The Asociación Mexicana de Hepatología A.C. carried out the Consensus on the Management of Complications of Cirrhosis of the Liver in Pediatrics to provide physicians with useful information for treating said complications. A group of pediatric gastroenterologists and experts in nutrition, nephrology, and infectious diseases participated and reviewed the medical literature. The Delphi method was applied to obtain the level of agreement on the statements that were formulated. The statements were sent to the participants to be analyzed and voted upon, after which they were discussed in virtual sessions, and the final versions were produced. The aim of the consensus results was to issue indications for the management of pediatric patients with liver cirrhosis, to prevent or control complications.
La Asociación Mexicana de Hepatología A.C. llevo a cabo el Consenso del manejo de las complicaciones de la cirrosis hepática en población pediátrica con el fin de proveer a los médicos de información útil para su tratamiento. Participaron un grupo de gastroenterólogos pediatras y expertos en nutrición, nefrología y enfermedades infecciosas, quienes revisaron la literatura médica. El método Delphi fue aplicado para obtener el nivel de acuerdo de los enunciados emitidos; estos fueron enviados a los participantes para ser analizados, votados y en sesiones virtuales fueron discutidos, obteniendo así, las declaraciones finales. Los resultados del consenso fueron emitir las indicaciones para el manejo de un niño con cirrosis hepática, con el fin de prevenir o controlar las complicaciones.
Cirrhosis in children presents as a common result of a broad spectrum of genetic, infectious, inflammatory, vascular, and cholestatic diseases1. It tends to be the consequence of two main processes: hepatotoxic lesion (infectious, metabolic, fatty liver) or cholestatic lesion. The latter usually results from bile flow obstruction, mainly biliary atresia (BA)1,2. The majority of cases of chronic liver disease (CLD) can progress to liver fibrosis, cirrhosis, and even to the development of hepatocellular carcinoma3.
In Mexico, liver diseases and cirrhosis of the liver are the fourth cause of death in the general population. There are no specific registers of liver cirrhosis, regarding its incidence, in the pediatric population4,5. There is little high-quality evidence on the diverse complications of the disease, as well as on their diagnosis and treatment, resulting in a lack of strong recommendations for their management. Therefore, the Asociación Mexicana de Hepatología summoned a group of pediatric gastroenterologists with expertise in the area of hepatology and healthcare professionals specializing in pediatric infectious diseases, nephrology, and clinical nutrition, to formulate a consensus that would serve as a useful guide for the management of the complications of liver cirrhosis in pediatric patients.
MethodologyEight roundtables on the different complications of cirrhosis, each with its own coordinator, were organized. The participants were 22 pediatric gastroenterologists and 3 specialists (infectious diseases, nephrology, nutrition). The review committee was composed of three pediatric gastroenterologists (JFC, JACH, and RVF) that developed the protocol and reviewed the statements. The initial information search was carried out on the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed (MEDLINE), and Ovid (EMBASE) and included articles published within the time frame of January 1, 1990 and March 1, 2021. The search keywords were: chronic liver disease in children, cirrhosis in children, combined with the following terms: “epidemiology”, “incidence”, “prevalence”, “Mexico”, “mortality”, “diagnosis”, “differential diagnosis”, “treatment”, “antibiotics”, “infection”, “therapy”, “management”, “nutrition”, “review”, “guidelines”, “transplant”, “meta-analysis”, “portal hypertension”, “esophageal variceal hemorrhage”, “hepatic encephalopathy”, “malnutrition”, “hepatorenal syndrome”, “ascites”, “spontaneous bacterial peritonitis”, “hepatoportal syndrome”, “portopulmonary”, “cholangitis”, and their Spanish equivalents. All publications in English and Spanish were identified (original articles, consensuses, guidelines, systematic reviews, and meta-analyses), along with articles the coordinators considered relevant, and were shared with all the participants.
After the information was reviewed, the statements were formulated by each roundtable and were proposed, analyzed, and evaluated through the Delphi process. Anonymous online voting (with the option of writing comments) was carried out to determine the level of agreement on the statements. Each statement was evaluated on a 5-point Likert scale: a) in complete agreement, b) in partial agreement, c) uncertain, d) in partial disagreement, and e) in complete disagreement.
Only the pediatric gastroenterologists took part in the voting. After the first voting round, the results were presented at several online work sessions (4 for the first round), during which the participants commented on the statements presented. The statements that reached a consensus (agreement >75%) were accepted and those that did not reach a consensus (agreement <75%) were re-evaluated, to be either eliminated or reformulated by the members and sent through a second anonymous voting round. The results of the second round were presented at 2 online work sessions, in which the participants made their comments, utilizing the same statement acceptance method described for round one. A third voting round was carried out, and at the final online working session, the participants gave their comments, and the final consensus statements were established. The coordinators of each roundtable analytically and synthetically formulated their corresponding parts of the manuscript.
ResultsA total of 90 statements were formulated, and after the third voting round and discussion, 66 statements were included in the present document.
Clinical diagnosis and severity evaluationLiver cirrhosis is characterized by 2 clinical stages: compensated cirrhosis, which is not clinically significant (asymptomatic, stable patients), and decompensated cirrhosis, in which there are complications related to portal hypertension and/or the presence of liver failure. On occasion, it can present as acute-on-chronic liver failure secondary to a precipitating event, such as viral, bacterial, or toxic infection. In children, there is usually a history of previous liver damage, often undetected, that progresses and continues its course up to the development of liver failure and patient death6.
There are multiple causes of liver cirrhosis in children (Table 1). Depending on the age group, BA is the most frequent in children under 2 years of age and autoimmune hepatitis is the most frequent in older children. When no cause is found, the origin is called cryptogenic, and its frequency has decreased with the advances in knowledge about new liver diseases, especially the metabolic/genetic diseases and their diagnostic techniques1,7.
Causes of liver cirrhosis in pediatric patients.
| Biliary obstruction | Familial intrahepatic cholestasis | Genetic-metabolic disease | Vascular alterations |
|---|---|---|---|
| Biliary atresiaCholedochal cystLithiasisBile duct stricture | Alagille syndromeFIC1 (ATP8B1) gene deficiencyBSEP (ABCB11) gene deficiencyMDR3 (ABCB4) gene deficiencyBile acid synthesis alteration | A1AT deficiencyType III and IV glycogenosisGalactosemiaFructosemiaTyrosinemia type 1Cystic fibrosisWilson diseaseMitochondrial hepatopathyPorphyria cutanea tardaHemochromatosisWolman disease | Budd-Chiari syndromeVeno-occlusive diseaseCongenital heart disease (heart failure)Constrictive pericarditis |
| Drugs and toxins | Autoimmune | Hepatotropic virus | Other diseases |
|---|---|---|---|
| TPNIsoniazidMethotrexateIntoxication Vitamin A | Autoimmune hepatitisSclerosing cholangitis | Hepatitis B and DHepatitis C | NAFLDNeonatal hepatitisZellweger syndrome |
A1AT: alpha-1 antitrypsin; NAFLD: nonalcoholic fatty liver disease; TPN: total parenteral nutrition.
The pattern of progression of a chronic liver disease to the development of cirrhosis differs, depending on etiology. Children with neonatal cholestasis, such as BA and progressive familiar intrahepatic cholestasis, can develop cirrhosis very early on, whereas patients with autoimmune diseases, hepatitis B virus, hepatitis C virus, and nonalcoholic fatty liver disease can progress with nonspecific signs that go undetected and do not manifest clinically until there is liver decompensation7,8. The search for the causal agent of cirrhosis is important, given that there is specific treatment for some of the causes that can delay or stop progression. Table 2 shows the diagnostic tests for the most common causes of cirrhosis in children1,7,8.
Frequent causes of cirrhosis in children with chronic liver disease and the studies carried out for its diagnosis.
| <2 years of age | >2 years of age | ||
|---|---|---|---|
| Cause | Diagnostic studies | Cause | Diagnostic study |
| Biliary atresia | Intraoperative cholangiogram | Autoimmune hepatitis | ANAASMAAnti-LKM (IgG) |
| Progressive familial intrahepatic cholestasis (PFIC) | GGTBiopsy with immunohistochemistryElectron microscopyGenetic study | Primary sclerosing cholangitis | UltrasoundERCPLiver biopsy |
| Alagille syndrome | Clinical phenotypeLiver biopsyGenetic study | Hepatitis B virus | HBeAg/HBV-DNA |
| Cystic fibrosis | Chloride sweat testGenetic study | Hepatitis C virus | Anti-HCVHCV-RNA |
| Glycogen storage disease | Fasting lactic acid, blood glucose, and lipid profiles Uric acidGenetic study | Wilson disease | Ceruloplasmin24-h copper urineCu in liver biopsy Kayser-Fleischer ring |
| Galactosemia | Reductor substances in urineGalactose-1-phosphate in erythrocytes uridyltransferase level | ||
| Tyrosinemia | Urine succinylacteone Serum amino acids | ||
| A1AT deficiency | A1AT phenotype serum levels | NAFLD | LFTs, lipid profile, blood glucose and serum insulin, HOMA index |
ANA: antinuclear antibody; anti-HCV: antibody against hepatitis C virus; anti-LKM (IgG): liver-kidney microsomal antibody (IgG); ASMA: anti-smooth muscle antibody; Cu: copper; ERCP: endoscopic retrograde cholangiopancreatography; GGT: gamma-glutamyl transpeptidase; HBeAg: hepatitis B e antigen; HBV-DNA: hepatitis B virus deoxyribonucleic acid; HCV-RNA: hepatitis C virus ribonucleic acid; HOMA: homeostasis model assessment; LFTs: liver function tests.
Level of agreement: in complete agreement 95.45%; in partial agreement 4.55%.
The clinical presentation of cirrhosis depends on the cause. Up to 40% of cases can be asymptomatic until the appearance of complications, such as liver failure or portal hypertension7,9,10. Half of the cases at an advanced stage of cirrhosis can have abnormal liver function tests, before diagnosis11. There is no specific biomarker for cirrhosis but the combination of clinical data (anthropometric parameters, failure to thrive, jaundice, hepatosplenomegaly, and ascites), biochemical data (abnormal liver function tests and altered hepatic synthesis), and noninvasive fibrosis markers are helpful as complements to the diagnosis and evaluation of severity7,9,10.
2. The most widely used marker for evaluating the severity of liver disease, its prognosis, and the prioritization for liver transplantation (LT), is the Pediatric End-Stage Liver Disease (PELD) score, for children <12 years of age, and the Model for End-Stage Liver Disease (MELD) score, for patients >12 years of age.Level of agreement: in complete agreement 95.45%; in partial agreement 4.55%.
A PELD or MELD score >25, or signs of decompensation, are indications for LT evaluation. Referral criteria in such cases are acute-on-chronic liver failure and complications, such as lack of weight gain, failure to thrive, variceal bleeding, untreatable ascites, recurrent cholangitis, episodes of peritonitis, uncontrollable pruritus, uncorrected coagulopathy, and progressive encephalopathy12.
3. The Child-Pugh scale and CLIF-SOFA score have been used in adults to estimate prognosis and the need for liver transplantation, but evidence in children is insufficient.Level of agreement: in complete agreement 83.33%; in partial agreement 16.67%.
The Child-Pugh scoring system (ascites, bilirubin, albumin, prothrombin time, international normalized ratio [INR], and encephalopathy) has been underestimated due to the use of subjective variables, such as encephalopathy grade and ascites grade, even though Child-Pugh class B has been described to be an indication for LT13,14. Bolia et al. evaluated the pCLIF-SOFA score in children with decompensated chronic liver disease (CLD) (Table 3), based on the measurement of organ failure, reporting that a score above 11 predicted 28-day mortality, with 94.9% sensitivity and 91.5% specificity15. The factors that worsened prognosis were creatinine levels 0.3mg/dl higher than the previous value (more than a total creatinine level above 2mg/dl)15,16 and a decrease in the serum sodium level17. More studies are needed to validate the pCLIF-SOFA score, but it can be useful in severe cases.
pCLIF-SOFA scale.
| Organ failure evaluation | |||||
|---|---|---|---|---|---|
| Scale | 0 | 1 | 2 | 3 | 4 |
| Respiratory (PaO2/FiO2) | >400 | <400 | a <300 | a <200 | a <100 |
| Neurologic | No HE | Grade 1 HE | Grade 2 HE | Grade 3 HE | Grade 4 HE |
| Circulatory | Nohypotension | aSystolic pressure <5th percentile for age | aDopamine <5mcg/kg/min | aDopamine >5 or epinephrine ≤0.1 or norepinephrine ≤0.1mcg/kg/min | aDopamine >15 or epinephrine >0.1 or norepinephrine>0.1mcg/kg/min |
| Hematologic (INR) | ≤1.1 | >1.1 to <1.25 | ≥1.25 to <1.5 | ≥1.5 to <2.5 | a≥2.5 |
| Kidney(serum creatinine) (mg/dl) | Normal for age | >1 to <2 upper limit of normal for age | a>2 to <3 upper limit of normal for age | a>3 upper limit of normal for age | aUse of renal replacement therapy |
| Liver(serum bilirubin) (mg/dl) | <1.2 | ≥1.2 to <2 | ≥2 to <6 | ≥6 to <12 | a>12 |
HE: hepatic encephalopathy; PaO2/FiO2: partial pressure of oxygen/fraction of inspired oxygen.
Level of agreement: in complete agreement 91.67%; in partial agreement 8.33%.
The AST-to-platelet ratio index (APRI) is a simple method that has been studied in children with different causes of CLD. In the few published studies conducted on children, an APRI score ≥0.51 could predict the presence of advanced fibrosis (F≥3). There is insufficient evidence on the method in the pediatric population, but given its accessibility, it can aid and guide in the decision to perform a liver biopsy18–20.
FibroTest measures serum markers (alpha-2 macroglobulin, haptoglobin, apolipoprotein A1, total bilirubin, and gamma-glutamyl transpeptidase [GGT]) and combines the results with patient age and sex. The score is obtained through those data by accessing a license from a website. The test can only be performed in validated laboratories and distinguishes between patients with significant fibrosis and those with no fibrosis or mild fibrosis. A value >59 is correlated with a METAVIR score >F221. FibroTest has been reported to have an AUROC of 0.97, for differentiating advanced fibrosis (F2–F4) from mild fibrosis or no fibrosis (F0–F1), in children with hepatitis due to the hepatitis C virus22.
5. For the evaluation of advanced fibrosis and liver cirrhosis, elastography and magnetic resonance elastography detect the degree of liver stiffness and its correlation with portal hypertension, but more studies are required for their validation in the pediatric population.Level of agreement: in complete agreement 86.36%; in partial agreement 13.64%.
Transitory elastography (TE) with FibroScan® detects the degree of liver stiffness23. Few studies have been conducted on children that determine standardized values24,25. According to age, the upper limit of normal in toddlers, school-age children, and adolescents, was reported at values of 5.96, 6.65, and 6.82kPa, respectively24.
In children with different CLDs, TE showed variations in the cutoff levels for distinguishing the presence of cirrhosis (F4); in BA, 18.1kPa was reported, in hepatitis C virus, 12.5kPa, and in CLDs of different etiologies, 14.1kPa26–28. In addition, TE has been studied for detecting portal hypertension29,30. TE is a useful technique for detecting fibrosis in children, but it does not distinguish between mild and moderate stages. It can be a complementary study, together with the clinical and biochemical evaluation of the patient at diagnosis and during follow-up, in children with CLD.
Magnetic resonance elastography (MRE) examines entire sections of the liver and can measure both hepatic stiffness and splenic stiffness, as well as comprehensively evaluate advanced liver fibrosis/cirrhosis and portal hypertension. Nevertheless, the majority of studies have been conducted on adults and there is scant pediatric information. A study utilizing MRE to measure splenic stiffness reported that a cutoff value of 9.9kPa could predict esophageal varices post-Kasai procedure, in children with BA31.
6. Liver biopsy provides the definitive diagnosis of cirrhosis, evaluates the severity and prognosis of the disease, and is useful for treatment response follow-up. Because it is an invasive procedure, it should be individualized..
Level of agreement: in complete agreement 91.67%; in partial agreement 4.17%, uncertain 4.17%.
Liver biopsy is the gold standard for evaluating the grade of liver fibrosis or cirrhosis. There are several histologic staging classifications. The METAVIR score is the most widely used in pediatrics and classifies fibrosis into 5 stages: F0: absence of fibrosis, F1: portal fibrosis, F2: periportal fibrosis, F3: bridging fibrosis in the portal areas, and F4: cirrhosis32. The disadvantages of liver biopsy are its high cost, sampling error, and interobserver variability. In addition, it is not exempt from complications, including bleeding (2.8%), bile duct leak (0.6%), pneumothorax (0.2%), and mortality in up to 0.6% of cases33. With respect to the risk of bleeding after the procedure, even though there are no parameter cutoff values for identifying alterations in hemostasis, platelets <60,000/mL and an INR >1.5 are indicators of risk for bleeding34. The indication for the procedure should be individualized, and used after thorough evaluation with noninvasive methods has not clarified the diagnosis.
Nutritional considerations in complications of cirrhosis in pediatricsMalnutrition and sarcopenia have an elevated prevalence in the cirrhotic patient (40–80%). They have similar physiologic mechanisms. Sarcopenia is specifically caused by muscle mass loss, with a subsequent loss of strength and reduced physical performance. It has been more frequently described in patients with a higher Child-Pugh score. Both entities play a fundamental role in the morbidity and mortality of the cirrhotic patient, and so their opportune diagnoses and treatments are determining factors in patient progression35,36. Surveillance of the nutritional status in the pediatric patient is essential for ensuring growth and development. This is especially true in patients with an added chronic condition, such as cirrhosis, whether compensated or decompensated, mainly because many of the metabolic cycles and biochemical alterations present in its development are closely associated with nutritional status.
7. The weight of cirrhotic pediatric patients should be carefully considered, given that it is affected by ascites and visceromegaly.Level of agreement: in complete agreement 100%.
The anthropometric evaluation of the pediatric patient with cirrhosis should include weight, height, head circumference (in children under 3 years of age), mid-arm circumference (MAC), and tricipital skin fold.
Weight should not be the only marker of nutritional status, principally in patients whose height is below 2 standard deviations (<2 SDs). Likewise, the body mass index or weight-for-height do not distinguish body components, such as lean mass or fat mass. The MAC and tricipital skin fold measurements are excellent tools for evaluating the changes in body composition because they correlate well with body composition measurements carried out through bone densitometry. Importantly, arm anthropometry principally enables the identification of lean mass loss and is not affected by ascites or visceromegaly37–41.
8. The frequency with which the anthropometric evaluation of the pediatric patient with cirrhosis should be carried out depends on his/her liver compensation and nutritional status.Level of agreement: in complete agreement 87.5%; in partial agreement 12.5%.
In severely nutritionally compromised patients or those with liver decompensation, monitoring should be strict, from twice a week to twice a month, whereas patients with moderate or mild nutritional compromise can be evaluated at least every 3 months9,37,42. An adequate dietary history, together with a complete anthropometric and clinical evaluation, enables any type of nutritional deficiency to be identified and opportune intervention to be carried out. The primary aim is always to prevent malnutrition-sarcopenia, or establish their reversal as quickly as possible, if they already exist, to prevent the development of morbidities that worsen the prognosis of the cirrhotic patient7,9,37,43.
9. Predictive equations for establishing the calorie goal in the pediatric patient with cirrhosis are not recommended.Level of agreement: in complete agreement 79.17%; in partial agreement 20.83%.
One of the main challenges, principally in patients with end-stage disease, is establishing the calorie goal for achieving an ideal nutritional status because it is dependent on the severity and complications of the liver disease. The requirements of the World Health Organization (WHO), according to age and sex, can be used as a starting point, followed by evaluating the nutritional progression and making the corresponding adjustments37,44,45. One of the most important considerations is estimating the energy requirements that depend on different factors, such as a) the metabolic imbalance caused by hepatocyte dysfunction, affecting the macronutrient metabolic pathways, b) increased catabolism, due to the liver dysfunction itself and the complications of the cirrhotic patient, and c) energy loss due to malabsorption, secondary to cholestasis, pancreatic insufficiency, or protein-losing enteropathy. All those conditions increase the energy requirement, in varying degrees, according to the condition of each individual37,45,46.
To establish the energy requirements, the guidelines proposed by the WHO can be used as a starting point, but those requirements can be increased by 120 to 150%, or even to more than 150%, depending on the patient’s nutritional status and the compensation status of the cirrhosis, to achieve nutritional recovery. Energy supply modifications are made in line with the anthropometric progression of the patient, dietary adherence, and the identification of other factors that affect compliance with the planned calorie goal37,43,44.
10. The dietary energy supply from carbohydrates is recommended to be 50–65%, preferably from complex carbohydrates that are especially useful for glucose control in hypoglycemic patients or in those with insulin resistance.Level of agreement: in complete agreement 100%.
A dietary composition related mainly to carbohydrates and proteins is essential in the cirrhotic patient, due to metabolic cycle alterations, more frequently seen in infants and in patients with advanced stages of cirrhosis. Simple carbohydrate intake can favor those alterations in glucose control37,38,47.
11. Protein restriction is not recommended in pediatric patients with cirrhosis.Level of agreement: in complete agreement 90.91%; in partial agreement 4.55%; uncertain 4.55%.
Protein requirements surpass those established by the WHO for age and sex and range from 2 to 4g/kg/day. Branched-chain amino acid oral supplements have been shown to be useful in nutritional recovery38,47–50. Protein requirements are increased due to metabolic dysfunction secondary to liver damage and to compromised nutritional status. Therefore, to prevent protein catabolism, the diet should contain at least 2g/kg/day of protein, keeping in mind that the protein supply should be directly related to nutritional status, sarcopenia, and the compensation status of the cirrhosis47,48. Patients with encephalopathy, with ammonia levels between 150 and 200μmol/l, are at greater risk for brain edema and could require protein restriction. However, said restriction should not last longer than 48h because prolonged fasting and the stress those patients suffer increase protein catabolism, and in turn, ammonia levels. Protein restriction does not justify reducing the dietary energy supply, which should be met 100%, with even an extra 20% to reverse catabolism. In pediatric patients, branched-chain amino acid supplements in powder have not been shown to be useful in the management of encephalopathy. However, their oral administration can aid in the nutritional recovery of malnourished patients with advanced liver disease37,51,52.
12. In cirrhotic patients with cholestasis, supplementation with liposoluble vitamins and medium-chain triglycerides is recommended, at a proportion of 30–70% of total dietary fat, and not exceeding 80%, to prevent essential fatty acid deficiency.Level of agreement: in complete agreement 90.91%; in partial agreement 4.55%; uncertain 4.55%.
Fat intake should be normal in patients with compensated or decompensated cirrhosis, at between 25 and 30% of the total energy supply and should only be increased in patients with malabsorption37,38,47,49.
13. The use of a feeding tube, preferably nasogastric or transpyloric, should be considered for providing high energy-dense enteral nutrition (1–1.5kcal/mL) to all patients that do not meet their calorie intake goal on their own.Level of agreement: in complete agreement 79.17%; in partial agreement 16.67%.
Enteral nutrition is also indicated in patients with severe malnutrition or a MAC<the 5th percentile, or in patients that have decompensated cirrhosis with comorbidities that limit their nutrient intake. The use of a feeding tube is safe and does not increase the risk of rupture of esophageal varices; ideally, it should be made of a soft material, such as silicone. Initially, enteral diet support can be given through the oral route, if the patient has not reached his/her calorie goal in 2 weeks37,47,48,53.
14. Parenteral nutrition should be utilized when the oral or enteral routes cannot be used for more than 72h or as a caloric complement when adequate height and weight growth cannot be achieved, despite full enteral support.Level of agreement: in complete agreement 81.82%; in partial agreement 13.64%; in partial disagreement 4.55%.
Importantly, the cirrhotic patient must cover 100% of his/her estimated dietary energy support, regardless of the compensation status of the cirrhosis37,53,54.
15. Sodium restriction should be established only in cases of decompensated ascites.Level of agreement: in complete agreement 91.67%; in partial agreement 8.33%.
Routine sodium restriction in all cirrhotic patients can compromise food acceptance and calorie intake. Restriction is considered to be <2mEq/kg of sodium per day, together with the corresponding management of diuretics and fluids1,37,43,54,55.
Hepatic encephalopathyHepatic encephalopathy (HE) is a brain dysfunction conditioned by liver failure and/or portosystemic shunting. It can manifest as overt hepatic encephalopathy (OHE) or as minimal hepatic encephalopathy (MHE). The prevalence of HE in cirrhotic children is between 30 and 40%. In OHE, the clinical spectrum includes neuropsychiatric abnormalities, from subclinical alterations to coma, and all extrinsic diseases should be ruled out. In severe HE, intensive care with strict surveillance of glucose levels and the fluid and electrolyte balance is of the utmost importance1,56–61.
MHE can go by undetected because the neuropsychiatric manifestations are not clinically apparent and only show up as abnormalities on neurologic or electrophysiologic tests1,56,59,62. The exact prevalence of MHE in children is not known but may be present in up to 50% of cases63.
16. Overt HE is diagnosed through the West Haven clinical criteria adapted for children and the Glasgow coma scale for infants and adolescents. Psychometric and neurophysiologic tests are utilized to diagnose MHE, but there is no standardized pediatric detection test.Level of agreement: in complete agreement 91.3%; in partial agreement 8.70%.
In HE, attention, work memory, psychomotor speed, visual-spatial capacity, electrophysiologic capacity, and brain function are altered. Its progression presents as apathy, irritability, disinhibition, motor function alterations, asterixis, alertness alterations, disrupted sleep cycle, time-space disorientation, inappropriate behavior, acute confused state with agitation or somnolence, stupor, and finally, coma60,64.
The West Haven criteria are the gold standard for the clinical evaluation of HE (Table 4)60. In cases of suspected MHE, at present there is no gold standard, and psychometric and neurophysiologic tests, the number connection test, psychometric hepatic encephalopathy score, critical flicker frequency measurement, continuous reaction time test, inhibitory control test, Stroop test, and the behavior rating inventory of executive function (BRIEF-2) evaluation are all instruments that have been utilized to detect MHE in adults. There are no data on the pediatric population that enable deciding which of those methods is the most appropriate, and evidence on their use in children is currently insufficient64–66.
West Haven criteria and clinical description.
| Clinical picture | Description | Criteria | Comments | |
|---|---|---|---|---|
| Minimal | Asymptomatic | Alterations in the psychometric and neuropsychologic tests that explore psychomotor speed, executive functions, or neurophysiologic alterations with no clinical evidence of mental changes | Abnormal psychometric and neurophysiologic test results with no clinical manifestations | There are no universal criteria for the diagnosis and the local standards and expertise required are limited |
| Grade I | Asymptomatic | Trivial lack of awarenessEuphoria or anxietyShort attention span Impaired ability to add and subtractAltered sleep rhythm | Temporal and spatial disorientationBehavioral and cognitive decay reported by the caregiver and corroborated by the physician | Clinical findings are not usually consistent |
| Grade II | Symptomatic | Lethargy or apathyTemporal disorientation Personality change Inappropriate behavior Dyspraxia and asterixis | Temporal disorientation (at least three of the following are answered incorrectly: month, day of the week, year, season)±the other symptoms mentioned | Clinical findings are variable, but consistent to some extent |
| Grade III | Symptomatic | Somnolence or semistuporous state Responsive to stimuli Confused Gross disorientationBizarre behavior | Spatial disorientation (at least three of the following are answered incorrectly: place, city, state, country) | Clinical findings are consistent to some extent |
| Grade IV | Symptomatic | Coma | Non-responsive to pain | Comatose state |
Level of agreement: in complete agreement 95.65%; in partial agreement 4.35%.
18. A normal ammonia level does not exclude the presence of HE.Level of agreement: in complete agreement 100%.
The determination of ammonia levels is not a reliable indicator for the diagnosis of HE or for its evaluation or resolution. Ammonia levels have been shown to not always correspond to the clinical status of the patient and there may be coexisting factors, such as bacterial overgrowth, elevated protein intake, portosystemic shunt, zinc deficiency, or inadequate sample (prolonged tourniquet, lack of transport on ice), among others, that modify ammonia levels60,67,68.
19. An electroencephalogram detects changes in cortical brain activity. The study is not age-dependent, is nonspecific, and does not necessarily determine the grade of encephalopathy.Level of agreement: in complete agreement 86.36%; in partial agreement 9.09%; uncertain 4.55%.
Electroencephalogram alone is nonspecific for diagnosing HE. It can detect nonspecific changes in cortical brain activity. Its performance does not involve patient cooperation and so can be carried out on patients of any age. Its findings include the slowing of wave frequency, desynchronization of the dominant activity, reduced activity in the θ and/or δ bands, and the appearance of triphasic waves60,64.
20. For the differential diagnosis of HE, imaging studies, such as computed tomography and magnetic resonance imaging (MRI), are used to rule out other pathologies. Magnetic resonance spectroscopy can detect neurotoxic metabolites and scintigraphy has prognostic value in MHE.Level of agreement: in complete agreement 81.82%; in partial agreement 18.18%.
At present, there is no imaging method that can diagnose HE or MHE. Tomography, MRI, and scintigraphy have been used as part of their study protocol, and spectroscopy has been employed to obtain diffusion tensor images of the brain and mean diffusivity and fractional anisotropy maps, as possible biomarkers of MHE.
The neuropsychologic tests have been reported to have a significant positive correlation with choline and cerebral myoinositol identified through MRI. The increase in intracellular osmolarity caused by hyperammonemia produces a reduction in the resonances of choline and myoinositol and an increase of glutamine-glutamate in the cirrhotic patient. Those metabolic anomalies are correlated with clinical severity and are reversible with treatment.
Scintigraphy has been shown to have a positive prognostic value, given that it has the capacity to measure the speed and precision for carrying out a memory task of growing complexity60,63,69,70.
21. The aims of HE prevention measures are to optimize nutritional status, identify precipitating factors, and if necessary, establish pharmacologic prophylaxis.Level of agreement: in complete agreement 86.96%; in partial agreement 13.04%.
The presence of OHE in cirrhotic patients represents a status of disease decompensation that is usually associated with precipitating factors that should be treated. Those factors, according to the time at which they present, are of the following types: a) episodic (the last presentation of HE in more than 6 months): infections, gastrointestinal bleeding, diuretic overdose, electrolyte disorders, and constipation and b) recurrent (the last presentation of HE in fewer than 6 months): electrolyte disorders, infections, constipation, diuretic overdose, gastrointestinal bleeding, and other unidentifiable factors59,60,71.
22. First-line pharmacologic treatment for HE is the administration of lactulose (by enema or oral route) and intraluminal antibiotics, in children and adults.Level of agreement: in complete agreement 100%.
The majority of studies have been conducted on adult patients. Studies on children with cirrhosis reported that 73% of the cases treated with lactulose had complete recovery from OHE61,72.
23. After a recurrent episode of HE, the administration of lactulose and/or intraluminal antibiotics (rifaximin) is recommended as secondary prophylaxis.Level of agreement: in complete agreement 81.82%; in partial agreement 13.64%; uncertain 4.55%.
OHE should be treated routinely, whereas MHE, because it is not obvious upon physical examination, is treated only when there are special circumstances that are significant in the daily life of the patient, such as quality of life and/or cognitive difficulties60.
The recommendation for preventing OHE recurrence is secondary prophylaxis with nonabsorbable sugars, of which lactulose, lactitol, and polyethylene glycol (PEG) are those that have been utilized in children56,61,62,73. Despite the fact that there are no randomized placebo-controlled trials on the subject, lactulose has been recommended for maintaining remission. The combination of lactulose and rifaximin has been reported to maintain remission in patients that have had one or more events of HE. The recommended prolonged cyclical therapy of 3–6 months reduces episodes of symptomatic HE59,71.
Antibiotics reduce the quantity of ammonia-producing bacteria, and those with luminal effects (neomycin, rifaximin) should be preferred, albeit others, such as metronidazole and vancomycin, can also be used74–76.
l-ornithine l-aspartate (LOLA) has been used for MHE management. It improves encephalopathy and has been shown to reduce mortality73,75,76.
Probiotics have also been used for preventing and improving MHE, but they have been reported to not have an impact on mortality. Evidence is scarce, and so more controlled studies are needed to recommend their routine use62,73,75,76.
24. The combination of l-carnitine and zinc has been shown to improve HE grade.Level of agreement: in complete agreement 80.95%; in partial agreement 19.05%.
l-Carnitine has been shown to have a protective effect against the neurotoxicity of ammonia and to improve the energy of patients and the grade of encephalopathy. Zinc improves neurotransmission and aids the conversion in the liver of amino acids to urea but has not shown an effect on encephalopathy if used alone62,73,76.
25. Liver transplantation is a treatment option in cases in which HE does not improve with maximum medical therapy or affects the quality of life of the patient, regardless of whether liver function remains stable.Level of agreement: in complete agreement 78.26%; in partial agreement 17.39%; uncertain 4.35%.
All patients with decompensated cirrhosis or advanced liver damage and with a first episode of HE should be evaluated for LT. Patients experiencing poor quality of life and deteriorating scholastic activity should also be considered. The opportune performance of LT can improve school performance and neurocognitive functions in the long term, but it will not have the same impact at the neurologic level if it is performed late, especially if the deterioration began in the first years of life61,77,78.
Portal hypertension and coagulopathyPortal hypertension and variceal bleeding are the main causes of morbidity and mortality in pediatric patients with cirrhosis. The mortality rate for variceal bleeding ranges from 7.3 to 20%. The associated morbidity of bacteremia, peritonitis, and acute kidney failure has a great impact on mortality due to variceal bleeding78,79.
26. The use of nonselective beta-blockers to prevent a first event of variceal bleeding is a sustained indication in adults, but there are no clinical trials in pediatrics that demonstrate their efficacy. The risk-benefit must be considered for their use.Level of agreement: in complete agreement 100%.
The pharmacologic treatment goal in portal hypertension in the cirrhotic patient is to modify the hepatic and splanchnic vascular bed. In adults, primary prophylaxis (to prevent the first episode of variceal bleeding) is a well-established therapy, supported by clinical trials that demonstrate the benefit of nonselective beta-blockers at doses that reduce the heart rate by 25% or portal venous pressure by 20% and the use of endoscopic variceal ligation, achieving a reduced blood loss rate. Given that there are no controlled clinical trials on pediatric patients, the risk-benefit of their use must be considered80–83.
27. Variceal ligation can be considered in the prevention of a first episode of bleeding, in cases with a high risk for bleeding, such as multiple grade 2 or 3 esophageal varices (EVs) with cherry-red spots.Level of agreement: in complete agreement 95.65%; in partial agreement 4.35%.
Observational studies have evaluated variceal endoscopic ligation in children with cirrhosis as primary prophylaxis of a first bleeding episode, with good results, at a short-term follow-up of 16 months. However, ligation is limited to children that weigh more than 10kg84.
28. The treatment of an acute episode of bleeding includes resuscitation, blood volume restoration, vasoactive therapy (octreotide), and endoscopic therapy, in a stable patient.Level of agreement: in complete agreement 91.3%: in partial agreement 8.7%.
The treatment of an acute episode of bleeding includes resuscitation and volume restoration to achieve hemodynamic stability, as well as conservative packed red blood cell transfusion. The goal is a hemoglobin level of 7–8g/dl that should be individualized, taking into account the associated comorbidities. Octreotide administration should be started before endoscopy, at a dose of 2–5μg/kg/h, as continuous infusion for 5 days78,84,85.
29. Antimicrobial prophylaxis within the first 48h of admission can have a positive impact on reducing bacteremia and the 30-day readmission rate.Level of agreement: in complete agreement 78.26%; in partial agreement 21.74%.
Prophylaxis with antibiotics is an integral part of treatment and should be considered from the time of patient admission, in cases of advanced disease (Child-Pugh class B and C), in accordance with individual risk factors and the local antimicrobial susceptibility patterns. The use of antibiotics within the first 48h of hospital admission, including third-generation cephalosporin (ceftriaxone 100mg/kg/day), could have a positive impact on the percentage of bacteremia-free children and the 30-day readmission rate86.
30. Secondary prophylaxis is the treatment after the first episode of bleeding, and sclerotherapy and/or EV ligation is indicated.Level of agreement: in complete agreement 95.65%; in partial agreement 4.35%.
In the pediatric patient, both sclerotherapy and endoscopic variceal ligation (EVL) have been reported to eradicate esophageal varices in 91 and 96% of the cases, respectively. Different sclerosing agents have been used in children, including ethanolamine at 5%, sodium tetradecyl sulfate at 1 or 1.5%, sodium morrhuate at 5%, and polidocanol at 1–1.5%. An average of 4 sessions are required for the eradication of varices through ligation, compared with 6 sessions of sclerotherapy (p≤0.0001). There may be rebleeding in 25% of patients undergoing sclerotherapy and in 4% of those undergoing ligation (p=0.49). After undergoing endoscopic sclerotherapy or EVL, the patient should be monitored, checking for probable acute complications. Acid suppression with proton pump inhibitors or local therapy with sucralfate should be administered, to promote cicatrization of the ulcer87–89.
31. Treatment for gastric varices (GVs) is ligation for esophagogastric varices (GOV1) and cyanoacrylate application for GOV2 varices and isolated GVs. The performance of the latter procedure is limited in pediatrics but has shown good results.Level of agreement: in complete agreement 82.71%; in partial agreement 17.39%.
In case series with pediatric patients, good response in the control of bleeding has been reported with the use of cyanoacrylate as secondary prophylaxis of GV bleeding. Variceal obturation or obliteration is performed using tissue adhesive (such as N-butyl-cyanoacrylate or isobutyl-2-cyanoacrylate), which polymerizes immediately upon contact with blood. It is strictly injected into the varix and obliterates large GVs90,91.
32. In cases of severe rebleeding after endoscopic treatment, the application of Hemospray® is useful as a rescue procedure, whether for EVs, GVs, or portal hypertensive gastropathy.Level of agreement: in complete agreement 78.26%; in partial agreement 8.7%; uncertain 13.04%.
The use of Hemospray® for gastrointestinal bleeding achieves hemostasis rates above 90%, with low recurrence. Although initially employed for peptic ulcer, there are publications on its usefulness even in variceal bleeding, thus it can be used as rescue therapy in cases in which the patient has presented with rebleeding after ligation or sclerotherapy92,93.
33. In cases of bleeding due to portal hypertensive gastropathy, coagulation with argon or laser reduces the need for transfusions in 85% of cases with localized involvement, and a lower response in diffuse disease.Level of agreement: in complete agreement 86.36%; in partial agreement 9.09%; uncertain 4.55%.
Argon plasma coagulation for the treatment of cirrhotic patients with portal hypertensive gastropathy achieves control of bleeding in one session in 75.4% of cases and significantly improves hemoglobin levels, reducing the need for transfusions in 85% (p<0.0001)94,95.
34. Compared with pharmacologic and endoscopic treatment, balloon tamponade is less efficacious and should be reserved as a rescue measure and bridge therapy before a definitive (surgical) procedure.Level of agreement: in complete agreement 86.36%; in partial agreement 9.09%; uncertain 4.55%.
The insertion of a double-balloon tube (esophageal and gastric) has a role in the treatment of variceal bleeding, in hemodynamically unstable patients with high transfusion requirements that do not respond to medical and endoscopic treatment, and so are candidates for double-balloon tube placement. If the device remains in place for more than 4 days, pressure necrosis or aspiration pneumonitis can develop, and so it should be considered temporary salvage therapy until a definitive procedure, such as LT or a portal shunt, is carried out96,97.
35. Coagulopathy is a complication in patients with cirrhosis. The usefulness of correcting laboratory values with blood products in patients that are not presenting with bleeding is questionable and can expose them to higher risk.Level of agreement: in complete agreement 82.61%; in partial agreement 13.04%; uncertain 4.35%.
There is little information on the risk of bleeding from invasive procedures, in the context of acute liver failure, nor are there clinical guidelines, with respect to a corrected INR goal, for preventing bleeding. Currently, the general consensus is that the INR should be corrected to ≤1.5, for controlling the risk for bleeding before a procedure. Aggressive treatment of an abnormal INR, with large volumes of fresh frozen plasma (FFP) in this type of patient can be harmful, given that volume overload increases the hepatic venous pressure gradient, with the subsequent risk for variceal bleeding. In the case of thrombocytopenia in the cirrhotic patient that undergoes variceal ligation, the administration of a unit of platelets produces a slight increase in the platelet count, with no significant effect on thrombin production or thromboelastometry98–100.
36. The management of bleeding due to coagulopathy includes the administration of FFP, vitamin K for correcting prolonged prothrombin time (PT), infusion of cryoprecipitates for hypofibrinogenemia, and platelet concentrates when platelet figures are < 50,000, if the patient is going to undergo an invasive procedure.Level of agreement: in complete agreement 90.91%; in partial agreement 4.55%; uncertain 4.55%.
In children, a dose of 15ml/kg of FFP, ranging from 10 to 20ml/kg, with PT monitoring after FFP infusion, to ensure reaching the desired INR correction, is recommended. In cases of acquired coagulopathy in a patient with suspected vitamin K deficiency, he/she should receive supplementation, but if there is no response, the repeated administration of large doses should be avoided. The pediatric dose for treating vitamin K deficiency is 30μg/kg, administered intravenously, subcutaneously, or orally. In children and adults, there is general agreement in the guidelines that fibrinogen should be replaced, when the figure is below 100g/dl. The dose of cryoprecipitate in pediatrics is 4−5ml/kg or 1 unit (10−20ml) for every 10kg of body weight. Maintaining a platelet figure above 50,000/dl before an invasive procedure with a high risk for bleeding, or for treating overt bleeding, is recommended101,102.
37. If the PT is not corrected with FFP and there is a risk for circulatory overload, the use of prothrombin complex concentrate is recommended as an alternative.Level of agreement: in complete agreement 90.91%; in partial agreement 4.55%; uncertain 4.55%.
Prothrombin complex concentrate has been used in the context of liver disease for controlling bleeding and preparing for elective surgery that has a risk for bleeding. It is also useful when the risk of circulatory overload limits the use of FFP. The dose is 20–25 units/kg103.
38. Surgical portosystemic shunting and LT should be considered when variceal bleeding persists, despite the combination of pharmacologic and endoscopic therapy. If those interventions are not feasible, a palliative transjugular intrahepatic portosystemic shunt is indicated.Level of agreement: in complete agreement 86.96%; in partial agreement 13.04%.
There are different surgical approaches for treating variceal bleeding secondary to liver cirrhosis that is refractory to medical and endoscopic treatment. They range from the mesorenal, splenorenal, and mesenteric/left portal vein (meso-Rex shunt) diversion surgeries that can be considered, depending on the anatomy of the portal hypertension of each patient (collateral development, presence of venous thrombosis) and the stage of liver disease, to LT as the definitive treatment104,105.
Acute kidney injury and hepatorenal syndromeAcute kidney injuryAcute kidney injury (AKI) is a complication in patients with decompensated liver cirrhosis that has an incidence of up to 20% of adult hospitalized patients. Its incidence in children is not known106,107.
AKI is defined as the increase of serum creatinine (sCr) ≥0.3mg/dl (26.5μmol/l) from the baseline in the first 48h or an increase ≥50% in the sCr baseline value within 7 days prior to diagnosis, according to the Kidney Disease Improving Global Outcome criteria, adapted and validated in pediatrics108–110.
39. The treatment of AKI should be started as soon as the diagnosis is made and includes the suspension of diuretics and nephrotoxic medications, the treatment of infectious processes, and the maintenance of adequate intravascular volume.Level of agreement: in complete agreement 90.91%; in partial agreement 9.09%.
The treatment of AKI in patients with cirrhosis should be started at diagnosis to prevent progression. It is important to identify and treat precipitating factors, such as infections, nephrotoxic medications (nonsteroidal anti-inflammatory drugs and β-blockers), and gastrointestinal bleeding, to prevent later renal complications107,109,111,112.
Diuretics should be suspended, and intravascular blood volume should be expanded through the administration of intravenous fluid and/or albumin at a dose of 1g/kg/day. Albumin tends to be the recommended volume expander due to its anti-inflammatory and antioxidant effects. In patients with AKI and tense ascites, therapeutic paracentesis combined with albumin infusion should be performed, even when a low volume of ascitic fluid is removed108–110,113.
Hepatorenal syndromeHepatorenal syndrome (HRS) is prerenal failure due to a decrease in kidney perfusion in patients with severe chronic liver disease, cirrhosis, or acute liver failure. Incidence in children has been reported at 5%109,111.
HRS is classified as HRS-AKI, formerly type 1 HRS, defined by an increase in baseline sCr >0.3mg/dl within 48h from onset, or a 50% increase in the baseline value, over the past 3 months. HRS-NAKI (“non-AKI”), formerly type 2 HRS, is associated with the existence of acute (fewer than 90 days) or chronic (more than 90 days) renal dysfunction106,109.
40. In a cirrhotic patient with AKI and no response to initial treatment, the presence of HRS-AKI criteria should be evaluated, and treatment started with a vasoconstrictor, such as terlipressin or norepinephrine plus albumin.Level of agreement: in complete agreement 90.91 %; in partial agreement 9.09%.
The diagnosis of HRS-AKI should be considered if kidney function does not improve after adequate volume expansion, and treatment started with albumin at a dose of 1g/kg/day. Systemic vasoconstrictors are first-line treatment109,111,114. Terlipressin is recommended as the first option in HRS-AKI in adults and has only been reported in case reports on children. Doses used in children vary from 15 to 20μg/kg every 4h, combined with albumin infusion, until kidney function improves. In cases of recurrence of HRS-AKI upon treatment cessation, the therapy should be repeated. Noradrenaline is another vasopressor used in intensive care units. Some studies recommend its use, in conjunction with albumin, in the initial treatment of HRS-AKI. The recommended dose is 0.5–1μg/kg/min109,113–115.
41. Renal replacement therapy (RRT) can be indicated for patients with HRS-AKI that do not respond to pharmacologic treatment and that present with volume overload or uremia. Its use should be reserved for bridging therapy to LT.Level of agreement: in complete agreement 86.36%; in partial agreement 13.64%.
Extracorporeal RRTs can be useful in the treatment of HRS. There are few studies providing guidance for identifying pediatric patients with cirrhosis that are candidates for RRT. Some have reported that up to 40% of patients with AKI require treatment with RRT. RRT is indicated in patients with HRS-AKI that do not respond to treatment with albumin and vasopressors and that also present with signs of uremia, hypervolemia, severe metabolic acidosis, or hypercalcemia110,116. RRT does not improve survival in HRS, but can be useful as a bridge to LT, when said transplant is indicated. Hemodialysis continues to be the recommended technique in patients that do not respond to medical treatment, mainly when they are on the waiting list for LT110,116,117.
42. LT is the treatment of choice for HRS.Level of agreement: in complete agreement 86.36%; in partial agreement 13.64%.
In patients that do not respond to any medical treatment modality, LT becomes the only option for survival108,117,118.
43. Liver/kidney transplantation should be considered in patients with significant chronic kidney disease (HRS-CKD), or acute persistent disease, including sepsis-associated AKI with no response to pharmacologic treatment.Level of agreement: in complete agreement 95.45 %; in partial agreement 4.55%.
Even though many cirrhotic children with kidney disease recover after LT, a large number require support with RRT after transplantation, which is why simultaneous liver-kidney transplant (LKT) has been considered an option116,119. Numerous studies have recommended guidelines for establishing the criteria of LKT indication, but it has not yet been defined. Patients with cirrhosis and AKI, regardless of the type, are considered candidates for LKT, when they do not respond to RRT >4 weeks or have an estimated glomerular filtration rate (GFR) ≤35ml/min or measured GFR ≤25ml/min ≥4, as well as patients with pre-existing chronic kidney damage116,119.
Hepatopulmonary syndromeHepatopulmonary syndrome (HPS) is a vascular pulmonary disorder characterized by the clinical triad of intrapulmonary vascular dilation, hypoxemia (Pa02 <80mmHg and alveolar-arterial difference >15mmHg). In the context of advanced liver disease, with or without portal hypertension, it tends to be more frequent and severe in patients with cirrhosis120–124.
Patients can be asymptomatic in the early stages. Dyspnea has been described as the cardinal symptom. Its onset is generally insidious, and it is exacerbated by exercise121,124. Platypnea and orthodeoxia have been reported in one-fourth of the patients122,123.
44. Pulse oximetry is not recommended for the diagnostic screening of HPS because normal values do not rule out mild or moderate HPS. The test is used in children because it is noninvasive and is useful in severe stages, but it does not replace gasometry.Level of agreement: in complete agreement 95.45%; in partial agreement 4.55%.
The recommended values for the diagnosis of HPS are: partial arterial pressure of oxygen (PaO2) <80mmHg or alveolar-arterial oxygen gradient (PAaO2) >15mmHg125,126. Given that the use of arterial gasometry is problematic because of its invasive nature, an attempt has been made to substitute it with pulse oximetry, which is well accepted in children. Reports of saturations <97% reflect hypoxemia confirmed by arterial gasometry (PaO2 <70mmHg) in adults with cirrhosis, with up to 100% sensitivity and 65% specificity125,127,128. However, according to the classification of severity, pulse oximetry would fail to identify all patients with mild HPS and some with moderate HPS, and so it not recommended as a diagnostic screening test128,129.
45. Contrast echocardiography using microbubbles is the best method for confirming intrapulmonary vascular dilations and is considered the gold standard for diagnosing hepatopulmonary syndrome.Level of agreement: in complete agreement 90.91%; in partial agreement 9.09%.
Contrast echocardiography is the gold standard in the diagnosis of intrapulmonary shunts, with 67–80% sensitivity and 93–100% specificity. It is positive, if after the injection of 10mL of shaken saline solution into a peripheral vein, microbubbles appear in the right atrium, and after 3–6 cardiac cycles, the bubbles are seen in the left atrium. It should be kept in mind that the normal diameter of the pulmonary capillaries is <8μm and the shaken saline solution creates microbubbles >10μm in diameter, and so under normal conditions, the bubbles do not pass through the pulmonary capillaries120,123–125,130.
46. There is no efficacious medical therapy for reversing HPS; LT is the only therapy for its reversal.Level of agreement: in complete agreement 100%.
No medical treatment has yet been able to reverse HPS in children. General measures include providing symptom relief, improving quality of life, providing the capacity to do exercise, and facilitating LT, if indicated. LT enables the reversal of HPS, with success rates that vary from 70 to 84%127. HPS is listed as a MELD score exception, to prioritize those patients for LT131. However, very severe hypoxemia (PaO2 <50mmHg) increases the risk for complications and post-LT mortality132.
Portopulmonary hypertensionPortopulmonary hypertension (POPH) is a rare and severe pulmonary vasculopathy, defined by the presence of pulmonary arterial hypertension, in the context of portal hypertension, with or without background liver damage, and in the absence of other causes of pulmonary hypertension132,133.
47. Transthoracic echocardiography is recommended as screening for POPH and the definitive diagnosis is made through cardiac catheterization.Level of agreement: in complete agreement 100%.
Clinical presentation varies widely. Patients are predominantly asymptomatic and dyspnea is the most frequent symptom134. The definitive diagnosis is made through cardiac catheterization, but transthoracic echocardiography has been established as the screening study of choice due to its noninvasiveness and because it enables indirect measurement of pulmonary artery pressure135. Therefore, the guidelines of both the European Respiratory Society and the European Society of Cardiology recommend the performance of transthoracic echocardiography in all patients that are candidates for LT and in symptomatic patients with cirrhosis and portal hypertension133.
48. The recommended medical treatment for portopulmonary hypertension is based on pulmonary vasodilators, with the goal of a mean pulmonary artery pressure (mPAP) under 35mmHg.Level of agreement: in complete agreement 100%.
There has been a marked advance in recent decades in the different modalities of the medical management for pulmonary arterial hypertension. Bronchial vasodilators are currently used and are classified into 3 different groups: prostanoids, endothelin receptor antagonists, and phosphodiesterase type 5 inhibitors136,137. They are recommended in the adult population and should be started after POPH confirmation to maintain and/or decrease pulmonary artery pressure below 35mmHg, monitoring said pressure periodically, at least every three or six months, until LT138. Those indications have been considered in pediatrics but they cannot be recommended due to insufficient clinical trials.
49. Liver transplantation can resolve portopulmonary hypertension but severe forms with mPAP >45mmHg have high perioperative mortality and contraindicate liver transplant.Level of agreement: in complete agreement 100%.
LT could be the only available treatment option for definitively reducing pulmonary artery pressure and pulmonary vascular resistances139. There are authors that consider it the only effective treatment option139,140, as long as the mPAP is under 35mmHg, thus preventing acute right ventricular failure precipitated by the post-transplant hemodynamic changes, which occurs when the mPAP goes above that value141.
While POPH is not a sole indication for LT in adults142, the 2014 Practice Guidelines for Pediatric Liver Transplantation recommend that all listed children with severe POPH qualify for a model for end-stage liver disease score exception and LT prioritization and should be immediately referred to specialized centers143,144.
Cholangitis and other infectionsAcute cholangitis (AC) is a morbid condition resulting from acute inflammation and infection of the bile ducts. The main predisposing factor is biliary obstruction of any cause145.
50. AC in children mainly occurs in patients with a history of biliary surgery, with symptoms that vary according to age, such as fever, abdominal pain, and jaundice, in the absence of any other infectious process.Level of agreement: in complete agreement 95%; in partial agreement 5%.
AC in children occurs mainly in patients with a history of biliary surgery, such as children with BA that underwent portoenterostomy (Kasai surgery), defects in the pancreaticobiliary junction, or after undergoing LT. Up to 50% of patients that undergo Kasai surgery develop AC146. Fever, vomiting, frequent abdominal pain in the right hypochondrium or epigastrium, and jaundice are important clinical data that serve as a guide for reaching the diagnosis of AC147.
51. Leukocytosis and elevated C-reactive protein, direct bilirubin, GGT, alkaline phosphate, and aminotransferases serve as a guide in the diagnosis of cholangitis.Level of agreement: in complete agreement 81.82%; in partial agreement 18.18%.
Biochemical tests in AC show elevated values with respect to leukocytes, C-reactive protein, plasma bilirubin, GGT, alkaline phosphatase, and aminotransferases. To determine disease severity, other tests, such as platelet count, blood urea nitrogen, creatinine, coagulation times, albumin, and blood gas studies are essential. Hemocultures are helpful for selecting antimicrobial treatment148.
52. Abdominal ultrasound enables the documentation of bile duct dilation and can sometimes identify its cause (bile duct stones).Level of agreement: in complete agreement 95%; in partial agreement 5%.
Abdominal ultrasound enables the detection of bile duct dilation in AC, and in some cases, can identify the cause, as in the case of bile duct stones. The imaging study is limited by the fact that it is operator-dependent147. Computerized axial tomography, magnetic resonance cholangiopancreatography, and even endoscopic ultrasound, have been employed to determine biliary obstruction and its level of obstruction and origin, as well as to detect complications, such as liver abscesses or portal thrombosis149.
53. Cholangitis is predominantly caused by Gram-negative bacteria, and to a lesser degree, by Gram-positive bacteria. Empiric treatment with piperacillin/tazobactam, ceftriaxone plus metronidazole, and third-generation cephalosporins have shown favorable results.Level of agreement: in complete agreement 95%; in partial agreement 5%.
Patients with AC should be hospitalized and started early on intravenous solutions and antibiotics. Empiric antimicrobial treatment includes activity against Gram-negative, Gram-positive, and anaerobic microorganisms. The most frequently isolated organisms are Escherichia coli, Klebsiella spp., Enterococcus spp., and Enterobacter spp.145–150. The antibiotic regimens employed include piperacillin-tazobactam, ticarcillin-clavulanate, third-generation cephalosporins, ceftriaxone with metronidazole, amoxicillin with clavulanate, ampicillin sulbactam, carbapenems, and gentamycin with metronidazole150,151. Antibiotics should be adjusted in relation to the results of hemocultures, considering that they are positive in only 21–71% of cases of AC145.
54. Hospitalization should be considered when an infectious process is suspected in the patient with cirrhosis.Level of agreement: in complete agreement 86.36%; in partial agreement 4.55%; uncertain 9.09%.
Up to 35% of the patients with liver cirrhosis present with bacterial infection at the time of their hospitalization and the infections are the most important cause of death in patients with decompensated liver cirrhosis. The most frequent infections, after cholangitis and spontaneous bacterial peritonitis (SBP), are urinary tract infections, pneumonia, bacteremia, skin infections, and soft tissue infections145,152.
55. Fever and abnormal leukocyte values may be absent in 30% of the patients with infection and liver cirrhosis. In such cases, reliable markers, such as procalcitonin and C-reactive protein, should be determined.Level of agreement: in complete agreement 81.82%; in partial agreement 18.18%.
Fever may be absent, and the leukocyte count is difficult to interpret due to the presence of hypersplenism and pancytopenia. Therefore, procalcitonin levels >0.5ng/ml can serve as a guide for reaching the diagnosis of bacterial infection in children with liver disease.151 The opportune starting of empiric antibiotic treatment with broad-spectrum drugs is recommended, until the culture results that enable directed treatment are available, and it is particularly important in patients with signs of septic shock. When the patient does not improve with the antimicrobial regimen, an antimycotic agent should be added151,152.
56. There is insufficient evidence in BA patients, after the Kasai procedure, for starting antimicrobial prophylaxis for cholangitis. Secondary oral prophylaxis reduces the recurrence of cholangitis, albeit evidence is limited.Level of agreement: in complete agreement 80%; in partial agreement 20%.
AC presents in more than 50% of children after Kasai surgery, despite the use of prophylactic antibiotics. In the reports on primary and secondary prophylaxis in AC, different antibiotics and administration time and route, including immunoglobulin and steroid administration, have been employed. The results have not been consistent, even when a decrease in the occurrence of cholangitis is reported. Evidence is insufficient and more studies are needed to make a recommendation153–157.
Ascites and peritonitisAscites is the pathologic accumulation of fluid in the peritoneal cavity, and it is the most common complication in cirrhosis. Survival in patients with cirrhosis is significantly reduced, after presenting with ascites158,159.
Ascites is classified according to its severity and response to diuretics. In grade 1 (mild), patients can be asymptomatic. The condition is usually detected through follow-up ultrasound and patients can be treated as outpatients. In grade 2 (moderate), there is abdominal distension and complications resulting from serum electrolyte alterations or infections may arise. Management is dynamic and carried out in relation to the clinical evaluation and assessment of the extracellular compartment, diuresis, and serum electrolytes. In grade 3 (severe), or tense ascites, hospitalization is recommended due to the high risk for severe complications and the need for opportune therapeutic interventions160,161.
57. Hospitalization criteria are tense ascites, with or without respiratory restriction, fluid and electrolyte alterations, failed diuretic treatment, and suspected SBP.Level of agreement: in complete agreement 86.36%; in partial agreement 13.64%.
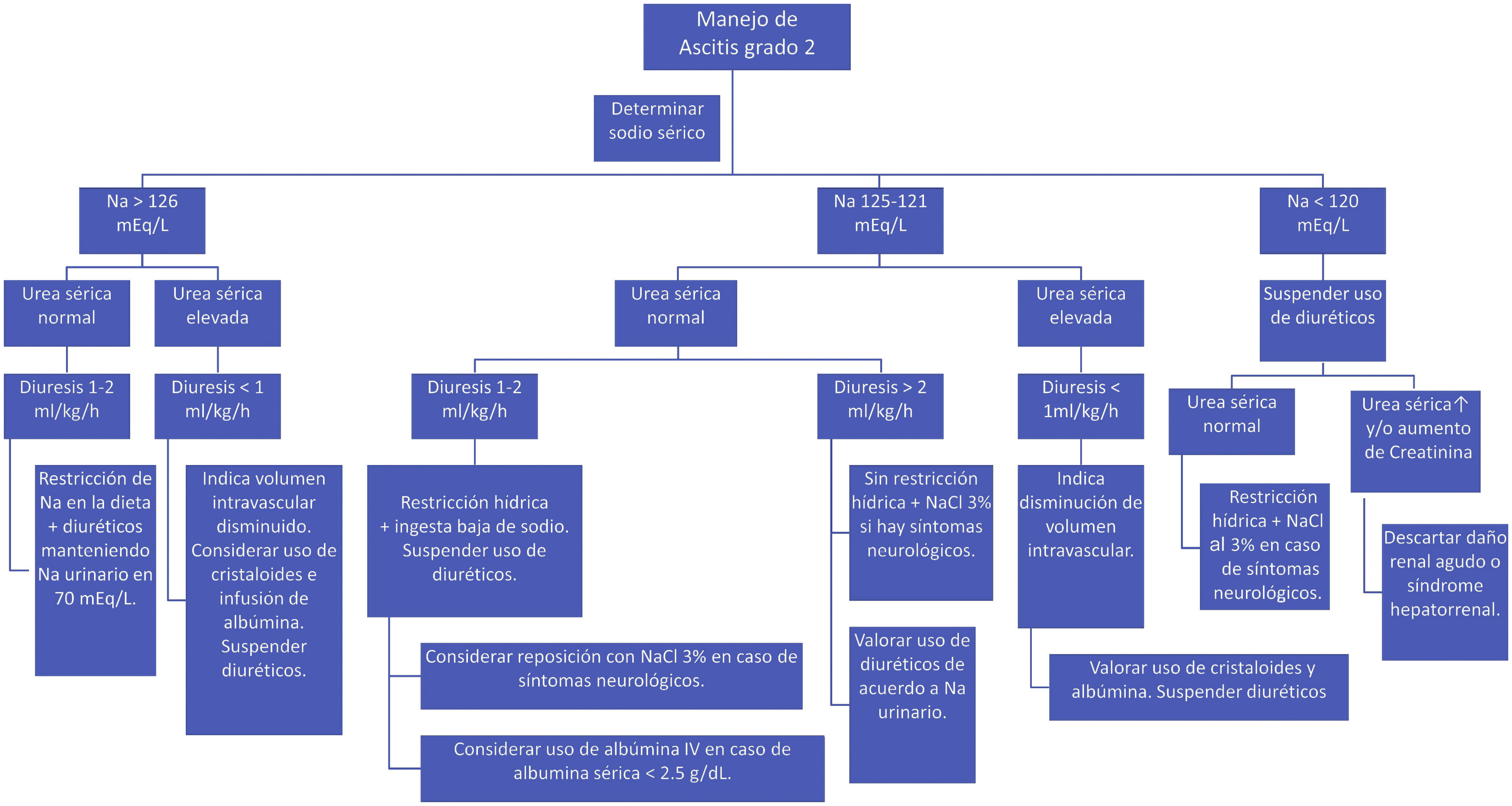
Hospitalization is also recommended in patients with refractory or untreatable ascites. Refractory ascites refers to the absence of clinical response after 7 days of adequate treatment with a low-sodium diet (1–2mEq/kg/day) and maximum doses of spironolactone and furosemide, or when fluid accumulation recurs after the first month of treatment. Untreatable ascites is defined when diuretics are contraindicated due to the presence of adverse events, such as encephalopathy or hyponatremia (serum Na <125mEq/l, kidney failure, hypokalemia and hyperkalemia (K <3 or >6mEq/l)162.
58. Adequate response to the treatment of ascites includes reduced weight due to ascitic fluid loss and resolved edema, reduced abdominal circumference, and increased diuresis.Level of agreement: in complete agreement 77.27%; in partial agreement 22.73%.
The treatment goal is to reduce weight by approximately 0.5–1%, dependent on daily ascitic fluid until its disappearance, and to prevent its re-accumulation. An inadequate response indicates that the negative balance of sodium was not achieved. The clinical data that should be monitored in the follow-up are weight, diuresis, vital signs (including temperature), peripheral edema, and abdominal circumference. Physical examination should be carried out at the time of diagnosis, during consultations, and daily in hospitalized patients161.
59. The recommended management of grade 1, or mild, ascites is a low-sodium diet (1–2 mEq/kg/day).Level of agreement: in complete agreement 76.19%; in partial agreement 23.81%.
Sodium intake of 1–2 mEq/kg/day is recommended for the treatment of mild ascites. There is no need for sodium restriction in exclusively breastfed patients. In patients with complementary feeding, no sodium should be added to the other foods. A more restricted diet is unnecessary and poorly tolerated160,163.
60. The management of grade 2, or moderate, ascites includes dietary sodium restriction and diuretics.Level of agreement: in complete agreement 81.82%; in partial agreement 18.18%.
Monotherapy with diet is insufficient in grade 2 ascites, and so diuretic treatment is added.160 Spironolactone is effective, given its effect on hyperaldosteronism. Due to its prolonged half-life, therapeutic effects can be seen after the third day. The dose is 2–4mg/kg/day, increasing up to 6mg/kg/day every 3–5 days, according to response. If there is no response after the maximum dose of spironolactone, furosemide at 1 mg/kg/day should be added and increased to the maximum dose. Because it is a loop diuretic, furosemide can increase sodium excretion up to 30%, but should be used with caution because it reduces the effective circulating volume. The dose is 1–4 up to 12 mg/kg/day164–166.
During treatment with diuretics, monitoring hydration status, blood chemistry, and serum and urine electrolytes is recommended. Once the ascites is resolved, diuretics can be reduced to half the dose, and if possible, suspended. Fig. 1 shows the recommendations for the treatment of ascites, according to the different clinical scenarios.
61. The management of grade 3 ascites, or tense ascites, is paracentesis, as first-line treatment in adults. The procedure is controversial in the pediatric population, albeit some centers utilize it. However, there is a lack of scientific evidence for its recommendation.Level of agreement: in complete agreement 94.1%; in partial agreement 5.9%.
Paracentesis is a procedure through which fluid in the peritoneal space is drained. In patients with tense ascites, therapeutic strategies include large-volume paracentesis (≥50mL/kg) and high doses of diuretics. Although there are no studies comparing the two interventions in children, the evidence in adults supports paracentesis as first-line treatment, because it is more effective, safer, and has fewer adverse effects167.
In adults with cirrhosis and large-volume ascites, the use of therapy with transjugular intrahepatic portosystemic shunt has been described. However, there is no evidence of its use in children, and so cannot be recommended168.
62. Albumin infusion is indicated for the management of ascites in cases with hypoalbuminemia <2.5g/dl, together with the administration of diuretics.Level of agreement: in complete agreement 90%; in partial agreement 10%.
Albumin is the ideal volume expander in ascites of hepatic origin because it has a better response rate, improves quality of life, and results in shorter hospital stay and a lower risk for readmission. The administration of albumin, combined with diuretics, is more effective than diuretics on their own. In patients with SBP, single infusion of 1g/kg of albumin at 20–25% and systemic antibiotics reduce renal compromise and death162,163.
Albumin infusion during large-volume paracentesis (≥50ml/kg) reduces circulatory dysfunction, ascites recurrence, and the development of hepatorenal syndrome or dilutional hyponatremia. The recommended dose is 0.5–1g/kg of body weight, infusing half in the first 2h and the rest within 6–8 of the following hours. After mobilization of the ascitic fluid, the use of diuretics at the minimum dose necessary to prevent re-accumulation is recommended169,170.
In HRS, intravenous albumin reduces secondary morbidity and mortality. In patients with hypoalbuminemia (<2.5g/dl), the intervention favors the extravascular reabsorption of water, improving the circulatory volume, cardiac output, and kidney function. In cases of hypervolemic hyponatremia, kidney function deterioration is prevented, when fluid restriction is combined with albumin infusion169,170.
63. Empiric treatment with third-generation cephalosporins is indicated in cases of first-time ascitic fluid infection, community-acquired infection, and no antimicrobial use in the past 4 weeks.Level of agreement: in complete agreement 86.36%; in partial agreement 13.64%.
SBP presents in one out of every three children with cirrhosis158,171. The clinical manifestations are poorly sensitive and specific, and up to 50% of the infected patients are asymptomatic. Symptomatic patients present with fever, abdominal pain, diarrhea, and hemodynamic and/or metabolic decompensation162,171,172.
Culture and cytochemistry of the ascitic fluid should be carried out in patients whose ascites is detected for the first time and in those that have signs and symptoms of infection. The best culture performance is obtained when the ascitic fluid is inoculated in culture jars at the site where the paracentesis is carried out, with an aliquot ≥10ml172.
64. In children with suspected SBP, diagnostic paracentesis is recommended for cytochemical study, cultures for aerobic and anaerobic bacteria, hemoculture, and urine culture.Level of agreement: in complete agreement 90.91%; in partial agreement 9.09%.
The diagnosis of SBP is made with ascitic fluid that has polymorphonuclear (PMN) cell count ≥250 cells/mm3 and a positive bacterial culture. Other variants of infection are neutrocytic ascites (PMN count ≥250 cells/mm3) and a negative culture, with no presence of another cause of inflammation, such as pancreatitis and/or intra-abdominal bleeding, and monobacterial ascites with no increase in PMN cells in the ascitic fluid159,171,172.
65. Empiric antimicrobial treatment should be administered before the suspicion of infection (SBP), in cases of recurrence, in-hospital acquisition, and antibiotic use in the past month.Level of agreement: in complete agreement 86.36%; in partial agreement 13.64%.
The empiric use of antimicrobials in ascitic fluid infection should cover a broad spectrum for the prevalent bacteria, such as Escherichia coli, Klebsiella pneumoniae, and Streptococcus pneumoniae, and be administered immediately after infection is suspected162,171,173–176. The antibiotics of first choice are third-generation cephalosporins158,173. Before choosing the empiric treatment, identifying the risk factors for infections from resistant pathogens, such as those associated with healthcare services, previous antimicrobial use, and prolonged hospital stay, is recommended158,173. The therapeutic options in those cases are cefepime, piperacillin/tazobactam, cefoperazone/tazobactam, and meropenem. In cases of bacterial isolation and sensitivity test availability, the recommendation is to prescribe the antibiotic that has sensitivity and the lowest antimicrobial resistance, suggesting third-generation cephalosporins as the medication of choice158. Intravenous antibiotic administration for at least 5 days is recommended. The parameters that define improvement are the signs and symptoms, acute phase reactants, and PMN cell count in the ascitic fluid176.
66. There is no evidence for antimicrobial prophylaxis for SBP in children. In adult patients, infection events have been reported to decrease with trimethoprim-sulfamethoxazole or rifaximin administration.Level of agreement: in complete agreement 86.36; in partial agreement 13.64%.
After the first event of ascites, the prescription of antimicrobial prophylaxis for an indefinite period of time, or until LT, has been proposed by different authors100, but the benefit of the intervention is uncertain in children. A disadvantage related to the use of prophylactic antibiotics is the colonization and/or infection by resistant bacteria173,175,176.
ConclusionThe primary management goal in pediatric patients with liver cirrhosis should be to prevent or control complications. We believe it is important to publish the advances in knowledge, to detect the children with cirrhosis, and thus offer opportune management of their complications, stopping their progression, and if necessary, provide the opportunity for their referral and evaluation for LT.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Flores-Calderón J, Cisneros-Garza LE, Chávez-Barrera JA, Vázquez-Frias R, Reynoso-Zarzosa FA, Martínez-Bejarano DL, et al. Consenso del manejo de las complicaciones de la cirrosis hepática en pediatría. Rev Gastroenterol Méx. 2022;87:462–485.