Methane (CH4) is an inert gas produced by colonic anaerobes and has been associated with different intestinal diseases, including irritable bowel syndrome (IBS). According to geographic region, the prevalence of methanogens varies, being higher in Africa (80%) and lower in the United States (35-40%). In Mexico, the prevalence of methanogens is unknown.
AimTo evaluate the prevalence of CH4 producers and associated factors in a group of patients with IBS and controls in a Mexican population.
Materials and methodsA baseline fasting measurement of alveolar H2 and CH4 gas was carried out, by gas chromatography (stationary phase), in consecutive patients diagnosed with IBS and a control group. Subjects with baseline levels of H2 of 0 ppm and CH4 ≥ 5 ppm were classified as methanogenic.
ResultsA total of 132 controls (53.8% women) and 67 patients with IBS (76% women) were included. The overall prevalence (n = 199) of methanogenic subjects was 38% (n = 76) (95% CI: 0.31-0.45) and they had a greater prevalence of overweight/obesity (56.5 vs 39.8%, P = .028). The prevalence of methanogens in the healthy controls was 41.6% (95% CI: 0.33-0.49), whereas, in the patients with IBS, it was 31.4% (n = 21, 71% IBS-C and 29% IBS-M).
ConclusionsThe prevalence of methanogens in our study on a Mexican population was comparable to that reported in other populations and was associated with overweight/obesity. One-third of the patients with IBS presented with methanogens. Said microorganisms were particularlyassociated with the constipation-predominant IBS subtype.
El metano (CH4) es un gas inerte producido por anaerobios colónicos y se ha asociado a diferentes patologías intestinales incluyendo al síndrome de intestino irritable (SII). De acuerdo con la región geográfica la prevalencia de metanógenos es variable siendo mayor en África (80%) y menor en Estados Unidos (35-40%). En México, se desconoce cuál es la prevalencia de metanógenos.
ObjetivoEvaluar la prevalencia de productores de CH4 y los factores asociados en un grupo de pacientes con SII y controles en población mexicana.
Material y métodosSe realizó de forma basal la medición en ayuno de gas alveolar de H2 y CH4, a través de cromatografía de gases estacionaria a pacientes consecutivos con diagnóstico de SII y un grupo control. Se clasificaron como metanógenos a aquellos sujetos con niveles basales de H2 de 0 ppm y CH4 > 5 ppm.
ResultadosSe incluyeron 132 controles (53.8% mujeres) y 67 pacientes con SII (76% mujeres). De forma global (n = 199) la prevalencia de metanógenos fue de 38% (n = 76) (IC 95% 0.31-0.45). Los sujetos metanógenos tuvieron mayor prevalencia de sobrepeso/obesidad (56.5 vs 39.8%, p = 0.028). La prevalencia de metanógenos en controles sanos fue de 41.6% (IC 95% 0.33-0.49) mientras que en los pacientes con SII fue de 31.4% (n = 21, 71% SII-E y 29% SII-M).
ConclusionesLa prevalencia de metanógenos en México es comparable con lo reportado en otras poblaciones y se asoció con sobrepeso/obesidad. Una tercera parte de los pacientes con SII son metanógenos y esto se asoció en especial con el subtipo estreñimiento.
Different gases released by the microbiota at the level of the intestine have been detected, and the three most abundant are carbon dioxide (C02), hydrogen (H2), and methane (CH4). Those gases were initially thought to be inert, but Ignarro et al.1 found that nitic oxide (NO), another gas produced by endothelial cells, controls smooth muscle tone, increasing the relevance of the effect of those gases produced at the intestinal level on intestinal function.
Carbohydrate fermentation at the intestinal level results in the production of different gases: H2, CO2, hydrogen sulfide (H2S), and CH4. A portion of H2 and CH4 is absorbed in the colon, transported to the lungs, and excreted in exhaled air,2 through breath, and their remaining portions are expelled through flatus.3 Those small molecules can cross the plasma membranes, and move into tissues by concentration gradients. Thus, they can have local access to the components of the intestinal wall, including the neuromuscular pathway, and consequently, can modulate intestinal function.4
CH4 can be measured through breath tests and exhaled levels correlate well with CH4 obtained by stool-based qPCR testing.5 In a study conducted by Rezaie et al.6 that evaluated 12,183 subjects, utilizing a baseline methane cutoff value ≥ 5 ppm, they obtained 96% sensitivity, 99.7% specificity, 98.5% PPV, and 99.3% NPV for detecting a methanogenic population. A subject is considered “methanogenic” if his/her baseline exhaled levels are above 3-5 ppm,7 with an increase ≥ 10 ppm over the baseline value, after the administration of a carbohydrate as a substrate.8
CH4 production in humans takes place exclusively in the intestine, carried out by colonic anaerobes. The primary microorganisms are Methanobrevibacter smithii,9Methanobrevibacter oralis, Methanobacterium ruminantium,10 and Methanosphaera stadtmaniae. Multiple animal models and studies on humans have shown the effect of methane on intestinal and colonic motility. For example, in studies on animals, the infusion of CH4 has been shown to increase the noncontractile activity of the small bowel and delay intestinal transit.11 Cloarec et al.,12 utilizing lactulose breath tests in humans, found that intestinal transit time in methanogenic patients, compared with non-methane producers, was much higher (111 min versus 68 min). The presence of intestinal CH4 has been associated with different intestinal diseases, such as chronic functional constipation, irritable bowel syndrome (IBS) that is constipation-predominant, obesity, constipation, and colon cancer, among others. Attaluri et al.13 evaluated patients with chronic constipation that had slow transit and those that had normal transit, through colonic markers and the comparison with healthy controls, and found a significantly higher prevalence of methanogens in the group with constipation, as well as a higher prevalence of methanogens in the slow transit group, compared with the normal transit group.
The prevalence of methanogens in open populations varies from 36 to 63%. It is higher in Africa, reaching 80%, and lower in the United States14 and Europe, with reports of 35-40%. In the Mexican population, the prevalence of methanogens and their associated factors is unknown. Therefore, the aim of our study was to evaluate the prevalence of CH4 producers and associated factors in a group of patients with IBS and healthy controls, in a Mexican population.
Materials and methodsPopulation studiedAdult patients (above 18 years of age) referred to our motility laboratory, having a diagnosis consistent with IBS, according to the Rome III criteria (within a 6-month period), were consecutively examined. Patients with recent antibiotic use (< 4 weeks before the test), with pulmonary diseases, and/or gastrointestinal surgery were excluded. The control group was made up of subjects considered asymptomatic due to negative results in gastrointestinal symptom questionnaires, the absence of the Rome III criteria, and no previous diseases or medication use. They came from an open population in response to an invitation as volunteers and provided informed consent, authorized by the institutional committees.
InterventionsPrior to the breath test, the subjects were asked to fast for 8 h, 24 h before the test, and to follow the dietary recommendations of abstaining from a high-fat, low-residue diet. They were instructed to not drink alcoholic beverages, smoke, chew gum, or consume menthol candy the day of the test. The patients were asked to arrive with their teeth brushed, and they gargled with an antiseptic mouthwash before the study to prevent false positives due to the fermentation of carbohydrate substrates by bacteria in the oral cavity.
Breath test protocolAt the beginning of the test, the following clinical characteristics were evaluated: weight, height, body mass index (BMI), grade of overweight/obesity. The patients were subclassified according to the IBS subtypes and they answered a gastrointestinal symptom questionnaire that assessed the presence of abdominal pain, postprandial fullness, postprandial distress, bloating, belching, diarrhea, flatus, and nausea, as well as a history of lactose intolerance. They were asked about the number of bowel movements per day and/or week and stool type, according to the Bristol Stool Chart.1–7
Only fasting alveolar H2 and CH4 were measured, through gas chromatography (stationary phase) (GastroCH4ECKl, Bedfont® Scientific Ltd, UK) and the patients were classified as methanogenic when they had H2 levels of 0 ppm and CH4 ≥ 5 ppm, according to the “baseline” cutoff point proposed by Rezaie et al.
Statistical analysisDescriptive statistics were utilized for the nominal variables, with absolute and relative frequencies, and the categorical variables were described using mean, median, and standard deviation. Group comparisons (methanogenic subjects versus non-methanogenic subjects, obese subjects versus non-obese subjects) were made, utilizing the Student’s t test, chi-square test, and ANOVA test, as required. Statistical significance was set at a p < 0.05.
Convenience sampling of all the patients with IBS that came to our laboratory, within a 6-month period, was carried out. The control group calculation was made, according to the study by Levitt et al.14 on 212 control subjects, in which, over a 35-year period, the prevalence of methanogenic subjects was close to 35%. Taking those figures into account and considering a 95% CI, with a 5% margin of error, between 105 and 133 subjects would be needed.
Ethical considerationsA written statement of informed consent was required for participation in the present study. The study met current regulations in bioethical research and was approved by the ethics committee of the Instituto de Investigaciones Médico Biológicas of the Universidad Veracruzana, with registration number IIMB-UV-2017-018. The authors declare that this article contains no information that could identify the patients.
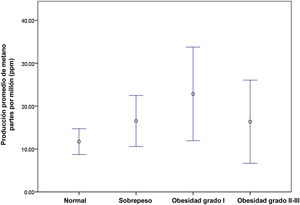
ResultsOverall analysisA total of 199 subjects were included, 132 (66%) of whom were healthy controls (53.8% were women, with a mean age of 28 ± 9 years) and 67 (34%) were patients with IBS (76% were women, 34.4 ± 14 years of age). Overall, i.e., the patients with IBS and the controls, we found a prevalence of methanogenic subjects of 38% (n = 76/199) (95% CI: 0.31-0.45). The methanogenic subjects produced a mean of 30 ± 13 ppm of methane, compared with the non-methanogenic subjects (3 ± 2 ppm, p = 0.001). Regarding the clinical characteristics of those two groups, there were no significant differences in relation to sex (60% men vs 63% women, p = 0.299) or age (29.13 ± 12 years vs 28.1 ± 10 years, p = 0.29), but there was a significant difference, with respect to a greater prevalence of overweight/obesity (56.5 vs 39.8%, p = 0.028), especially in the subjects with grade II-III obesity (23% vs 10%, p = 0.011). The mean BMI of the methane-producing patients was higher than that of the non-producers (26.7 ± 5 vs 24.9 ± 4, p = 0.023) (Table 1). We found greater methane production, the higher the grade of overweight/obesity (p = 0.04) (Fig. 1).
In the analysis according to groups, the prevalence of methanogenic subjects in the healthy controls was 41.6% (95% CI: 0.33-0.49), whereas said prevalence in the IBS patients was 31.4% (n = 21) (95% CI: 21.5 -0.43, p = 0.16) (Tables 2 and 3).
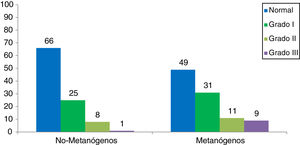
In the control population analysis, there were no significant differences in relation to sex (56% men vs 52% women, p = 0.37) or age (27 ± 11 years vs 25 ± 7 years, p = 0.7) but there was a significant difference regarding a greater prevalence of overweight/obesity (51 vs 32%, p = 0.02). The methanogenic control subjects produced a mean of 30 ± 12 ppm of methane, compared with the non-methanogenic controls (3 ± 1 ppm, p = 0.001). The mean BMI in the methane-producing control subjects was higher than that of the control non-producers (26 ± 4 vs 24 ± 3, p = 0.04) (Table 2). Fifty-one percent of the methanogenic controls presented with obesity (p = 0.09) and the most frequent type was grade I obesity, at 31% (Fig. 2).
With respect to the patients with IBS, there were no significant differences regarding sex (81% men vs 74% women, p = 0.38), age (35 ± 14 years vs 33 ± 14 years, p = 0.5), or a greater prevalence of overweight/obesity (71 vs 52%, p = 0.11). The methanogenic IBS patients produced a mean of 29 ± 4 ppm of methane, compared with the non-methanogenic IBS patients (3 ± 2 ppm, p = 0.001). The mean BMI of the methane-producing IBS patients was higher than that of the non-methane producing IBS patients (28 ± 5 vs 26 ± 4, p = 0.05) (Table 3).
Regarding the constipation, mixed, and diarrhea-predominant IBS subgroups, of the 67 patients with irritable bowel, 32 patients presented with the IBS-C subtype (47.8%), 25 with the IBS-M subtype (37.3%), and 10 with the IBS-D subtype (14.9%). Of the 21 methanogenic IBS patients, 71% (n = 15) belonged to the IBS-C subgroup, 29% (n = 6) to the IBS-M subgroup, and none (n = 0) to the IBS-D subgroup (p = 0.012).
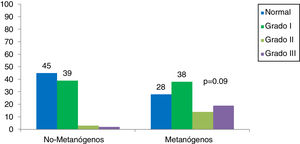
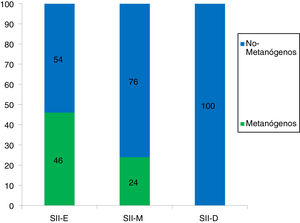
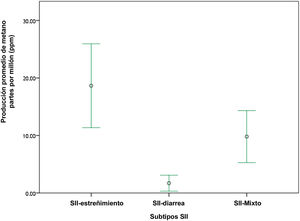
Of the patients with IBS, 72% of the methanogenic patients presented with obesity (p = 0.09) and the most frequent type was grade I obesity, at 38% (Fig. 3). With respect to the IBS subtypes, of the 32 patients with IBS-C, 46% were methanogenic; of the 25 patients with IBS-M, 24% were methanogenic, and of the 10 patients with IBS-D, none were methanogenic (Fig. 4). The IBS-C patients had higher levels of methane than the IBS-M and IBS-D patients (18.6 ± 5 ppm vs 9.8 ± 6.3 vs 1.5 ± 1, p = 0.008) (Fig. 5). In our total population, there were 12 subjects (5 controls and 7 IBS patients) that produced no H2 or CH4.
DiscussionThe prevalence of methanogenic subjects worldwide varies and depends on the geographic region, as illustrated by the wide gap between the prevalence reported in the African countries of Nigeria (77%) or South Africa (87%) and the prevalence reported in Norway (34%) or the United States (40%).15 The causes of said differences are not yet completely established, but they are thought to depend on genetic and/or dietary factors that influence the specific microbiota of each population.
Our study is the first to explore the prevalence of CH4-producing subjects in Mexico. We found an overall prevalence of CH4-producing subjects of 38%, in an open population. Those findings are consistent with that reported in other populations of North America. Nevertheless, in the analysis by groups, the prevalence of asymptomatic healthy controls producing CH4 was greater (non-significantly), when compared with IBS patients (41 vs 31%), i.e., more than one-third of the healthy controls were methanogenic.
There is an obesity epidemic in Mexico, with a prevalence of 32% in the adult population.16 In the present study, we found that methane production was higher in patients with overweight and/or obesity, which should be highlighted. As stated above, methane production is associated with obesity in some populations but whether a methanogenic microbiota is a cause, or a consequence, of obesity remains to be clarified. One hypothesis posits that, in the production of methane by M. smithii, hydrogen requirements involve other microorganisms, becoming a symbiotic act, in which their productivity and metabolism is increased, facilitating short-chain fatty acid production and increasing the availability of calories for the organism. In a study by Mathur et al.,17 those authors significantly related the production of methane and hydrogen to an obese population. They were also the first to demonstrate the relation between the presence of methane in breath tests and obesity, reporting that the BMI in the methanogenic obese subjects was 6.7 kg/m2 higher than in the non-methanogenic obese subjects.18
In our study, the methane-producing subjects had a higher BMI than the non-methane-producers, and more than 50% of our total methanogenic population presented with obesity. Obesity in the methanogenic subjects was more frequent in the IBS patients. In particular, we found that the grade of obesity that was more prevalent, overall, in the highest methane producers, was grade I.
Regarding age, neonates and children under 2 years of age have been reported to not excrete methane. Methane production begins at 2 years of age and increases up to 10 years of age, at which time it remains constant up to the eighth decade of life.19 In a Nigerian population, Hudson et al.15 demonstrated a significant difference in the prevalence of methanogens between children and adults. In our study population we found no difference with respect to age in the methanogenic subjects vs the non-methane producers.
Many factors have been identified that appear to increase the possibility of producing methane. Differences in sex appear not to be significant. According to the current literature, there are apparently no significant differences in methane production regarding sex,16 and in our population, we found that age was not a risk factor for increasing or decreasing the prevalence of methanogens. In our overall population, the methanogenic subjects produced 10-times more methane than the non-methanogenic subjects.
The IBS patients with a greater methanogenic propensity were those presenting with the constipation-predominant subtype. Methane was thought to be an inert gas, but there is current experimental and clinical evidence stating that it can alter intestinal transit.6 There is an important relation between methane production and its action on intestinal transit, especially in patients with chronic functional constipation.13 However, there are publications reporting that methane affects intestinal neuromuscular function. In canine models, small bowel transit is reduced, increasing the ileal circular muscle contraction through the mechanical stimulation of the mucosa.9
In an Italian population, a relation of intestinal disorders to methane and hydrogen production was sought, finding that methane was related to IBS-C,20 as was the case in our study population. We found that 71% of the IBS-C patients were methanogenic, a result that concurs with those described in previous studies. We also found that none of our IBS-D patients (albeit there were only 10) had a methanogenic microbiota, making it appear that said microbiota is not associated with the presence of diarrheic stools or intestinal transit. Nevertheless, based on our results, it would appear that methane is related to intestinal disorders, such as IBS, even though its correlation with symptoms is still uncertain. In a recent study by Di Stefano et al.21 on 103 subjects with IBS and 28 controls, they found that despite the fact that 46% of the IBS patients excreted methane in their stools, breath methane was detected in only 26% of them. Those authors concluded that the clinical role of methane is still uncertain and should be re-evaluated, utilizing new technologies, such as microbiota analysis, and especially emphasizing the association of symptoms.
Finally, we believe it is important to underline the fact that 12 subjects (5 controls and 7 IBS patients) produced neither H2 nor CH4, suggesting a microbiota that mainly produces H2S. Nevertheless, there are currently few tools available for evaluating that gas at the intestinal level.
Among the limitations of our study is the fact that it was conducted on a specific population, given that there can be variations in the microbiota that are dependent on local diet and the genetic component. Regarding IBS subtypes, the methanogenic microbiota is described as more prevalent in the constipation-predominant subtype, as confirmed by our study, but that subtype was the most prevalent in our study subjects and we had few cases of the diarrhea-predominant subtype. Despite those limitations, we consider our results relevant, novel, and comparable, regarding those published in other studies. Conducting a multicenter study at different regions of the country is important, to provide conclusions that are more applicable at the national level and to generalize data.
ConclusionsIn our study, the prevalence of methanogens in an open population and in patients with IBS was similar to that reported worldwide. Within the methanogenic population, IBS-C was the most prevalent subtype. In addition, obesity was related to methane production, and BMI was higher in the methane producers than in the non-methane producers. The most prevalent obesity grade was grade I.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Aja-Cadena MG, Amieva-Balmori M, Taboada-Liceaga HA, Cobos-Quevedo OJ, Hernández-Ramírez GA, Reyes-Huerta J, et al. Prevalencia de metanógenos y factores asociados en pacientes con síndrome de intestino irritable y controles sanos en una población del sureste de México. Rev Gastroenterol Méx. 2023;88:50–56.