Helicobacter pylori (H. pylori) is known to be capable of causing chronic inflammation of the gastric mucosa that slowly progresses through the premalignant stages, reaching localized gastric adenocarcinoma (GAC). Its outcome is closely related to the stage at which diagnosis is made. The aim of the present study was to determine cost-benefit by comparing esophagogastroduodenoscopy, serum pepsinogen detection, and no screening at all.
Material and methodsUtilizing Markov chains and Monte Carlo simulation, the costs and effects of various detection modalities were simulated to analyze the cost-benefit of each strategy. For our population, we used the published data of patients with gastric cancer, applicable to the Mexican population.
ResultsThe results were reported as incremental cost-effectiveness ratios. The best strategy was serum pepsinogen determination, followed by the strategy of endoscopic examination with continued monitoring every 3 years.
ConclusionsThe performance of serum pepsinogen serology and directed endoscopic examination (and continued monitoring, if necessary) for GAC screening could be a cost-effective intervention in Mexico, despite the low-to-moderate general prevalence of the disease.
Se sabe que Helicobacter pylori (H. pylori) es capaz de provocar inflamación crónica de la mucosa gástrica, que progresa lentamente a través de las etapas premalignas hasta llegar al adenocarcinoma gástrico (ACG) localizado, su pronóstico está estrechamente relacionado con la etapa en la que realiza el diagnóstico. El propósito de este estudio es determinar el costo-beneficio mediante la comparación de la esofagogastroduodenoscopia, la detección de pepsinógeno en suero o no realizar ningún cribado.
Material y métodosUtilizando cadenas de Markov y simulación de Monte Carlo se simularon los costos y los efectos de varias modalidades de detección para analizar el costo-beneficio obtenido de cada una. Para nuestra población, utilizamos los datos publicados de pacientes con cáncer gástrico, aplicables a la población mexicana.
ResultadosLos resultados se informaron en proporciones incrementales de costo-efectividad (ICER). La mejor estrategia fue buscar pepsinógeno en suero, seguido de una prueba de estrategia de examen endoscópico con vigilancia continua cada 3 años.
ConclusionesLa detección de pepsinógeno en suero, así como la detección endoscópica dirigida (y la vigilancia continua, si es necesario) para el cribado de ACG podría ser una intervención rentable en México a pesar de una prevalencia general de la enfermedad de baja a moderada.
Gastric cancer is the fifth most common malignant tumor and the third cancer-related cause of death worldwide1,2. Incidence of and mortality due to gastric adenocarcinoma (GAC) are currently particularly high in certain regions of the world, as is the case in Eastern Asia (especially in Korea, Mongolia, and Japan), in Eastern Europe, and in Latin American countries, such as Chile, Costa Rica, and Colombia3. In Mexico, in 2017, malignant tumors were in third place as cause of death, following heart disease and diabetes mellitus4, and in second place in the age group of 45 to 64 years, following diabetes mellitus. Stomach cancer was in second place for all tumors in that same age group. GAC development is known to follow a multistage carcinogenesis process. Within that process, Helicobacter pylori (H. pylori) is capable of causing chronic inflammation of the gastric mucosa that slowly progresses through the premalignant stages of atrophic gastritis, intestinal metaplasia (IM), and dysplasia, reaching localized GAC, and then presenting with local and distant metastasis5. Its outcome is closely related to the stage at which it is diagnosed. The opportunity for curative resection is possible when the disease is detected at the early stages of carcinoma that are limited to only the mucosa or submucosa. Said treatment tends to be accessible endoscopically, through endoscopic dissection of the submucosa. Other interventions have not been widely studied, such as the screening strategy of serum pepsinogen measurement for detecting gastric atrophy (GA), which could be useful in the prevention and early diagnosis of gastric cancer. In countries with an intermediate risk for gastric cancer, the decision regarding endoscopic detection is less clear, and cost analyses are needed to define the best strategy in terms of health benefits and the use of economic resources6,7.
Materials and methodsA cost-effectiveness economic analysis was conducted. Due to the type of analysis, a study population was not required. A baseline scenario was simulated for GAC screening in the Mexican population in individuals 50 years of age. The selection criteria were:
Inclusion criteria:
- •
Articles published in indexed journals.
- •
Publications accessible in the PubMed/MEDLINE, Ovid, Embase, and Cochrane databases.
- •
Articles in English and Spanish.
- •
Articles with the keywords and MeSH terms: “Costs and cost analysis, Cost analysis, Early detection of cancer, Endoscopy procedures, Esophagogastroduodenoscopy, Gastrointestinal endoscopy, Gastric cancer, Helicobacter pylori, Markov chains, Neoplasm, Precancer, Preneoplasia, Pepsinogen, Standard direct cost, Stomach neoplasm” and their Spanish equivalents.
Exclusion criteria:
- •
Articles with epidemiologic data not applicable to the target population.
- •
Documents written in languages other than English or Spanish.
The data for the variables were obtained from articles published in indexed journals in English and Spanish, from the Instituto Nacional de Estadística y Geografía (INEGI), the “Unitary Costs by Level of Medical Care” of the Instituto Mexicano del Seguro Social (IMSS), projected to 2019 and published in the Diario Oficial de la Federación (DOF) on December 28, 20178, and information obtained through the official websites of the different institutions, as well as from the PubMed/MEDLINE, Ovid, Embase, and Cochrane databases. The variables were entered into the Microsoft® Excel program, version 16.22 for Mac, for their later analysis, and a statistical model was built, utilizing TreeAge Pro 2019 R1.1 (TreeAge, Williamstown, MA, USA) software.
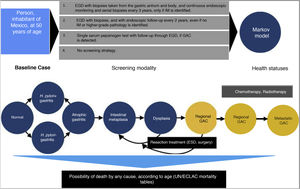
A mathematical simulation of the natural history of GAC, through the development of a Markov decision process state transition model, was utilized. Given the limited resources available on the Mexican population, the Markov model was developed by adapting models previously published by other authors, based on published data applicable to the Hispanic population9–11. All 50-year-old individuals were entered into the model, with the possibility of transitioning between different states of health, depending on the different probabilities designated for each event. The different states of health included: normal gastric mucosa, gastritis (with or without concomitant H. pylori infection), GA, IM, dysplasia, asymptomatic localized GAC (resectable), asymptomatic regional GAC, asymptomatic metastatic GAC, symptomatic localized GAC (resectable), symptomatic regional GAC, symptomatic metastatic GAC, and death.
Comparing strategies with not employing any GAC detection test, we evaluated the following screening strategies first implemented, once a person reaches 50 years of age (given that most of the cases in Mexico are diagnosed in advanced stages in patients older than 55 years)12:
- 1)
Upper endoscopy (esophagogastroduodenoscopy [EGD]), with biopsies taken from the gastric antrum and body, and continuous endoscopic monitoring and serial biopsies every 3 years, only if IM is identified (or as resection management of the lesion, if a more severe pathology is diagnosed).
- 2)
EGD with biopsies, and with endoscopic follow-up every 2 years, even if no IM or higher-grade pathology is identified.
- 3)
Serum pepsinogen detection.
A hypothetic cohort of 50-year-old individuals was simulated on a 30-year time horizon, with one-year long cycles. The cost-effectiveness of each of the 4 detection algorithms previously described was reported from the public healthcare perspective. The result measures were described in terms of the incremental cost-effectiveness ratios (iCERs) (2019 USD per quality-adjusted life year [QALY]), with a willingness-to-pay threshold of $9,000 USD/QALY. There is no consensus on determining willingness-to-pay in any country. In the United States a willingness-to-pay of $100,000 USD/QALY is generally used.
Statistical analysisA Monte Carlo probabilistic sensitivity analysis was carried out with 10,000 iterations, utilizing gamma distributions for costs and beta distributions for transition probabilities and utilities.
Ethical considerationsGiven that the present work is an economic analysis study based on epidemiologic data and costs available to the general public, in accordance with the Declaration of Helsinki I developed at the 29th World Medical Association (WMA) General Assembly (Tokyo, 1975), the Declaration of Helsinki II amended at the 35th WMA General Assembly (Venice 1983), and at the 41st WMA General Assembly (Hong-Kong 1989), no approval by an ethics committee was required.
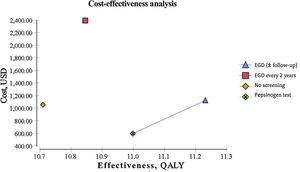
ResultsEndoscopic examination with continuous monitoring every 3 years was cost-effective, with an iCER of 129.23/QALY and an accumulated cost of $1,123.66, which was slightly higher than the accumulated cost of the current no-screening strategy of $1,056.25. The serum pepsinogen determination strategy for detecting gastric atrophy had an iCER of –$1,589.59/QALY and an accumulated cost of $596.76, which was lower than that of no screening. The serum pepsinogen strategy dominated the no-screening strategy in the general population.
Biannual endoscopic examination produced an iCER of $9,798.85/QALY and an accumulated cost of $2,391.30. All the strategies described were compared individually with the current no-screening policy for gastric cancer. Table 1 summarizes the iCER reported in USD by QALY. The iCER could not be directly calculated due to the rounding applied. All the interventions were compared individually with the no-screening strategy. QALY and costs were discounted at 3% annually (Table 1) (Fig. 1).
Detailed analysis of the iCER in using a given screening technique for GAC, compared with no screening.
| Screening modality | Accumulated cost (USD) | Additional cost (USD) | Effectiveness (QALY) | Incremental effectiveness (QALY) | iCER (USD/QALY) |
|---|---|---|---|---|---|
| No screening | 1056.25 | – | 10.7 | – | – |
| EGD (±follow-up) | 1123.66 | 67.41 | 11.22 | 0.52 | 129.23 |
| EGD every 2 years | 2391.3 | 1335.05 | 10.84 | 0.14 | 9798.85 |
| Pepsinogen test | 596.76 | 459.49 | 10.99 | 0.29 | 1589.59 |
EGD: esophagogastroduodenoscopy; GAC: gastric adenocarcinoma; iCER: incremental cost-effectiveness ratio (defined as the additional cost of a specific strategy divided by its additional clinical benefit or incremental effectiveness, compared with the no-screening strategy); QALY: quality-adjusted life year; USD: United States dollars.
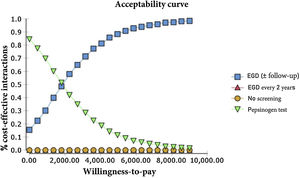
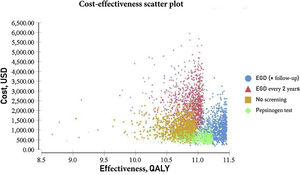
The probabilistic sensitivity analysis was derived from 10,000 iterations in a Monte Carlo simulation for the Markov model (Fig. 1). Cost-effectiveness acceptability curves were generated, based on a willingness-to-pay of $9,000 USD/QALY, as were the scatter plots for each of the strategies (Figs. 2 and 3).
For our baseline scenario of a healthy, asymptomatic, 50-year-old Mexican, both the endoscopic examination for GAC with continuous monitoring for IM (or the management of more advanced lesions with later monitoring) and the serum pepsinogen test were cost-effective in the predetermined willingness-to-pay threshold of $9,000/QALY.
The only strategy of our analysis that was not cost-effective regarding the variables and costs determined for the Mexican population was the biannual endoscopic examination, given that it surpassed the predetermined willingness-to-pay threshold of $9,000/QALY and the current figures for the no-screening strategy.
The results of the Monte Carlo simulation, based on the Markov model, showed that the strategy with the best cost-effectiveness was serum pepsinogen determination, followed by the endoscopic examination with continuous monitoring every 3 years. Both tests had significant cost-effectiveness, with a willingness-to-pay of $9,000 USD/QALY, and presented costs below that amount, in 100% of the iterations in the Monte Carlo simulation. The biannual endoscopic examination was cost-effective in 52% of the iterations in said simulation (Fig. 4).
We discovered that in Mexico, a country with a much less elevated prevalence of GAC than some of the Asian countries, carrying out efforts to endoscopically detect premalignant and malignant lesions or serologically detect serum pepsinogen was cost-effective13,14. When high-risk individuals are selected at the asymptomatic stage (corresponding to the age group selected in our study), there is a greater probability of an early diagnosis of GAC that is susceptible to curative resection.
The implementation of national screening programs for GAC in countries like Japan and South Korea has been correlated with a significant benefit regarding mortality. In those countries, 5-year overall survival is 60 to 70% (and 90 to 95% for early gastric cancer), compared with 20 to 30% before the implementation of such strategies, which are the current figures in the United States and Mexico, where there are no established screening strategies13,15,16.
Biannual upper endoscopy is one of the current detection practices in Japan and South Korea and we included it as a strategy in our model.16 However, it was not cost-effective in the Mexican population, most likely due to the lower incidence of GAC in our general population and the higher cost that is a product of repeat interventions in the entire selected population.
As is true with any decision process model, the incidence and prevalence of the states of disease and the estimated progression rates influence the results. Given the scant epidemiologic evidence, great variability of results, and lack of progression rates described for the Mexican population, the estimated progression rates of the states of health we used in our study were based on publications from the United States, focused on that country’s Hispanic population, which does not precisely represent the incidence and progression of GAC in Mexico. For example, the reported incidence in the United States is 13.3 cases per 100,000 Hispanic inhabitants, whereas data from the WHO states that incidence of GAC in the Mexican population above 50 years of age is 25 per 100,000 inhabitants and is 5.8 in the general population, without establishing a specific age group. Another limitation of our model was having taken into account only the direct costs of medical attention, without including the indirect costs that could positively affect cost-effectiveness. In addition, we obtained the costs from the “Unitary Costs by Level of Medical Care” of the IMSS, to provide a public health perspective, and because it is the most standardized source of public health medical costs in Mexico, with a greater variety of procedures and interventions per level of care. Despite those limitations, the advantages of our study include the fact that the Markov model reflected therapeutic management and continuous monitoring over a 30-year time span, accompanied by probabilistic and sensitivity analyses that took into account the present heterogeneity in the medical literature and the uncertainty of the progression rates in the natural history of the disease. They also took into consideration the endoscopic and surgical complication rates, the probability of not detecting lesions through EGD or serum pepsinogen determination, and the risk for recurrence after early gastric cancer resection.
ConclusionWe found that serum pepsinogen determination and directed endoscopic examination (and continuous monitoring, if indicated) as screening strategies for the detection of GAC could be a cost-effective intervention in Mexico, despite the low-to-moderate general prevalence of the disease. Due to the availability of curative surgical techniques, as well as the growing availability of endoscopic options, GAC is now a potentially curable disease, when diagnosed in the early stages. Nevertheless, it must be kept in mind that diffuse gastric cancer, which is more prevalent in urban zones, cannot be detected through those strategies, and in nonurban zones, pepsinogen measurement is more difficult to perform due to lack of access to the reactant, thus limiting GAC detection.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Enríquez-Sánchez LB, Gallegos-Portillo LG, Camarillo-Cisneros J, Cisneros-Castolo M, Montelongo-Santiesteban JJ, Aguirre-Baca DA, et al. Costo-beneficio de cribado de adenocarcinoma gástrico por pepsinógeno sérico en la población mexicana. Revista de Gastroenterología de México. 2022;87:285–291.