Childhood obesity is a pandemic with significant morbidity and mortality implications, impacting both quality of life and the economic burden on healthcare systems. Given the effects on health for the pediatric population, and aligned with the multidisciplinary management approach, the Obesity Working Group of the Latin American Society for Pediatric Gastroenterology, Hepatology and Nutrition (LASPGHAN) summoned a group of healthcare professionals to develop a consensus on overweight and obesity. The aim of this document is to present those consensus results.
Material and methodsThe LASPGHAN Obesity Working Group organized 6 working panels to formulate statements on diagnostic approach, treatment, and follow-up. We conducted a comprehensive review of the current literature across several databases utilizing different search criteria. Thirty-four panelists from the countries that make up the LASPGHAN were selected. They participated in an anonymous online voting process using the Delphi method. A priori consensus for each statement was defined as 80% agreement on a 3-point Likert scale.
ResultsTwenty-six statements were discussed and voted upon, resulting in the final approval of 22 statements.
ConclusionsThere is a lack of uniformity in diagnosing overweight and obesity in Latin America, especially in the choice of growth charts and cutoff points for the pediatric population.
La obesidad infantil es una pandemia con importantes implicaciones de morbimortalidad, impactando en la calidad de vida y la economía de los sistemas de salud. Debido a las implicaciones para la salud en la edad pediátrica y uniéndose al esfuerzo en la materialización del manejo multidisciplinario, el grupo de trabajo de Obesidad de la Sociedad Latinoamericana de Gastroenterología, Hepatología y Nutrición Pediátrica (LASPGHAN, por sus siglas en inglés, Latin American Society for Pediatric Gastroenterology, Hepatology and Nutrition) integró un grupo de profesionales de la salud para desarrollar un consenso sobre sobrepeso y obesidad. El objetivo de este documento es mostrar el resultado de dicho consenso.
Material y métodosEl grupo de trabajo de obesidad de la LASPGHAN organizó 6 mesas de trabajo para elaborar declaraciones de abordaje diagnóstico, tratamiento y seguimiento. Se realizó una revisión exhaustiva de la literatura actual en diversas bases de datos con diferentes criterios de búsqueda. Se seleccionaron 34 panelistas de cada país que conforman LASPGHAN, quienes votaron en línea de forma anónima mediante un proceso Delphi. Se definió consenso a priori para cada enunciado con el 80% de acuerdo en la escala Likert de 3 puntos.
ResultadosSe discutieron y votaron un total de 26 declaraciones, quedando finalmente 22 enunciados.
ConclusionesEn Latinoamérica hace falta uniformidad respecto al diagnóstico de sobrepeso y obesidad, en relación a que tablas de crecimiento y puntos de corte utilizar en la edad pediátrica.
Childhood obesity is one of the major healthcare challenges of this century.1,2 Its prevalence has reached epidemic proportions worldwide, affecting the economic burden of healthcare systems due to its direct impact on nontransmissible chronic diseases in the adult.3,4
In Latin America, there is no consensus on the diagnosis, treatment, and prevention of overweight and obesity. Due to the health implications for the pediatric population and joining the effort to establish multidisciplinary management, the Obesity Working Group of the Latin American Society for Pediatric Gastroenterology, Hepatology and Nutrition (LASPGHAN) brought together a group of healthcare professionals made up of pediatricians and pediatric gastroenterologists to develop a necessary consensus on overweight and obesity.
The aim of this document is to present the results of that endeavor, offering healthcare professionals a useful tool for the prevention and management of overweight and obesity.
Material and methodsThe LASPGHAN Obesity Working Group was made up of a group of specialists (pediatricians and pediatric gastroenterologists). They were organized into 6 working panels to address different aspects of overweight and obesity. Each working panel was made up of 2–3 members whose job was to formulate statements on the different topics, then search for evidence supporting the different statements. The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.5 A thorough review of the current literature was carried out on the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (PubMed), and EMBASE (Ovid), for articles published within the time frame of January 1, 1990, to November 4, 2024. The bibliographic review was conducted using these MeSH terms as keywords: «adolescents», «anthropometric», «anthropometry», «bariatric surgery», «body mass», «childhood obesity», «childhood overweight», «children», «complications», «diagnosis», «dietetic management», «fatty liver», «follow up», «index», «infant», «lifestyle», «MAFLD», «metabolic diseases», «metabolic syndrome», «NAFLD», «nutrition», «nutrition assessment», «obesity», «obesity environment», «pediatric», «pediatrics», «pediatric obesity», «prevention», «risk factor», «school», «toddler», «treatment», «transaminases», «vitamin D deficiency», and their Spanish equivalents. All the publications in English and Spanish (original articles, consensuses, guidelines, and systematic reviews) were identified, along with articles that the coordinators and members of the present consensus group considered relevant. Important additional studies cited in the selected article references were also evaluated and all articles were made available to the members of the consensus throughout the entire process. A first virtual meeting was carried out to explain the dynamics of developing the consensus. The information was analyzed by the corresponding working panel, adapting and perfecting the different statements that were considered pertinent for each module, discussing the recommendations and points of debate on the issues of diagnostic or therapeutic approach and prevention. Each of the working panels sent the statements and their justifications to the general coordinators of the consensus (YRS and JAM), who organized them and sent them to all the participants for their review. A second virtual meeting was conducted for the initial presentation of the statements (32 statements). Each subgroup created evidence tables for the information supporting the different statements, whenever possible. The third (on March 4, 2024, World Obesity Day), fourth, and fifth virtual meetings were held, at which the final statements and their justifications were presented. All participants in attendance had the opportunity to comment on and question the statements.
The Delphi process was carried out through an ad hoc platform, with anonymous online voting.6 A Delphi panel of 34 participants was selected, through a modification of the criteria for determining legal medical witness expertise, utilized by the Courts of California in the United States.7 All participants could comment on, suggest modifications, and grade each statement, utilizing a 3-point Likert scale (1- in agreement, 2- abstained, 3- in disagreement). Voting was conducted by one or two delegates from each participating country. The coordinators and members of the working panels did not vote. The study facilitator (CMTB) was not allowed to vote or comment on the statements. The consensus was defined a priori by 80% of the panelists that were in agreement or disagreement on the Likert scale. The results of the first voting round on the final 26 statements were presented at a virtual work meeting. The statements that achieved consensus ( ≥ 80% in agreement) were accepted. Those that did not reach consensus were re-formulated (6 statements) and voted on in an anonymous second round. Four of those statements reached consensus, 2 did not, and 2 were eliminated, resulting in a total of 22 statements. Cronbach’s alpha was utilized to determine internal consistency of the evaluation tool after each round.8 The final voting round of the consensus was defined as such, by reaching a Cronbach’s alpha value of 0.88. The members of each working panel analyzed and synthesized the information in the corresponding parts of the manuscript. The categorical variables were expressed as proportions (%).
ResultsThirty-four experts participated in the development of the consensus, representing the 21 member countries of the LASPGHAN: Argentina, Bolivia, Brazil, Chile, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Portugal, Spain, Uruguay, and Venezuela. Seventeen of the experts were specialists in gastroenterology and pediatric nutrition, 7 were experts in pediatric endocrinology (one with an advanced specialty in obesity and one in diabetes mellitus), 9 were pediatricians (3 specializing in nutrition), and one was a pediatric nutritionist.
Twenty-six statements were originally formulated. After a second voting round and discussion, a total of 22 statements were included in the consensus.
The consensus statements and their corresponding agreement percentages were determined using a total of 34 voters as the reference point and are presented below. The percentage of voters who abstained from voting in agreement or disagreement is also included.
Generalities: definition, epidemiology, etiology- 1
Obesity is a chronic, progressive, and recurrent neurometabolic disease of multifactorial origin, characterized by an abnormal accumulation (excessive and/or dysfunctional) of adipose tissue that has adverse health consequences and reduces life expectancy. (In agreement: 81%; abstained: 16%; in disagreement: 3%)
Obesity is a multicausal disease (polygenic heritability, obesogenic environment, lifestyles),9–12 with varying clinical phenotypes that have gut-brain axis modulation in common.4,13–15 It arises from a persistent surplus of energy intake over expenditure, disrupting neuroendocrine, inflammatory, and behavioral controls. This dysregulation affects adipose tissue function and contributes to adverse health conditions.16–18 Recognizing obesity as a chronic disease enables improved early and timely care of children/adolescents, for preventing the appearance of comorbidities that create economic, clinical, and psychosocial comorbidities in the long term.19,20
- 2
The worldwide prevalence of children and adolescents with overweight and obesity is increasing. Obesity is the most frequent nontransmissible chronic disease at any stage of life and should be considered a global public health problem. (In agreement: 100%)
Since 1980, studies have reported changes in human body composition, with registers of rising body mass index (BMI) values.21 The prevalence of obesity increases with age and one out of every 4 children with obesity between 6–9 years of age in the European countries belonging to the World Health Organization (WHO) has severe obesity. According to the available data, no country is on the path for reaching the sustainable development goals by 2030 that aim to reduce childhood overweight by 3% and maintain the results.1,3,22Fig. 1 shows the prevalence of overweight and obesity in children and adolescents in the member countries of the LASPGHAN. The lack of recent epidemiologic studies in several of those countries and the small population samples of others should be pointed out.
- 3
In addition to the host’s own conditions (genetic or epigenetic susceptibility), there also has to be a positive energy balance that interacts with environmental, psychologic, and socioeconomic factors to modify the accumulation of adipose tissue and produce harmful effects on the individual’s health. (In agreement: 94%; abstained: 6%)
The best strategy for evaluating and intervening in childhood obesity is comprehending the different interactions between the individual and his/her environment.23 The health status of both parents in the preconception period may have an impact that possibly transcends the health of several generations (polygenetic heritability of obesity >50%).23–25 Up to 1,100 genetic loci associated with obesity have been located, but those signals explain only 6% of BMI variability. Up to 24 genetic loci have been described that are related to changes in BMI and/or body composition, after multidisciplinary lifestyle interventions in children and adolescents with excess adiposity.26–28 Multiple factors can influence the adaptation of the gut microbiota, which exerts a variety of protective, structural, and metabolic effects on body weight regulation.29
Nutritional factors (breastfeeding, complementary feeding, and food quantity, frequency, quality, and availability) have been identified that interact with lifestyles (physical activity, sedentary lifestyle, sleep hygiene, management of emotions, and parenting styles) to regulate the trajectory of childhood growth, generating changes in quantity, quality, and function of adipose tissue. Said changes are considered the main risk factor for comorbidities, such as high blood pressure (HBP), type 2 diabetes mellitus (DM2), kidney disease, metabolic function-associated steatotic liver disease (MASLD), and osteoarticular problems, that reduce quality of life and have produced a high economic burden on healthcare systems.11,30,31
DiagnosisIn Latin America, there is a lack of uniformity regarding the clinical diagnosis of overweight and obesity. Agreement was not reached in the present consensus among the voters, with respect to the growth charts and cutoff points to be used in pediatrics.
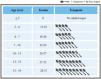
Based on a thorough review of the current evidence on the diagnosis of overweight and obesity in children and adolescents, the authors signing the present manuscript recommend using the WHO 2006 growth charts by age and sex for diagnosing children under 2 years of age, with the weight-for-length (WFL) cutoff points of ≥2 standard deviations (SD) or ≥ the 97.7th percentile for defining overweight. The WHO describes a WFL cutoff point of ≥ 3 SD or ≥ the 99.9th percentile (Fig. 2) for defining obesity.32,33 The WHO 2006 charts are preferred because they are the result of a high quality, multicenter benchmark study that reflects the growth patterns of breastfed children with adequate guidelines for starting complementary feeding.34–36
2006 World Health Organization breakdown of Z scores and corresponding percentiles in a normal study distribution.
There are definitions based on WFL or BMI from the WHO 2006 or Centers for Disease Control and Prevention (CDC) 2000 growth charts, for children under 2 years of age.32,34,37 For predicting obesity at the first 5 years of life, Rifas-Shiman et al. conducted a multicenter study that compared the CDC 2000 and WHO 2006 growth charts and included 15,488 children (92,928 somatometry measurements). They concluded that the cutoff point as a predictive factor for obesity was ≥ the 97.7th percentile, corresponding to 2 SD above the mean, using the WHO growth curves, with 54% sensitivity and 84% specificity.35 Likewise, Roy et al. conducted a retrospective study of 73,949 infants, evaluating WFL measurements up to age 24 months at different cutoff points, comparing the CDC 2000 WFL overweight classification of ≥ the 95th percentile with the WHO WFL and BMI of ≥ the 97.7th percentile. Upon comparing BMI and WFL, both were similar for diagnosing overweight.38
The CDC 2000 growth curves are a widely accepted tool for diagnosing overweight and obesity because they reflect genetic and environmental diversity.38 In patients above 2 years of age, we suggest using those curves with BMI charts specific for age and sex, with cutoff points of BMI ≥ the 85th percentile for overweight and ≥ the 95th percentile for obesity.39,40
The adequate use of those curves enables the growth trajectory to be monitored, comparing and interpreting anthropometric values, evaluating the risk for overweight and obesity, and classifying according to the different grades.40Table 1 shows the methodological differences between the CDC 2000 and WHO 2006 growth charts.36
Comparison of the CDC and WHO growth curves.
| Comparison of the populations used to develop the WHO and CDC growth curves for children under 24 months of age | ||
|---|---|---|
| Characteristics | CDC growth reference standard (2000) | WHO growth reference standard (2006) |
| Data source | National vital statistics (weight at birth) Missouri and Wisconsin vital statistics (length at birth) Pediatric nutrition surveillance system (length, 0.1 to <5 months) NHANES I (1971−1974) (12−23 months) NHANES II (1976−1980) (6−23 months) NHANES III (1988−1994) (2−23 months) | MGRS longitudinal component, included populations from Brazil, Ghana, India, Norway, Oman, the United States |
| Data collection type and frequency | Weight and length cross-sectional data from 2 months of age, with mathematical models used for connecting weight and height at birth with data from the survey | Longitudinal data with weight and length measurements at birth and at weeks 1, 2, 4, 6, and 8 and at months 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 16, 18, 20, 22, and 24 |
| Sample size | 4,697 observations for 4,697 different children | 18,973 observations for 882 different children |
| Exclusion criteria | Very low weight at birth (<1,500 g) | Low socioeconomic level Birth at an altitude above 1,500 m Birth before 37 or ≥42 weeks Multiple birth Perinatal morbidities Infant health conditions that are known to affect growth Mother who smoked during pregnancy or breastfeeding Breastfeeding for fewer than 12 months Complementary feeding introduced before 4 or after 6 months Weight-for-length measurements >3 standard deviations above or below the study median for sex |
| Breastfeeding | Approximately 50% were breastfed at some time Approximately 33% were breastfed up to 3 months | 100% breastfed at some time 100% breastfed predominantly up to 4 months 100% continue to be breastfed at 12 months Mean age of 5.4 months for complementary feeding |
CDC: Centers for Disease Control and Prevention; MGRS: Multicentre Growth Reference Study; NHANES: National Health and Nutrition Examination Survey; WHO: World Health Organization.
BMI is used as an estimated adiposity measurement but has the limitation of being unable to differentiate between lean mass and fat mass, making it necessary to take other anthropometric parameters into account, such as waist circumference (WC), mid-upper arm circumference, neck circumference and waist-to-height ratio (Table 2).37,41–43 WC is a predictive index for fat mass that has shown efficacy for estimating total adiposity. Routine measuring is suggested in all patients with overweight and obesity.43–45 The cutoff point associated with cardiometabolic risk is ≥ the 90th percentile for age and sex.37 In 2021, Marrodán-Serrano et al. conducted a study to provide WC percentiles for Hispanic children and adolescents. It included 13,289 healthy school-age participants between 6 and 18 years of age from Argentina, Cuba, Mexico, Spain, and Venezuela. Those authors concluded the 90th percentile cutoff values may be useful for evaluating central obesity in pediatric patients.43
Anthropometric indexes and indicators utilized in children and adolescents with overweight and obesity.
| Under 2 years of age | Above 2 years of age | ||
|---|---|---|---|
| Weight | Instrument: Calibrated or digital scale. Child should be weighed naked. Weight should be measured to the nearest 0.01 kg. | Weight | Instrument: Balanced or digital floor scale. Either a balanced floor scale or a digital floor scale can be used. Child/adolescent should be weighed wearing as little clothing as possible. |
| Length | Instrument: Infantometer. Child should be placed in a reclining position, aligning the head against the headboard. Straighten the body and legs with the feet parallel to the footrest. Repeat the measuring twice, within 0.2 cm. | Height | Instrument: Stadiometer. The child/adolescent should stand up straight with buttocks, shoulder blades and heels touching the vertical piece of the device. Feet should face outward at a 60°angle. In patients with genu valgum, separate feet enough to avoid knee overlap while maintaining contact between the knees. Arms should hang loosely at the sides with palms facing the thighs. The horizontal bar should be lowered until the hair is compressed to the crown of the head. The measurement should be read to 0.1 cm. Repeat the measurement twice to obtain 2 readings within 0.2 cm. |
| Weight/length | Reflects body weight in proportion to growth achieved in length or height. | Body mass index | BMI is the ratio of body weight in kilograms to height in meters squared. Weight in kilograms/(height in meters x height in meters). |
| Mid-upper arm circumference | Instrument: Non-stretchable fiberglass tape measure, no thicker than 5 mm. It should be placed on the non-dominant arm bent at a 90º angle and held close to the body. The distance between the bony protrusion of the acromion and the point of the olecranon is measured, marking the midpoint. With the arm hanging loose, measure around the upper arm at the midpoint making sure the tape measure is not too tight. Measure in centimeters. | ||
| Neck circumference | Instrument: Non-stretchable fiberglass tape measure, no thicker than 5 mm. Measured at the midpoint of the neck, at the level of the thyroid cartilage and perpendicular to the axis of the neck, with eyes looking forward, breathing normally. | ||
| Waist circumference | Instrument: Non-stretchable fiberglass tape measure, no thicker than 5 mm. Place the tape at the level of the midaxillary line, at the midpoint between the costal margin and the iliac crest or at the level of the umbilicus. Performed with the patient in a standing position, at the end of a normal expiration. Carrying out at least 2 measurements and averaging them is recommended. | ||
| Waist-to-height ratio | WHtR = waist circumference (cm)/height (cm). The cutoff point is > 0.5. | ||
WHtR: waist to height ratio.
Having knowledge of the specific charts that should be used for certain genetic syndromes is important.46
Comorbidities- 4
Obesity is associated with a greater risk of short-term and long-term complications, such as insulin resistance; metabolic, cardiovascular, respiratory, autoimmune, musculoskeletal, gastrointestinal, liver, and kidney diseases; fertility alterations; psychologic disorders; and depression; and in the long term, diseases such as cancer, early death, and disability in adulthood. (In agreement: 94%; abstained: 3%; in disagreement: 3%)
Numerous comorbidities are associated with obesity. Regarding cardiovascular disease, there is a 3-times higher possibility of presenting with HBP in children with obesity, compared with non-obese children.47 HBP and childhood obesity are related to early cardiac damage, such as left ventricular hypertrophy.48,49 The effects on lipid metabolism include elevated levels of low-density lipoprotein cholesterol (LDL-C), very-low-density lipoprotein cholesterol (VLDL-C), triglycerides, and low levels of high-density lipoprotein cholesterol (HDL-C).49 Obesity induces an altered immune system that can be seen from childhood. It is associated with greater susceptibility to surgical site, urinary, hospital-acquired, and dermatologic infections. In addition, there is a lower response to vaccines and evidence that the risk for rheumatoid arthritis, multiple sclerosis, psoriasis, and psoriatic arthritis is increased.50 Biliary acute pancreatitis has been linked to the increase in childhood obesity as an independent risk factor.51
Fig. 3 lists the main comorbidities that have been identified in pediatric patients with obesity.49,52 Obesity is considered one of the main reasons for the increased incidence of gastroesophageal reflux disease (GERD) and its associated conditions (Barrett’s esophagus and esophageal cancer).49 In women, it increases the risk of urinary incontinence and polycystic ovary syndrome (PCOS).53 PCOS is a frequent cause of female infertility and increases the complication rate during pregnancy. It is one of the main risk factors of osteoarthritis, with a major impact on the knees, but it also impacts the joints of the hands and contributes to lumbar pain. Men have a 2–3-times higher relative risk for presenting with gout.49 Adolescents with overweight and obesity have a greater likelihood of adopting risky behaviors, such as substance abuse, unsafe sexual activity, or violence.49
- 5
We recommend systematic screening for the diagnosis of high blood pressure in children and adolescents with overweight and obesity. (In agreement: 100%)
HBP is a syndrome of interrelated metabolic neuroimmune abnormalities that has hemodynamic consequences.54 Dysfunctional adipocytes release adipokines, such as leptin, resistin, and interleukin 6, that increase sympathetic nervous system (SNS) activity and diverse hormones and mineralocorticoid stimulating factors of the renin-angiotensin-aldosterone system, and as a consequence, increase arterial pressure.55 Adolescents are the most affected age group. BMI alterations associated with the increase in visceral fat, accelerated biologic maturity, metabolic abnormalities typical of metabolic syndrome (MetS), and SNS activation are part of the HBP phenotype.56
The reported prevalence of HBP is 5% in children with normal weight, 20% in children with overweight, 26% in children with obesity, and 39% in children with severe obesity.56,57
The American Academy of Pediatrics (AAP) recommends taking at least 3 measurements in children/adolescents with initially elevated blood pressure, given that the values of successive measurements often decrease58 (Table 3).17,58,59 Obesity-associated HBP increases cardiovascular risk in adulthood,60 making the timely detection of altered values for age and sex essential.
- 6
Metabolic function-associated steatotic liver disease has a high prevalence (33.7%) in children and adolescents with overweight and obesity, compared with the general population, and so we recommend a directed approach to those patients. The disease should be suspected when there is an increase in aminotransferase values. (In agreement: 86%; abstained: 9%; in disagreement: 6%)
Recommended method for measuring blood pressure in children and adolescents.
| Technique Seat the patient in a quiet room, relaxed, for 3−5 min with back supported, feet flat on the floor, legs uncrossed, before and/or during the measuring. Measure BP in the right arm, unless contraindicated. Arm should be held so that the middle of the cuff is at the level of the heart. Measure the mid-arm circumference at the midpoint between the acromion and olecranon to determine the correct cuff size. Place the cuff 2 cm above the brachial artery palpated in the antecubital fossa. To determine the maximum cuff inflation level, palpate the radial artery pulse and inflate the cuff to 20−30 mmHg above the point at which the pulse disappears. Palpate the brachial artery in the antecubital fossa and place the diaphragm or the bell of the stethoscope over the brachial artery. Make sure it is not positioned under the cuff. Inflate the cuff to the maximum inflation level. Deflate the cuff to 2–3 mm Hg/s. The first Korotkoff sound (K1) is the systolic BP, and the last audible sound (K5) is the diastolic BP. |
| Adequate cuff size The cuff length should be ≥80% of the mid-arm circumference, and the cuff width should be 37–50% of the mid-arm circumference. The arm width-to-girth ratio should be 0.45−0.55. In patients with severe obesity, the intermediate point between the acromion of the scapula and the olecranon of the elbow should be measured, with the shoulder in a neutral position and the elbow bent at a 90 ° angle. |
| Age | Width (cm) | Length (cm) | Maximum arm circumference (cm) |
|---|---|---|---|
| Neonate | 4 | 8 | 10 |
| Toddler | 6 | 12 | 15 |
| Child | 9 | 18 | 22 |
| Adolescent | 10 | 24 | 26 |
| Adult | 13 | 30 | 34 |
BP: blood pressure.
In 2024, a multi-society consensus, in collaboration with the LASPGHAN, proposed replacing the nomenclature of nonalcoholic fatty liver disease (NAFLD) with MASLD, given the systemic contribution to liver dysfunction in metabolic syndrome, correlating with liver fibrosis stages and noninvasive fatty infiltration markers.61
Previously, NAFLD diagnosis included an increase of twice the limit of normal of alanine aminotransferase (ALT) (>25 IU/l for boys and >21 IU/l for girls).62 Presently, MASLD diagnosis is made when there is liver steatosis and at least one cardiometabolic criterion (Table 4).61 An evaluation should be carried out that rules out other causes, such as autoimmune hepatitis, Wilson’s disease, alpha-1 antitrypsin deficiency, celiac disease, thyroid alterations, and hepatotropic B and C virus infections.61 In the absence of overweight and obesity, but in the presence of alarm symptoms and no cardiometabolic criteria, the approach should be broadened to determine the cause.61
Diagnostic approach to metabolic dysfunction-associated steatotic liver disease.
| Cardiometabolic criteria: one or more in the presence of liver steatosis |
| BMI ≥ the 85th percentile for sex/age (BMI ≥ 1 SD) or abdominal circumference ≥ the 95th percentile ± Fasting serum glucose ≥100 mg/dl serum glucose ≥200 mg/dl Glucose ≥140 mg/dl 2 h post-load glucose HbA1c ≥5.7% Diagnosis/treatment of DM2 Blood pressure <13 years of age ≥ the 95th percentile or ≥130/80 mmHg (the one that is lower) Blood pressure >13 years of age 130/85 mm/Hg Antihypertensive treatment Triglycerides ≥100 mg/dl <10 years of age Triglycerides ≥150 mg/dl >10 years of age HDL cholesterol <40 mg/dl Treatment with lipid-lowering agents |
| Alarm symptoms |
| Under 8 years of age Delayed neurodevelopment Altered hepatic synthesis function BMI ≤1 SD Splenomegaly Other etiology suspected |
BMI: body mass index; DM2: type 2 diabetes mellitus; HbA1c: glycated hemoglobin; SD: standard deviation.
Notably, the diagnosis of MASLD may coexist with other pathologies and include a broad spectrum of liver injuries, such as simple steatosis and nonalcoholic steatohepatitis (NASH) that can cause the severe complications of liver cirrhosis and liver cancer.61,63,64 Regarding imaging studies, ultrasound is operator-dependent and can detect liver steatosis >20%, with 60−96% sensitivity and 84–100% specificity. Computed tomography can detect liver steatosis >30%, with 100% sensitivity and specificity. Nevertheless, there is a 1B level of evidence for the two methods and they are not recommended in the 2017 Vos guideline.65 On the other hand, even though there are still no cutoff values for the pediatric population, transient elastography is increasing more widely studied and utilized in children and adolescents to provide estimates of stiffness and fatty liver content.61
Metabolic evaluationA recent review of 30 guidelines evaluating the metabolic complications in children/adolescents with overweight and obesity showed variations in the recommendations for the biochemical approach protocol.66 Because patients can be asymptomatic, a thorough clinical history and physical examination is essential for identifying etiology, planning laboratory tests, and identifying associated comorbidities.11,42,67
- 7
We suggest determining liver enzyme levels (ALT, aspartate aminotransferase [AST]) from the first visit, comparing the results with the normal parameters for age and sex. (In agreement: 80%; abstained: 6%; in disagreement: 14%)
In children/adolescents with obesity, evaluation is recommended from 2 years of age, and in those with overweight, from 10 years of age. When values are high, evaluation should be repeated every 3–6 months, broadening the approach for causes of chronic hepatopathy, when values are persistently elevated.65,66,68 The NASPGHAN recommends evaluating for MASLD, in the presence of overweight with additional risk factors: central adiposity, insulin resistance, pre-diabetes or DM2, dyslipidemia, sleep apnea, or a family history of MASLD/NASH, as well as considering evaluating siblings and parents of children/adolescents with MASLD, if there are risk factors (obesity, Hispanic ancestry, insulin resistance, pre-diabetes, DM2, dyslipidemia).65 MASLD should be looked for in children/adolescents with overweight that have a waist-to-height ratio (WHtR) >0.5, and repeated annually.65,67
- 8
We suggest screening for metabolic syndrome in children/adolescents with overweight and obesity. (In agreement: 97%; in disagreement: 3%)
Ever since the WHO provided the first official definition of MetS in 1999,69 different definitions have been proposed, the most accepted of which have been those made by the European Group for the Study of Insulin Resistance (EGIR)70 and the National Cholesterol Education Program, Adult Treatment Panel III (NCEP-ATP III) groups.71,72
MetS is defined as a pathologic condition characterized by central obesity, insulin resistance, dyslipidemia, and HBP that increase cardiovascular risk and are triggered by visceral obesity, systemic inflammation, and cellular dysfunction.69,73,74 The incidence of MetS is parallel to that of obesity and DM2.75
There are no standardized pediatric criteria for diagnosing MetS, and they are based on proposals adapted from the criteria in adults, which include the presence of the following cardiovascular risk factors: central obesity, hypertriglyceridemia, low HDL-C, and HBP.75–77 At present, the most widely accepted are the proposals from the International Diabetes Federation75 (Table 5).
- 9
We suggest determining glucose level from the first visit, comparing the results with normal parameters. (In agreement: 81%; abstained: 3%; in disagreement: 17%)
- 10
We suggest ordering glycated hemoglobin (HbA1c) when there are one or more risk factors associated with pre-diabetes and type 2 diabetes mellitus, to identify high-risk patients that require more extensive evaluation. (First voting round: In agreement 32%; abstained: 56%; in disagreement: 12%) (Second voting round: In agreement 88%; abstained: 3%; in disagreement: 9%)
Diagnostic criteria for metabolic syndrome in children and adolescents.
| NCEP-ATP III | NCEP-ATP III | Cook et al.77 (2003) | De Ferranti et al.76 (2004) | IDF (2007) | |
|---|---|---|---|---|---|
| Age | Adults | >10 years of age | 12−19 years of age | 12−19 years of age | 10−16 years of age |
| Waist circumference | >102 cm males >88 cm females | ≥ the 90th p both sexes | ≥ the 90th p both sexes | ≥ the 75th p | ≥ the 90th p |
| Triglycerides | ≥150 mg/dl | ≥110 mg/dl | ≥110 mg/dl | ≥ 97.34 mg/dl | ≥150 mg/dl |
| High-density lipoproteins | ≤40 mg/dl males ≤ 50 mg/dl females | ≤40 mg/dl | ≤40 mg/dl | ≤ 45 mg/dl ≤ 50 mg/dl (for children 15−19 years of age) | ≤40 mg/dl |
| Blood pressure (systolic or diastolic) | ≥130/85 mmHg | ≥ the 90th pa | ≥ the 90th pa | > the 90th pa | ≥130/85 mmHg |
| Fasting glucose | ≥100/110 mg/dl | ≥110 mg/dl | ≥110 mg/dl | ≥109.9 mg/dl | ≥100 mg/dl |
| Considerations | At least 3 criteria | Includes children with central obesity | At least 3 criteria | At least 3 criteria | Metabolic syndrome should not be diagnosed in children under 10 years of age but weight should be followed in children with central obesity with relatives with cardiovascular disease. |
p; percentile.
The incidence and prevalence of DM2 in children and adolescents has increased.78 In adolescents with obesity, 20–25 % present with pre-diabetes and DM2 affects 1–4%.66 For diagnosing pre-diabetes and DM2 (Table 6), the suggestion is to evaluate asymptomatic children/adolescents with overweight and obesity that present with one or more risk factors associated with DM2: maternal history of DM2 or gestational diabetes, history of DM2 in a first-degree or second-degree relative, and race/ethnicity (Native American, African, Hispanic, Asian, or Pacific Islander), with data or conditions associated with insulin resistance, such as acanthosis nigricans, HBP, dyslipidemia, PCOS, or low weight for gestational age.78 HbA1c, glucose, or the oral tolerance to glucose curve can be used for evaluating pre-diabetes and DM2 in pediatric patients. However, the American Diabetes Association (ADA) recognizes there is limited evidence on HbA1c for diagnosing DM2 in children/adolescents due to ethnic/racial variations.78 Ordering insulin levels as the initial approach to obesity is not recommended.34
- 11
We suggest determining the lipid profile (triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol) from the first visit, comparing the results with normal parameters according to age. (In agreement: 91%; abstained: 3%; in disagreement: 6%)
Diagnostic criteria for pre-diabetes and type 2 diabetes mellitus in asymptomatic children and adolescents.
| Complication | Age at evaluation | Signs/symptoms | Laboratory study |
|---|---|---|---|
| Pre-diabetes | ≥10 years of age or onset of puberty* | Insulin resistance (acanthosis nigricans), high blood pressure (frequent headache), dyslipidemia, polycystic ovary syndrome (acne, hirsutism) | Glucosea 100−125 mg/dl (5.6−6.9 mmol/l) or Oral glucose tolerance curveb 140−199 mg/dl (7.8−11.0 mmol/l) or HbA1c 5.7−6.4% (39−47 mmol/mol) |
| Diabetes | ≥10 years of age or onset of puberty* | Polyuria, polydipsia, blurry vision, fungal vaginitis/vaginal discharge (girls), unexplained weight loss | Glucosea ≥126 mg/dl (7.0 mmol/l) or Oral glucose tolerance curveb ≥200 mg/dl (11.1 mmol/l) or HbA1c ≥6.5% (48 mmol/mol) or Random glucose with classic signs of hyperglycemia/hyperglycemic crisis ≥200 mg/dl (11.1 mmol/l) |
HbA1c: glycated hemoglobin.
Despite a high prevalence of dyslipidemia (46–50.4%) in obese children,32 there is no consensus on when to start monitoring. The Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics recommend screening from 6 years of age.32 Lim et al. suggest evaluating children with obesity from 2 years of age and those with overweight from 10 years of age.66 The expert panel of the National Heart, Lung and Blood Institute (NHLBI) and the American Heart Association (AHA) recommend universal screening (given the predisposition to atherosclerosis) between 9−11 years of age and repeating it between 17−21 years of age. If results are normal, it should be performed every 2 years, if values are borderline every year, and if there are alterations, re-evaluation every 2 weeks to 3–6 months (Table 7).42,66,68
- 12
Vitamin D (25[OH]D) deficiency (<20 ng/mL) and insufficiency (20−29 ng/mL) are prevalent in children and adolescents with overweight and obesity and the relation of said deficiency/insufficiency to insulin resistance has been reported. (In agreement: 83%; abstained: 14%; in disagreement: 3%)
Reference values for defining dyslipidemia in children.
| Lipidsa | Acceptable, mg/dl | Borderline, mg/dl | Abnormal, mg/dl |
|---|---|---|---|
| Total cholesterol | <170 | 170−199 | ≥200 |
| LDL cholesterol | <110 | 110−129 | ≥130 |
| Non-HDL cholesterol | >120 | 120−144 | ≥145 |
| HDL cholesterol | >45 | 40−45 | <40 |
| Triglycerides | |||
| 0−9 years of age | <75 | 75−99 | >100 |
| 10−19 years of age | <90 | 90−129 | >130 |
HDL: high-density lipoprotein; LDL: low-density lipoprotein.
The inverse relation between 25(OH)D and body composition has been widely described.79 In Mexico, 24% deficiency and 30% insufficiency of vitamin D have been reported in preschoolers with overweight and obesity, and 10% deficiency and 18% insufficiency in school-age children with overweight and obesity.80
One of the outstanding hypotheses on the causes of the decrease in vitamin D is its lipophilic nature; it becomes diluted in the adipose tissue, reducing its biologic functions.81 It is well known that 25(OH)D participates in the regulation, differentiation, and metabolism of adipose tissue, with effects on lipogenesis and adipogenesis.82 Another hypothesis includes sedentary lifestyle, which involves reduced sunlight exposure and endogenous vitamin D synthesis,83 as well as insufficient dietary intake.84
Different studies have supported evidence of the negative association between vitamin D levels and adiposity parameters (WC, skin fold) and with insulin resistance markers, such as fasting insulin, oral glucose tolerance curve, and the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) index.81,84 Thus, measuring 25(OH)D levels in the patients in that risk group is recommended.
Vitamin D supplementation is recommended at a dose of 400 IU/day in children from 0 to 1 year of age, and at least 600 IU/day in children from 1 to 8 years of age. For children and adolescents with obesity, a dose at least 2 or 3-times higher than the daily requirements by age is recommended. Children from 1 to 18 years of age with vitamin D deficiency should receive 2,000 IU/day for at least 6 weeks or 50,000 IU/week of vitamin D2 for at least 6 weeks, to achieve levels above 30 ng/mL, followed by a maintenance dose of 600−1,000 IU/day.85
Medical and nutritional treatment- 13
Effective management of overweight and obesity requires a multidisciplinary approach encompassing psychologic support, nutritional guidance, and physical activity interventions, along with the timely identification of comorbid conditions. Active involvement of the family is a crucial component of the treatment process. (In agreement: 97%; abstained: 3%)
The main treatment goals are promoting weight loss, preventing and/or improving associated comorbidities, detaining progression to chronic diseases, and attenuating excessive weight gain in the long term.67,86,87
Multidisciplinary management involves caregivers, including parents, family, and school, as well as healthcare professionals (pediatricians or pediatric specialists) for diagnosing, treating, and following up of comorbidities (gastroenterologists, endocrinologists, nutritionists, psychotherapists, pneumologists).34,67
Interventions should be comprehensive and include lifestyle changes, nutritional and behavioral treatment, performing regular physical activity to modify sedentary behavior, and fomenting sleep routines. Pharmacologic and/or surgical treatment should be considered if there is no response to the initial multidisciplinary treatment.67,86,88
- 14
Nutritional treatment should be individualized, focusing on lifestyle changes, such as reducing ultra-processed food intake, following healthy eating guidelines, and establishing personalized nutritional plans, considering the age and sex of the child/adolescent and the baseline grade of overweight or obesity. Restrictive diets should be avoided. (In agreement: 100%)
Nutritional treatment should be carried out by a nutritionist specialized in pediatrics,87 aiming to cover the dietary needs for optimum growth and development,67 avoiding the use of restrictive diets,89 but at the same time, conditioning a nutritional regimen that enables weight loss of 0.5 kg/week to be achieved.87
In addition, strategies should be started for avoiding or limiting added sugar consumption. The WHO,90 LASPGHAN,91 ESPGHAN,92 and AAP1 recommend no added sugar intake before 2 years of age and limiting added sugar consumption to < 5% of the daily energy intake in children 2–18 years of age. In an effort to guide parents and/or caregivers, the present Obesity Working Group adapted the measurement of simple sugar in grams to the equivalent in teaspoons for children above 2 years of age, recommended by the Nutrition Committee of the ESPGHAN (Fig. 4).92
Maximum sugar intake recommendations by age group from the ESPGHAN Nutrition Committee.
Drinks with added sugars should be substituted with plain water or unsweetened milk drinks, and sports drinks and/or energy drinks should be avoided. Increasing the intake of fruits and vegetables is recommended, along with reducing the portion size of snacks.92
Avoiding restricting calories before 6 years of age is suggested, opting instead for increasing physical activity and a balanced and healthy diet. Based on the MyPlate principles, this aids in maintaining healthy weight and can be adapted to different cultures.67,90 Healthy dietary strategy recommendations include reducing the frequency of eating out and increasing the frequency of family home meals, utilizing the SMART (Specific-what?, Measurable-how much?, Achievable-how?, Realistic-with what?, Time-bound-when?) goals, promoting self-control, and discouraging the use of food as a reward.17,66,89 The results of clinical trials that have utilized different dietary interventions for weight control have been controversial. Regarding calorie restriction, low-calorie diets, low-carbohydrate diets, and modified macronutrient diets should only be considered under strict supervision due to the risk of eating disorders and micronutrient deficiency.17,67,87
Family participation in adopting healthy habits (diet and physical activity) is essential for long-term success.89,93 The actions and eating styles of parents and/or caregivers have been shown to positively or negatively change the dietary behavior of the child/adolescent. Eating styles are influenced by cultural norms, the concern of the parents, and the characteristics of the child/adolescent. Dietary practices of parents are based on 4 well-described parenting and feeding styles: negligent, controlling, indulgent-permissive, and perceptive-receptive. Better results, reflecting more democratic and responsible behaviors, have been shown with the perceptive-receptive model, whereas the others generally produce negative consequences.34,93,94
- 15
Physical intervention should be individualized, taking into account physical limitations, preferences, and health status. Children and adolescents with overweight and obesity should perform 60 min/day of moderate-to-intense physical activity at least 5 days/week, combining aerobic and anaerobic exercise. (In agreement: 91%; abstained: 3%; in disagreement: 6%)
Regular physical exercise is a non-pharmacologic intervention that can delay obesity-related comorbidities.95 Cesa et al. conducted a meta-analysis on the effects of physical exercise, reporting a statistically significant decrease in cardiovascular risk (reduced HBP and cholesterol and triglyceride levels), without showing improvement in the BMI.96 The majority of studies suggest at least 60 min/day of exercise, but cognitive and metabolic improvement has been seen with even 20 min/day, 3−5 days/week.97
In 2023, the AAP recommended putting in practice the Intensive Health Behavior and Lifestyle Treatment (IHBLT), as a focus for achieving a decrease in the BMI or attenuating excessive weight gain in children and adolescents. The most consistently effective IHBLT programs offer 26 h or more of face-to-face family counseling on nutrition and physical activity for a period of at least 3–12 months. The IHBLT structure, adapted from the clinical practice guideline of the AAP,1 is summarized in Fig. 5. The physical activity component is more efficacious with the combination of aerobic and anaerobic exercise. Non-competitive, cooperative, and fun activities that develop motor skills and self-confidence are more appealing. Several studies have observed adaptations by children with obesity, including a preference for water activities and non-weight-bearing activities (bicycle riding), and consider physiotherapy or training if there is a low level of physical condition.1
Intensive Health Behavior and Lifestyle Treatment.
A sedentary lifestyle should be avoided. Walking, riding a bicycle, and taking the stairs, instead of using public or private transport, elevators, and escalators, are recommended.98,99
- 16
Pharmacologic treatment is indicated in obese adolescents above 12 years of age, when there is no response to a multidisciplinary intervention (specialist, pediatric nutritionist, psychologist, and physical therapist) focused on lifestyle changes. However, it should be considered from the initial evaluation, when there are severe complications. (First voting round: In agreement: 77%; abstained: 11%; in disagreement: 11%) (Second voting round: In agreement 85%; abstained: 6%; in disagreement: 9%)
The efficacy of pharmacologic treatment combined with lifestyle changes has been shown in patients with obesity, in whom a behavioral focus has been insufficient for reducing BMI and/or comorbidities. Even though therapies for weight loss in pediatric patients are limited, Table 8 describes the drugs approved by the US Food and Drug Administration (FDA).11,100–103
- 17
Bariatric and metabolic surgery is reserved for adolescents with a BMI ≥40 kg/m2 or a BMI >35 kg/m2, who present with severe comorbidities and have an adequate psychologic evaluation. It is essential that the surgery be performed at a bariatric surgery center with experience in pediatric patients. (First voting round: In agreement 76%; abstained: 15%; in disagreement: 9%) (Second voting round: In agreement 85%; abstained: 6%; in disagreement: 9%)
Approved weight-loss medications for adolescents with obesity.
| Drug Generic name | Mechanism | Dose | Side effects |
|---|---|---|---|
| Orlistat | Gastric and pancreatic lipase inhibitor | 120 mg/3 times a day with meals, administered orally | Abdominal pain, steatorrhea, flatulence, fecal urgency and incontinence, liposoluble vitamin deficiency |
| Liraglutide | GLP-1 receptor agonist; improves insulin secretion, decreases gastric emptying, directly affect the regions of the brain related to appetite | 3.0 mg, subcutaneously, once a day | Nausea, vomiting, diarrhea; increased baseline heart rate; hypoglycemia in adolescents with type 2 diabetes mellitus |
| Semaglutide | GLP-1 receptor agonist; improves insulin secretion, decreases gastric emptying, directly affect the regions of the brain related to appetite | 2.4 mg, subcutaneously, once a week | Nausea, vomiting, diarrhea |
| Phentermine/Topiramate | Topiramate: suppresses appetite increasing GABAergic activity | 7.5 mg/46 mg or 15 mg/92 mg, administered orally | Topiramate: reversible cognitive dysfunction, paresthesia, metabolic acidosis; teratogenic effect (orofacial birth defects), may decrease oral contraceptive efficacy |
| Phentermine | Reduces the norepinephrine reuptake that stimulates the neurons in the hypothalamus and reduces the serotonin and dopamine reuptake that improves appetite inhibitory control | 15 mg, 30 mg, or 37.5 mg daily, administered orally Do not use for more than 12 weeks | Insomnia, dry mouth, tremor, headache, dizziness, mood swings, changes in heart rate, and elevated blood pressure |
Approved by the US Food and Drug Administration (FDA) for persons above 12 years of age, except phentermine, which is approved as monotherapy for persons above 16 years of age.
BMI: body mass index; GLP-1: glucagon-like peptide type 1.
Bariatric and metabolic surgery can be considered in adolescents with failed medical-pharmacologic treatment.11,104 It is indicated in patients with a BMI ≥40 kg/m2 or a BMI >35 kg/m2, who present with the comorbidities of obstructive sleep apnea, DM2, idiopathic intracranial hypertension, MASLD, Blount’s disease, slipped upper femoral epiphysis, GERD, or HBP.105 Some authors recommend bariatric surgery at the early stages of adolescence to reduce the possible comorbidities related to age and obesity in adulthood.106
Currently, the most widely used procedures are laparoscopic Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG). Adjustable gastric band surgery was previously carried out but is now in disuse due to high re-intervention rates.107
Results vary according to the procedure. At a 5-year follow-up, weight loss of 26–29 % after RYGB and 27% after SG was reported. The Teen-LABS study showed that higher postoperative weight loss was related to better long-term cardiometabolic risk factor reversal.107 A multicenter analysis with a one-year follow-up after RYGB in adolescents with severe obesity showed a mean decrease in BMI of 37%, reduced MetS rate, as well as 50–100 % resolved DM2, 23–100% reduced dyslipidemia, and 53–100% reduced HBP.108
Contraindications include substance abuse, current or planned pregnancy (within 12–18 months after the procedure), and any psychiatric, cognitive, or psychosocial medical condition that impedes adherence to the postoperative dietary and/or pharmacologic treatment.109
Nutritional follow-up and vitamin and mineral supplementation are essential after the surgery to reduce the risk of deficiencies.110,111
Despite current evidence, further studies are needed that include populations with diverse ethnic, cultural, and geographic characteristics.112
Prevention- 18
Exclusive breastfeeding is one of the protective factors against overweight and obesity. (In agreement: 86%; abstained: 14%)
Exclusive breastfeeding continues to be the most widely recommended form of feeding during the first 6 months of life and should be continued with complementary feeding because of the multiple short-term and long-term benefits. The decision to continue breastfeeding during the second year of life or longer is suggested to be one that is made by the family, or more specifically, the mother and child.113,114
A meta-analysis reported that children breastfed for 9 months had 30% less probability of developing overweight, compared with children that were never breastfed.115 Another study found that breastfed children were less likely to be obese at different ages, compared with formula-fed children.116 Even though many studies have reported said protective effect, it remains a subject of debate, given that some studies have not been able to completely demonstrate it or have related it to other factors, such as epigenetics, hereditary factors, and complementary feeding.117,118 In a systematic review, breastfeeding was found to possibly have a protective effect in children up to 7–8 years of age, attributing it to components, such as ghrelin, adiponectin, leptin, insulin-like growth factor, and insulin, that guarantee satiety signaling and regulate appetite in the neonate, contributing to energy balance and adiposity. Other considerations include the difference in calorie intake between breastfeeding and formula feeding.118,119
Another meta-analysis showed that breastfeeding was inversely associated with the risk for early obesity in children 2–6 years of age. In addition, a dose-response effect was found between breastfeeding duration and a reduced risk of obesity in infancy: one month of breastfeeding was associated with a 4% decrease in the risk of obesity.120
- 19
Overweight and obesity prevention requires the opportune identification of groups with risk factors. (In agreement: 91%; abstained: 9%)
Effective interventions for preventing overweight and obesity and their comorbidities require knowledge of the risk factors for obesity in the early stages of life.121
Excessive weight gain during pregnancy is a predictive factor of childhood obesity.67,122 The risk of childhood obesity increases 2–3 times if one of the parents is obese and up to 15 times if both parents are obese.42 A history of prematurity, low birth weight (<2,500 g) or high birth weight (>4,000 g) in full-term babies, mother with pre-existing diabetes or gestational diabetes, a family history of obesity, dyslipidemia, or DM2, or early cardiovascular disease in first and second-degree relatives, are associated risk factors.67,123 Early suspension of breastfeeding (<6 months) increases the risk of obesity 6–12 times.67 Early intake and high dietary protein content,124,125 accelerated weight gain in the first 2 years of age (defined as a growth trajectory that crossed at least 1 percentile [≥0.67 standard deviation]),121 central/truncal adiposity,68 and sedentary lifestyle68 confer a high risk for developing greater adiposity and insulin resistance. A history of overweight in the first 2 years of life is a predictive factor for obesity at 5 years of age.35 In a systematic review that included 286,804 pediatric patients from 33 countries (including Argentina, Brazil, and Colombia), omitting breakfast was associated with overweight and obesity in 94.7% of patients and with alterations in lipid levels, blood pressure, insulin resistance, and MetS.126Table 9 summarizes other risk factors.
- 20
Strategies for promoting healthy lifestyle habits (diet, physical activity, sleep hygiene, screen use) are recommended for facilitating treatment adherence. (First voting round: In agreement 73%; abstained: 27%) (Second voting round: In agreement 94%; abstained: 3%; in disagreement: 3%)
Other risk factors for overweight and obesity.
| Risk factors | |
|---|---|
| Prenatal | Maternal malnutrition Maternal tobacco and alcohol use during pregnancy |
| Postnatal | Baby bottle volume (≥6 oz) is associated with greater weight-for-age and weight-for-length in 2-month-old infants Cesarean delivery Early antibiotic use |
| Family and home environment factors | Low parental educational level Non-parental caretakers Family member food preferences Parental lifestyle Foods available in the home Parental workload Parental upbringing style Family structure at meals Using food as a reward Sugary drinks Portion size Eating out and family meals Screen time Hours of sleep Environmental smoke exposure Psychosocial stress Living in a single-parent home Adverse childhood experiences |
| Neighborhood and community factors | School environment Lack of access to fresh foods Fast food consumption Access to safe physical activity Environmental health Ultra-processed food availability Socioeconomic level |
| Psychologic factors | Anxiety Depression Eating disorders |
| Political factors | Unhealthy food marketing Lack of nutritional assistance Lack of designated areas for exercise Low-income communities Food insecurity |
Dietary and physical activity interventions were addressed in previous statements (13 and 14).
Increased appetite after sleep restriction has been described. Some studies suggest there is an association between sleep disorders and obesity, reporting hormonal alterations regarding low levels of leptin (anorexigenic) and high levels of ghrelin (orexigenic). Therefore, it is important to recommend at least 8 h of sleep, daily.127–129
More than 2 h of screen time is associated with a higher risk of overweight and obesity, derived from mechanisms, such as increased adiposity, eating or selecting unhealthy foods, and displacement of the time available for physical activity.1,130 Evidence also shows prolonged screen time is associated with irritability, bad mood, depressive symptoms, and cognitive and socioemotional dysfunction.130,131 The screen time recommendations from different medical societies are summarized in Table 10.132–139
- 21
Strict and regular follow-up contributes to realistic and sustainable goals, promoting gradual and lasting lifestyle changes. (In agreement: 97%; abstained: 3%)
- 22
Including strategies in the areas of education and childcare directed at diet and physical activity that are habitually conducted in a family environment may be beneficial for adhering to healthy habits and preventing overweight and obesity. (In agreement: 100%)
Screen time recommendations from different societies.
| Society | Recommendation |
|---|---|
| CPS | Children under 2 years of age: avoid screen use. 2 to 5-year-olds: limit to 1 h/day. 5 to 19-year-olds: there are no recommendations. |
| AAP | Children from 18 to 24 months of age: avoid multimedia content use (except videochat). 2 to 5 years: limit to 1 h/day of high-quality content. Above 6 years of age: there are no recommendation on exact time, but exposure limits should be established. |
| KSPGHAN | There are no age recommendations. Limit non-academic screen use to 1−2 h/day. |
| WHO | Under 2 years of age: avoid screen use. Under 5 years of age: limit screen time to 1 h/day. Less is more. From 5 to 17 years of age: there are no recommendation on the exact amount of screen time. |
| ESPGHAN | Above 2 years of age: there are no recommendation on the amount of time; limited screen time is recommended, as well as avoiding the use of devices with screens during meals. |
| AEP | Under 2 years of age: avoid screen use, there is no safe time. 3 to 5-year-olds: less than 1 h/day of high-quality programming. 6 to 18-year-olds: there are no recommendation on the exact amount of time, and screens should be used purposefully and sparingly. |
| SIP | Under 2 years of age: screen use is not recommended; screens should not be used during meals and not used at least 1 h before going to bed; rapidly paced programs and applications with distracting or violent content are not recommended; screens should not be utilized as a limit pacifier to keep children quiet in public spaces. 2 to 5-year-olds: limit exposure to less than 1 h/day. 5 to 8-year-olds: less than 2 h/day of high-quality programming and only in the presence of an adult. |
| DHAG | Under 2 years of age: avoid screen use. 3 to 5-year-olds: less than 1 h/day. 5 to 17-year-olds: less than 2 h/day. |
AAP: American Academy of Pediatrics (5); AEP: Asociación Española de Pediatría (15); CPS: Canadian Pediatric Society (4); DHAG: Department of Health and Aging of Australia (17,18); ESPGHAN: European Society for Paediatric Gastroenterology, Hepatology and Nutrition (8); KSPGHAN: Korean Society of Pediatric Gastroenterology, Hepatology and Nutrition, Committee on Pediatric Obesity (6); SIP: Sociedad Italiana de Pediatría (16); WHO: World Health Organization (7).
Follow-up visits for children/adolescents with overweight and obesity should be monthly for a minimum of 2 years, lasting 15−60 min per visit.140–142
School and the home are key environments for the prevention of overweight and obesity because children/adolescents spend most of their time in those two places, and they have a significant influence on the dietary options and level of physical activity that children/adolescents develop. Therefore, strategies should be implemented that promote the consumption of plain water, avoid the availability of sugary drinks, promote the intake of healthy snacks and refreshments, avoid the consumption of processed and industrialized products with high energy density, and limit food vending machines in schools.1,143–145 Programs that promote physical activity and include activities or games that avoid sedentary behavior should also be developed and supported.145–147
The present Obesity Working Group created the diagram in Fig. 6, showing the elements to promote and avoid for achieving a healthy lifestyle.
ConclusionsThis document developed by the LASPGHAN Obesity Working Group does not pretend to be a normative instrument, but rather an aid in providing a comprehensive approach to and treatment and follow-up of pediatric patients with overweight and obesity.
Despite the fact that Latin America is a region that shares ethnic and cultural characteristics among its 21 countries, there is a lack of uniformity regarding the growth charts and cutoff points utilized in pediatrics, for clinically diagnosing overweight and obesity, as well as different considerations about the biochemical evaluation protocol in those patients.
With this consensus, our intention is to unify multidisciplinary approach criteria, for preventing and reducing associated comorbidities, identifying the at-risk population, and providing information on the available therapeutic innovations (pharmacologic and surgical). Promoting a healthy lifestyle, from all levels of healthcare, is imperative.
Ethical considerationsEthical approval was not necessary, given that the study did not use patient data or biologic material. No experiment was conducted on animals or humans.
Financial disclosureNo financial support was received in relation to this study/article.
The authors declare that there is no conflict of interest.
The authors wish to thank and recognize the support of the following specialists, for their invaluable participation in the voting rounds on the statements, making the present consensus possible:
Argentina: Daysi Mireya Toapanta Toapanta; Bolivia: Juan Pablo Hayes Dorado, Cristian Javier Rengel Retamoso; Brazil: Mauro Fisberg; Chile: Catalina Le Roy Olivos, Lorena Rodríguez González; Colombia: María Catalina Bages Mesa, Adriana Maria Jaramillo Villegas; Costa Rica: Roberto Bogarín Solano; Cuba: Ivette Pereira Venereo; Dominican Republic: Altagracia Páez Abreu, Pedro José Rijo; Ecuador: Alexandra Salvador Medina, Lissette Elizabeth Macías Salazar; El Salvador: Roberto Arturo Zablah, Ligia Marcela Portillo Canizalez; Guatemala: María del Pilar Gallardo, Marco Antonio Ortiz Guerra; Honduras: Alejandra Marissela Sabillón, Keyla Posadas Mendoza; Mexico: Rubén Peña Vélez, Jennifer Pamela García Pureko; Nicaragua: Milton Mejia Castro, Ivania Fabiola González Cerda; Panama: Judith Ho, Roderick Bejarano; Paraguay: Cristina Medina; Peru: María del Pilar Saénz Naranjo; Portugal: Susana Mesquita Campos; Spain: Marina Llobet Garcés, Montse Amat Bou; Uruguay: Laura Delgado; Venezuela: Sandra Emanuela Neri Ochoa, Livia Thais Machado Hernández.