement 26. The daily quantity of drinking water during the complementary feeding period is approximately between 60 and 150ml for infants from 6 to 8 months of age, between 240 and 300ml for infants from 9 to 11 months of age, and between 450 and 600ml for infants from 12 to 23 months of age (in agreement: 81%, abstained: 19%)
The liquid requirement for non-breastfed infants depends on the renal solute load of the complementary foods, estimated between 470 and 500ml/day for infants from 6 to 9 months of age, between 450 and 530ml/day for infants from 9 to 12 months of age, and between 340 and 470ml/day for infants from 12 to 24 months of age. The liquids can be provided by water and other drinks or foods.30 In a study conducted in Guatemala on breastfed infants from 7 to 12 months of age, the recommended liquid intake (797ml/day) was found to be met mainly by breast milk, followed by water in drinks, soups and broths, and then at a lower proportion in water contained in solid and semisolid foods, and only under 5% came from plain water at around 30ml per day. Those authors emphasized the need for a lower volume of high nutritionally dense complementary foods to not displace the contribution of breast milk.72 This could lead to increasing the volume of water, an essential nutrient that is not widely studied for this stage of life. Statement 27. Adding sugar to foods during the infant’s first two years of life is not recommended (in agreement: 100%)
The innate preference for the sweet flavor has been described in humans, even before birth.73 Breast milk has a sweetness intensity similar to that of a 2.12% sucrose solution, causing a hedonic response and favoring its higher intake.74 Likewise, very early on, infants learn to relate the sweetness of breast milk to affection and nurturing.75 Frequent exposure to complementary foods with sugar can increase the preference for sweetness and have an impact on the choice of foods, and the risk for dental caries,76 excess weight,77 and nontransmissible chronic diseases.78 In a cohort of low-income families from Porto Alegre, Brazil, the incidence of dental caries at 38 months of age was determined, according to 3 dietary patterns. Incidence was higher in the children that received sweet foods (candies, cookies, sugar-sweetened beverages) at 6 and 12 months of age and the risk increased the higher the intake. Those results were maintained even when sugar-sweetened drinks were eliminated.76 Likewise, around 40% of children were found to eat free sugar and natural sugar at 12 months of age, which was associated with a greater risk for excess weight at 30 months of age.77 Statement 28. Honey intake is not recommended in infants under 2 years of age, given the potential for its contamination with Clostridioides botulinum spores (in agreement: 100%)
Infant botulism is caused by Clostridioides botulinum (C. botulinum) spores that colonize the gastrointestinal tract and produce the botulinum toxin that is responsible for blocking voluntary and autonomic motor functions.79 Infants under 12 months of age are particularly susceptible, possibly due to their microbiota.80 The environment (dust and soil) is the main source of the C. botulinum spores that contaminate honey. Honey intake has been reported in 59.2% of the cases of infant botulism described in Europe.80 Therefore, its ingestion in infants under 12 months of age is not recommended. Following the same line of recommendations for added sugar, whose early introduction favors greater long-term ingestion and having an impact on the nutritional status, the introduction of honey is not recommended in the infant’s first 24 months.77 Statement 29. The intake of natural and industrialized juice, as well as sugar-sweetened beverages, is not recommended in an infant’s first 2 years of life (in agreement: 100%)
Sugar-sweetened drinks, specifically juices, are a complementary food group that is very frequently offered to infants. In Mexico, according to a 2012 national health survey, more than 35% of infants between 6 and 23 months of age drank sugar-sweetened beverages.81 That practice has been related to greater intake of those beverages in preschool and school age children,82 dental caries,83 the risk for overweight and obesity,84 greater adiposity,85 and cardiovascular diseases.78 Six-year-old children that drank at least one sugar-sweetened beverage daily had been exposed to those drinks some time before 12 months of age.82 The prevalence of obesity at 6 years of age is double in children that have drunk sugar-sweetened beverages in their first year of life. In turn, infants that drank sugar-sweetened beverages had a higher BMI-for-age z-score.82 Twelve-month-old infants whose juice intake was equal to or greater than 480ml, had a higher intake of sugar-sweetened drinks and a higher BMI-for-age z-score in the preschool and school-age periods.85 Along with the WHO,86 the European Society for Pediatric Gastroenterology, Hepatology and Nutrition,87 and the American Heart Association,78 the recommendation of the present guidelines is to avoid exposure to foods with sugar and sugar-sweetened beverages in children under 2 years of age. Statement 30. The intake of caffeinated beverages, teas, infusions, carbonated drinks, plant-based drinks (almond, oat, rice, soy, and coconut, among others), artificially sweetened drinks, and broths in the infant’s first 2 years of life are not recommended. Soups are allowed, when the preparation supplies a minimum ¾ of solid foods (in agreement: 90.5%, in disagreement: 9.5%)
Beverages with low nutritional value should be avoided in CF, given that they can displace foods with better and greater nutritional density. In addition, drinks, like tea and coffee, can interfere with the absorption of other critical nutrients, such as iron, due to their content of polyphenols.88 Plant-based drinks are not adequate substitutes for breast milk, infant formula, or cow’s milk, and their nutritional composition can be inadequate in relation to protein supply, added sugar, calcium, and vitamin D. Thus, they can increase the risk for malnutrition, anemia, electrolyte disorders, and other nutritional deficiencies.89,90 The use of plant-based drinks based on rice should be avoided, particularly due to their probable arsenic content. The use of foods and drinks with noncaloric sweeteners is not recommended, given that there is inconclusive and insufficient scientific evidence for making an evidence-based recommendation.91 Soups contribute to the daily liquid supply, but can affect the energy and nutritional density of complementary foods, and so a higher concentration of solids than liquids should be sought.30,70 Statement 31. The rational use of salt in food preparation is considered acceptable (only for the preparations) from 12 months of age (in agreement: 95.2%, abstained: 4.5%)
Little has been studied about the sodium requirement in infants and its necessity in complementary foods. The adequate recommended intake for infants from 7 to 12 months of age by the Institute of Medicine (IOM) is 370mg/day.70 That requirement is met on average with the adequate intake of breast milk and infant formula, as well as with the sodium content in the complementary foods, making it totally unnecessary to add salt to preparations. The inadequate use of certain complementary foods rich in sodium (baked goods, cheeses, and breakfast cereals) can contribute to excessive salt intake. In the majority of infants from 8 to 12 months of age, the adequate intake of 400mg/day is exceeded by cow’s milk as the main drink and three pieces of bread per day.92 Early salt intake favors the preference for salty foods,40 and so it is important that infants know the original flavor of foods before seasoning them with salt or other condiments. Likewise, there is evidence that the excess of sodium from this first stage of life can also impact blood pressure and cardiovascular risk in the long term.93 From one year of age, the adequate sodium intake is 1g per day,70 allowing the addition of salt to food preparations. In the same way, the majority of foods already should have been introduced to and known by the infant in their original presentation and flavor. Breast milk contains iodine, but its concentration can vary depending on intake and maternal reserves. Concentrations from 150 to 180mg/l in breast milk indicate sufficient mother-child iodine.94 Infants receive from 40-45% of the iodine requirement from breast milk.95,96 During the CF period, the supply from breast milk plus complementary foods is indispensable for preventing deficiencies.97 Iodine content in fruits and vegetables is dependent on the iodine from soil, the use of fertilizers, and irrigation practices, in turn, affecting the iodine content in products of animal origin.98 In infants from 6 to 23 months of age, in countries or regions in which iodized salt is not common (<90% of homes), and/or the median maternal urinary iodine in the population is <100μg/l, the WHO recommends maintaining breastfeeding, as long as the mother is supplemented with iodine, given that supplementation in the mother is more efficient than supplementation in the infant,99 together with eating iodine-enriched complementary foods.100 For non-breastfed infants, fortified formulas aid in meeting the iodine requirement, and in zones with adequately efficacious iodized salt programs, additional supplementation is not necessary.101 Statement 32. Spices may be used as condiments in food preparation, preferably after the infant has been exposed to the foods in their original taste (in agreement: 81%, in disagreement: 9.5%, abstained: 9.5%)
Complementary foods should not only meet nutritional requirements, but also offer a variety of textures, tastes, and temperatures that allows the infant to experience different sensations and responses. The innate preference for sweet and salty flavors 73 can be molded by flavor experiences in which umami, bitter, and sour tastes are offered. The use of condiments contributes to those experiences and can be used once the infant has been exposed to the original flavor of the complementary foods. The safety of fennel (in oil and/or tea form) has been a subject of debate in children, especially infants, due to its estragole content, its carcinogenic genotoxic effect, and the lack of evidence for that age group.102 Even though some studies suggest that the content of estragole in fennel teas or in foods containing its essence can surpass the maximum doses allowed, models have also been described in which estragole extraction and absorption is around 2.5% (compared with the 25–35% proposed by the EFSA in 2009) and that it would be practically impossible to surpass the maximum dose. For safety, the present guidelines recommend avoiding fennel tea and oil in the first two years of life, without contradicting the possibility of its introduction.
Vitamin supplementationGiven that a varied CF style implies that the required vitamin and mineral supplies are met, vitamin supplementation is not considered necessary during the CF period, unless a deficiency in any of them, secondary to a pathologic status, is shown. Statement 33. Exclusively breastfed infants can receive a daily supplementation of 400 IU of vitamin D3 for the first 12 months of life. Note: If biochemical analysis shows normal values for a nutrient, its supplementation should not be started or it should be suspended (in agreement: 95.2%, abstained: 4.7%)
Vitamin D deficiency is common worldwide, favoring rickets and osteomalacia, which have a substantial impact on the health, growth, and development of infants, children, and adolescents. Even when breast milk is the best option for feeding infants, it has a low level of vitamin D and exclusively breastfed infants are at risk for vitamin D deficiency. Vitamin D supplementation at a dose of 400 IU/day for infants increases the levels of 25-OH vitamin D and reduces its incidence of deficiency.103 Likewise, vitamin D deficiency has increased in infants due to changes in lifestyle, dressing habits, and the use of topical sunscreen.
For maintaining adequate serum vitamin D concentration, all exclusively or partially breastfed infants should receive daily oral vitamin D3 supplementation, starting the first days of life and continuing until the infant has been weaned and ingests at least 1l/day of vitamin D-enriched infant formula,104–106 considering that on average, 950ml of a breast milk substitute meets 85% of the vitamin D requirement.
With respect to iron supplementation, no consensus was reached, and so for the time being, it should be continued as traditionally carried out. Given the relevance of this topic, it needs to be more profoundly analyzed, and a recommendation will be emitted in another document dealing expressly with that theme.
Adoption and adaptation of this consensusThe Nutrition Working Group of the LASPGHAN invites all healthcare professionals to adopt the guidelines on CF presented herein, which are based on the best currently available evidence, and make the parents and/or caregivers of children of the region aware of the CF recommendations. In addition, knowing that there are differences among countries, even among different regions of the same country, we invite health professionals to make adaptations to this consensus that will enable it to be applicable to the populations each of us treats.
ConclusionsCF should be implemented utilizing a responsive feeding style. The time to start CF in an infant is from 4 to 6 months of age, favoring 6 months of age in exclusively breastfed infants. From the start of CF, diverse feeding should be offered that includes the different food groups, with foods that are available in the region and by season. CF is not different for children with or without a risk for allergies or even in infants diagnosed with other allergies. Studies conducted in the regions are needed, to increase the strength of the recommendations reached by consensus and presented herein.
Ethical considerationsGiven that the present consensus document is based on the best scientific evidence published, patient informed consent for receiving treatment to participate in the study was not required. No experiments were performed on animals and/or humans.
Due to the descriptive nature of the document and because it is a position paper of the association, authorization by an ethics committee was not required.
The authors declare this article contains no personal information that could identify the patients.
Please cite this article as: Vázquez-Frias R, Ladino L, Bagés-Mesa MC et al. Consenso de alimentación complementaria de la Sociedad Latinoamericana de Gastroenterología, Hepatología y Nutrición Pediátrica: COCO 2023. Rev Gastroenterol Méx. 2023;88:57–70.