There are differences, with genetic and embryologic support, in the clinical behavior of proximal colon cancer (PCC) (right colon: cecum, ascending colon, and transverse colon) and distal colon cancer (DCC) (left colon: descending colon, sigmoid colon, rectum). Our aim was to determine whether there was a divergent pattern in the demographic characteristics, risk factors, TNM stage, and clinical stage at diagnosis between patients with PCC and those with DCC.
Material and methodsA retrospective, analytic, and multicenter study was conducted. Medical records of patients diagnosed with colorectal cancer, confirmed by histopathology and with TNM staging, within the time frame of 2018-2023, were collected from two hospital centers in the city of Chihuahua. They were divided into the PCC and DCC groups, for evaluating the abovementioned characteristics.
ResultsFrom a total of 513 cases, 404 were included in the study. Significant differences were found in the demographic characteristics of female sex and a history of cholecystectomy, both with a greater relative frequency for PCC. Distant metastasis was present in 35.6% of patients, despite their younger age at diagnosis. The rectum was the most commonly affected segment in the DCC group, as was the ascending colon in the PCC group. There was a greater prevalence of peritoneal carcinomatosis in the PCC group. In contrast, the DCC group had a greater prevalence of distant metastasis to other organs, as both individual metastasis (M1a) and multiple site metastasis (M1b). There were no considerable differences in the KRAS, NRAS, or BRAF gene mutations between the two groups.
ConclusionsPCC was associated with a history of cholecystectomy and female sex and had more aggressive TNM staging, compared with DCC.
Existen diferencias entre el comportamiento clínico con sustento genético y embriológico entre el cáncer de colon derecho/proximal (CCP)(ciego, colon ascendente y transverso) e izquierdo/distal (CCD)(colon descendente, sigmoides y recto). Nuestro objetivo es determinar si existe un patrón divergente en las características demográficas, factores de riesgo, estadificación TNM y estadio clínico al momento del diagnóstico entre pacientes con CCP y CCD.
Material y métodosEs un estudio multicéntrico, retrospectivo y analítico. Se analizaron expedientes de dos centros de ciudad Chihuahua con diagnósticos de cáncer colorrectal durante el periodo 2018-2023, confirmados por histopatología y con estadificación TNM. Se agruparon en CCP o CCD, estudiando las características mencionadas.
ResultadosHubo un total 513 casos, 404 fueron utilizados para el estudio. Se encontraron diferencias significativas en las características demográficas de sexo femenino y antecedente de colecistectomía, ambos con mayor frecuencia relativa para el CCP. El 35.6% de pacientes presentaron metástasis a distancia a pesar de una menor edad al momento del diagnóstico. El recto fue el segmento más afectado en el grupo del CCD y el colon ascendente el principal en el CCP. El CCP demostró mayor prevalencia para carcinomatosis peritoneal, mientras que CCD mostró mayor prevalencia para diseminación a distancia a otros órganos, tanto para metástasis individuales (M1a) como a múltiples sitios (M1b). No se observaron diferencias considerables para mutaciones de KRAS, NRAS o BRAF entre ambos grupos.
ConclusionesEl CCP se asoció al antecedente de colecistectomía y sexo femenino, también demostrando una mayor agresividad por TNM respecto al CCD.
Colorectal cancer (CRC) holds third place in new cancer diagnosis frequency worldwide, with more than 1.8 million new diagnoses made in 2020.1 According to the Mexican Health Department, approximately 15,000 cases of CRC are diagnosed annually, making it the first cause of cancer death in Northern Mexico.2 Likewise, in 2020, CRC was the second cause of death due to cancer in men and women across the globe, with almost one million deaths per year or 10% of deaths secondary to cancer.1,3 In Mexico, the National Statistics and Geography Institute (INEGI, the Spanish acronym) places CRC as the first cause of death due to cancer in men and the fourth in women in the 30-59 year age group, and third place in persons of both sexes over 60 years of age.4 Global Cancer Observatory (GLOBOCAN) data predict an increase to 3.2 million new cases of CRC per year by 2040.3
Traditionally, CRC has been considered a malignant tumor affecting any segment of the colon, whether the cecum, ascending colon, transverse colon, descending colon, sigmoid colon, or rectum. However, since the 1990s, a difference between clinical behavior and the characteristics of right or proximal colon cancer (PCC) (cecum, ascending colon, and transverse colon) and left or distal colon cancer (DCC) (descending colon, sigmoid colon, and rectum) has been proposed. This proposal is based on the distinct embryologic origins of the proximal and distal segments, with the former originating from the middle intestine and the latter from the posterior intestine.5 Other variables, such as the load and type of microbiota specific to each segment, vascular supply, innervation, bile acid exposure, and physiologic function, have also been considered to influence the development of PCC or DCC, at varying degrees.6,7 Such observations result in the presence of two different entities, each with distinct epidemiologic, biologic, pathologic, carcinogenic, and prognostic behavior.5,8
Furthermore, this distinction appears to signify a difference in risk factors between the two segments for cancer development. Sex, obesity, a lack of physical activity, and smoking have recently been described to predispose, in varying degrees, to the appearance of cancer, regarding those colonic segments.9–11 Likewise, at the genetic level, different mutations and carcinogenic pathways implicated in cancer development have been reported for the proximal and distal segments of the colon.6,7,9 Current evidence shows that PCC and DCC are different entities, with their respective risk factors, carcinogenic pathways, presentation age, and clinical behavior, underlining the importance of more selective management for the two cancer types.
The present work aimed to determine whether there is a divergent pattern in the demographic characteristics, probable risk factors, TNM staging, and clinical staging at diagnosis between patients with PCC (cecum, ascending colon, and transverse colon) and DCC (descending colon, sigmoid colon, and rectum).
Material and methodsThe present work is an observational, analytic, multicenter study with a retrospective cohort, utilizing the STROBE checklist for observational studies. All patients diagnosed with CRC who received medical attention and follow-up at the Hospital Ángeles Chihuahua and Centro Estatal de Cancerología de Chihuahua (CECAN) from January 2018 to December 2023 were analyzed. Demographic data, history of cholecystectomy, pathologic TNM staging, tumor differentiation grade, clinical stage, distant metastasis sites, and KRAS, NRAS, and BRAF gene mutation evaluation in the corresponding metastatic cases were collected from the clinical records. The patients were placed in the PCC (cecum, ascending colon, transverse colon) group or the DCC (descending colon, sigmoid colon, and rectum) group, based on the anatomic location of the primary tumor in the colon.
The TNM system and clinical stages proposed by the 8th edition of the American Joint Committee on Cancer (AJCC)12 were utilized for uniform staging.
Inclusion and exclusion criteriaPatients above 35 years of age, diagnosed with CRC, differentiated as adenocarcinoma and confirmed by histopathology, were included in the study. Cases that did not have a complete clinical record were excluded. In addition, patients that had only a CRC diagnosis through colonoscopy but no consequent follow-up, or patients whose surgical specimens were analyzed after chemotherapy due to neoadjuvant therapy, were eliminated from the study.
Data acquisitionThe following demographic variables were registered: sex, age, body mass index (BMI), history of cholecystectomy, smoking, and family history of CRC or cancer at other sites. Imaging studies and reports were analyzed to corroborate the gallbladder’s absence. The definitive histopathologic reports of the colectomy or abdominoperineal resection specimens were reviewed. The anatomic location, cell differentiation grade (catalogued as low-grade [G1 and G2], high-grade [G3 and G4], and unevaluable), TNM staging, and clinical stage were identified for each tumor. The cases with distant metastasis were confirmed by pathology reports, particularly registering liver and lung metastases and/or peritoneal carcinomatosis, given that they were the more prevalent sites described for CRC metastasis.13,14 The rest of the metastases were grouped as “other sites” (e.g., ovaries, bone, central nervous system, stomach, skin, etc.). The mutation panel for the KRAS, NRAS, and BRAF genes performed in patients with distant metastasis was included.
Statistical analysisThe qualitative variables were presented as numbers and percentages and the quantitative variables as mean and standard deviation. The means of the demographic variables in the PCC and DCC patients were compared utilizing the Student’s t test for the independent samples and the Pearson chi-square test for the qualitative variables. Statistical significance was set at a p ≤ 0.05. Prevalence regarding the level of tumor differentiation, TNM stage, clinical stage, and the KRAS, NRAS, and BRAF gene mutation analysis was compared between the PCC and DCC cases, and a difference in prevalence above 5% between the two groups was considered significant. The statistical analyses were carried out using the STATA 8.0 program.
Ethical considerationsThe present study was evaluated and approved by the medical research committee and the medical research ethics committee of the Hospital Ángeles Chihuahua (registration number: CONBIOETICA-08-CEI-001-20160413) and the School of Medicine and Biomedical Sciences of the Universidad Autónoma de Chihuahua (registration number: Cl-014-23). Because this is a retrospective, observational, and analytic study, no interventions were carried out regarding management or decisions made about the study patients; it merely collected medical record data. The patients were codified to protect their anonymity. Likewise, the authors declare that this article contains no personal information that could identify patients.
ResultsThere were 513 cases of CRC between the two centers within the time frame of 2018-2023. Of those cases, 109 were eliminated due to not meeting the inclusion criteria established in the materials and methods section, leaving a total of 404 cases of colorectal adenocarcinoma to be evaluated in the present study. Table 1 shows the overall demographic characteristics of the study population.
Overall demographic characteristics of the colorectal cancer population treated at the Hospital Ángeles Chihuahua and CECAN between 2018-2023
| Demographic data | Number of cases | Percentage |
|---|---|---|
| n = 404 | ||
| Sex | ||
| Male | 233 | 57.7 |
| Female | 171 | 42.3 |
| Age at diagnosis | 58.6 ± 10.26 | |
| Body mass index | 26.7 ± 5.4 | |
| Smoking | ||
| Negative | 217 | 53.7 |
| Positive | 132 | 32.7 |
| Suspended more than 10 years ago | 55 | 13.6 |
| History of cholecystectomy | 52 | 12.9 |
| Family history of cancer | ||
| No history | 252 | 62.3 |
| History of cancer at other sites | 107 | 26.6 |
| History of colorectal cancer | 45 | 11.1 |
Tumor location was predominantly distal, consisting of 321 (79%) cases, mainly affecting the rectum and sigmoid colon. In the proximal colon, the most prevalent segment was the ascending colon. The diagnosis was made in stage III-IV in 270 (66%) of the cases; only 60 (15%) of the total number of cases were in stage I or in situ. There were 229 individual registers of metastasis, and the liver was the organ with the greatest prevalence of distant metastasis. Table 2 shows the overall clinical characteristics of the study population.
Overall clinical characteristics of the colorectal cancer population treated at the Hospital Ángeles Chihuahua and CECAN between 2018-2023
| Clinical characteristic | Number of cases | Percentage |
|---|---|---|
| n = 404 | ||
| Affected segment of the colon | ||
| Proximal colon | Cecum: 17 | 4.1 |
| n = 83 (20.5%) | Ascending colon: 51 | 12.6 |
| Transverse colon: 15 | 3.7% | |
| Distal colon | Descending colon: 24 | 5.9 |
| n = 321 (79.5%) | Sigmoid colon: 124 | 30.7 |
| Rectum: 173 | 42.8 | |
| Clinical stage at diagnosis | ||
| 0 | 4 | 1 |
| I | 56 | 13.8 |
| II | 1 | 0.25 |
| IIa | 49 | 12.1 |
| IIb | 17 | 4.2 |
| IIc | 7 | 1.7 |
| III | 2 | 0.5 |
| IIIa | 17 | 4.2 |
| IIIb | 64 | 15.8 |
| IIIc | 44 | 10.9 |
| IVa | 69 | 16.8 |
| IVb | 43 | 10.9 |
| IVc | 31 | 7.7 |
| Tumor differentiation grade | ||
| Low-grade | 339 | 84 |
| High-grade | 48 | 11.8 |
| Unevaluable | 17 | 4.2 |
| Metastasis site | Cases with metastasis = 229 | |
| Liver | 97 | 42.3% |
| Lung | 56 | 24.4% |
| Peritoneal carcinomatosis | 31 | 13.5% |
| Other sites | 45 | 19.6% |
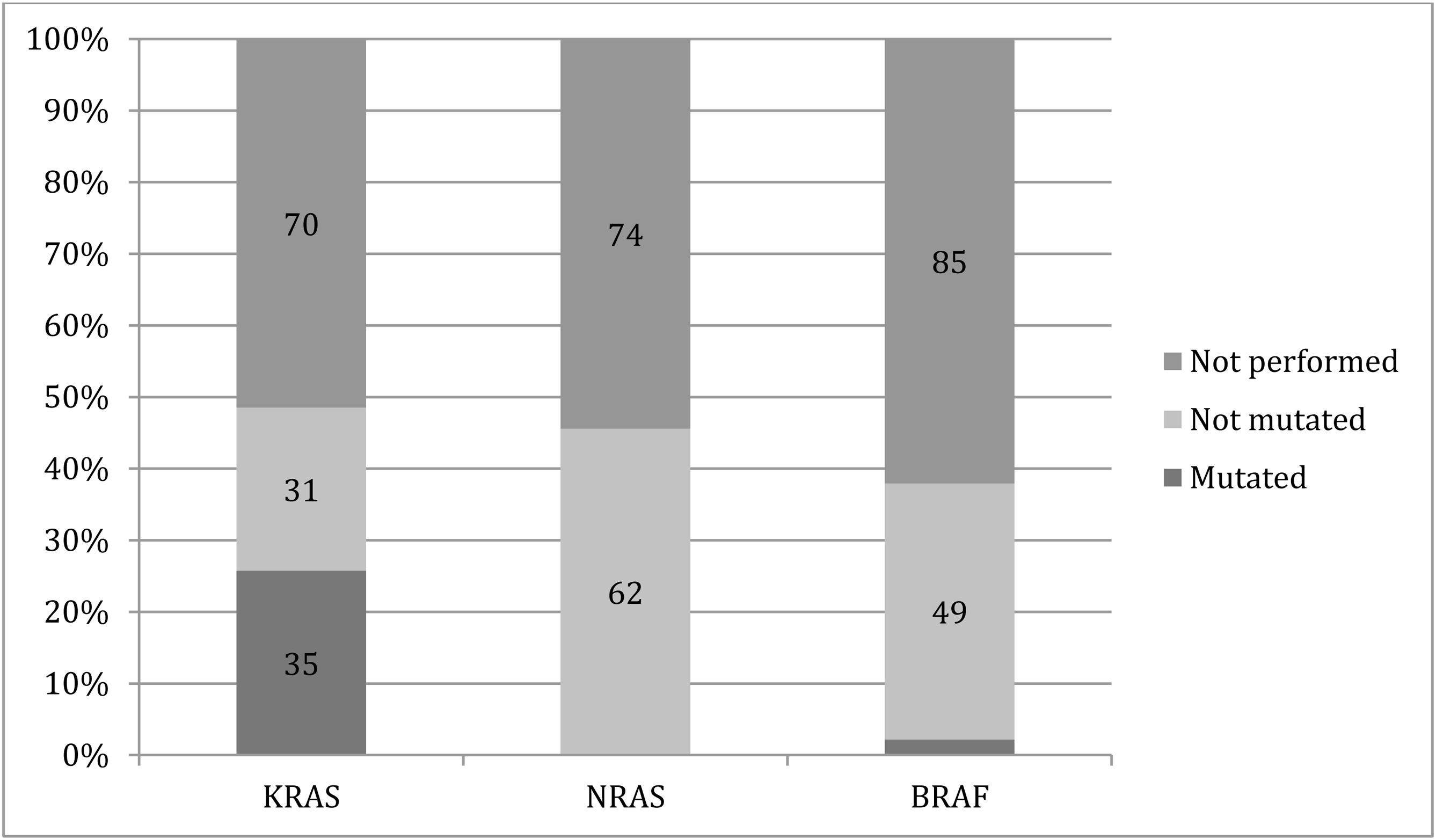
Thirty-five patients with distant metastasis had a mutation in the KRAS gene, no mutations in the NRAS gene, and only 3 (2.2%) patients had BRAF gene mutations. However, genetic testing was not performed in more than half of the patients who were candidates for it (Fig. 1).
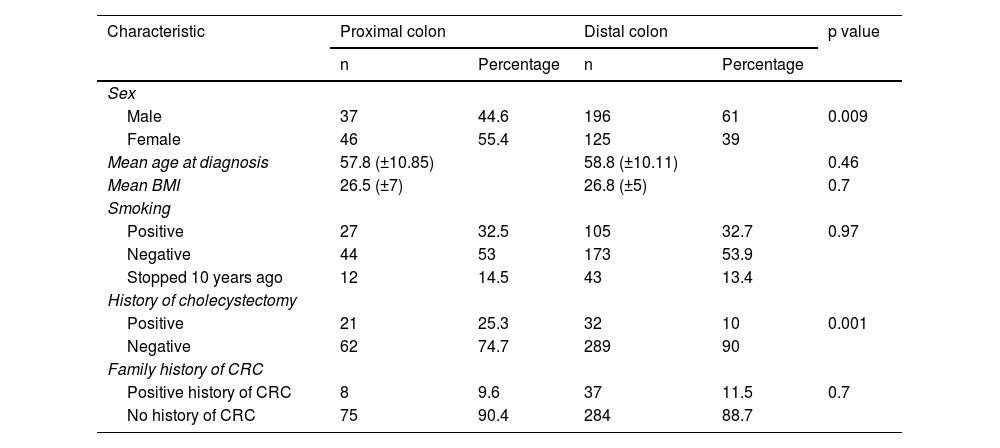
There were considerable differences in the relative frequencies of different risk factors for CRC between the PCC and DCC cases for sex and a history of cholecystectomy. The remaining characteristics were similarly distributed between the two groups (Table 3).
Differences in the demographic characteristics and their relative frequencies, regarding the proximal colon and distal colon
| Characteristic | Proximal colon | Distal colon | p value | ||
|---|---|---|---|---|---|
| n | Percentage | n | Percentage | ||
| Sex | |||||
| Male | 37 | 44.6 | 196 | 61 | 0.009 |
| Female | 46 | 55.4 | 125 | 39 | |
| Mean age at diagnosis | 57.8 (±10.85) | 58.8 (±10.11) | 0.46 | ||
| Mean BMI | 26.5 (±7) | 26.8 (±5) | 0.7 | ||
| Smoking | |||||
| Positive | 27 | 32.5 | 105 | 32.7 | 0.97 |
| Negative | 44 | 53 | 173 | 53.9 | |
| Stopped 10 years ago | 12 | 14.5 | 43 | 13.4 | |
| History of cholecystectomy | |||||
| Positive | 21 | 25.3 | 32 | 10 | 0.001 |
| Negative | 62 | 74.7 | 289 | 90 | |
| Family history of CRC | |||||
| Positive history of CRC | 8 | 9.6 | 37 | 11.5 | 0.7 |
| No history of CRC | 75 | 90.4 | 284 | 88.7 | |
BMI: body mass index; CRC: colorectal cancer.
The prevalence distribution for the T stages showed that 69 (83%) of the PCC cases were diagnosed in stages T3-T4, considerably higher than the 211 (65.7%) reported for the DCC cases. Likewise, there were other considerable differences between the PCC and DCC cases for N2a-c (20 [24%] vs. 49 [16%] cases, respectively) and M1c (14 [18%] vs. 17 [5.6%] cases, respectively). On the other hand, DCC showed a greater prevalence for M1a-b, with 94 (29%) cases, compared with PCC with 18 (20%) cases, as well as for Tx-T2 (80 [34%] vs. 14 [16.8%] cases, respectively).
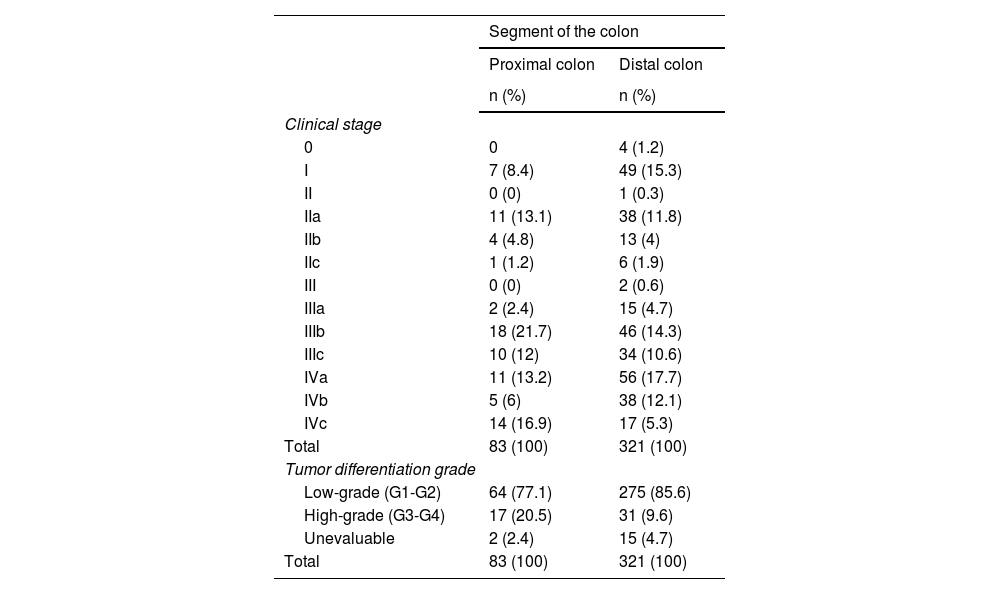
Distribution according to clinical stage showed that 72% (n = 60) of the PCC cases were diagnosed in a regional-metastatic stage versus 65% (n = 208) of the DCC cases. Regarding individual stages, the greatest differences were seen in stages I, IIIb, and IVc. Likewise, with a significant finding of p = 0.03, the proximal colon showed a greater relative frequency for diagnosis of a high-grade tumor at 20% (n = 17), compared with 9% (n = 31) in the distal colon (Table 4).
Prevalence distribution for clinical staging and tumor differentiation grade, regarding proximal colon cancer and distal colon cancer
| Segment of the colon | ||
|---|---|---|
| Proximal colon | Distal colon | |
| n (%) | n (%) | |
| Clinical stage | ||
| 0 | 0 | 4 (1.2) |
| I | 7 (8.4) | 49 (15.3) |
| II | 0 (0) | 1 (0.3) |
| IIa | 11 (13.1) | 38 (11.8) |
| IIb | 4 (4.8) | 13 (4) |
| IIc | 1 (1.2) | 6 (1.9) |
| III | 0 (0) | 2 (0.6) |
| IIIa | 2 (2.4) | 15 (4.7) |
| IIIb | 18 (21.7) | 46 (14.3) |
| IIIc | 10 (12) | 34 (10.6) |
| IVa | 11 (13.2) | 56 (17.7) |
| IVb | 5 (6) | 38 (12.1) |
| IVc | 14 (16.9) | 17 (5.3) |
| Total | 83 (100) | 321 (100) |
| Tumor differentiation grade | ||
| Low-grade (G1-G2) | 64 (77.1) | 275 (85.6) |
| High-grade (G3-G4) | 17 (20.5) | 31 (9.6) |
| Unevaluable | 2 (2.4) | 15 (4.7) |
| Total | 83 (100) | 321 (100) |
The KRAS, NRAS, and BRAF gene mutation analysis was not performed in over half of the patients with distant metastasis; in the patients in whom the analysis was carried out, there were no significant differences in mutation prevalence between the two segments of the colon.
Discussion and conclusionsThe present study aimed to establish if there were differences in the demographic characteristics, probable risk factors, and pathologic properties at diagnosis between the patients with PCC and those with DCC.
CRC was more frequent in the male sex, similar to epidemiologic analyses carried out in the United States (US), unlike the results of other studies conducted in Central and Southern Mexico that showed a trend toward a higher prevalence of CRC in the female sex.11,15–18 A possible explanation is that the population in Northern Mexico shares risk factors for CRC with the US population.
The mean age at diagnosis (58 years) was lower than that reported in the US,16 with a difference of approximately 5 years, with respect to other epidemiologic reports from different Mexican states.15,18,19 Likewise, the highest mean of our study population differed from that of other analyses of the demographic characteristics of CRC in Mexico, in agreement with a higher overweight rate described in the border states of Northern Mexico in the National Health Census.15,18,20 Likewise, a history of active smoking was positive in over one-fourth of the study population, which is a shared history in Mexican CRC patients.15,18 Even though the prevalence of a family history of CRC has not been solidly established, a low prevalence of this risk factor has been documented.15
A majority of DCC cases were found in our study, similar to the prevalence distribution established in the literature, regarding the proximal and distal colon, with more than half of the cases (54%) involving the rectum.11,15,18,19,21 On the other hand, most of the cases in the proximal colon affected the ascending colon, unlike other distribution analyses, in which the cecum was the most affected part of that segment.21
Regarding clinical stage distribution, more than one-third of the cases (35.6%) presented with distant metastasis, despite a younger patient age at diagnosis, which was a significantly higher percentage, compared with epidemiologic studies conducted in the US (22%).11 This higher prevalence of advanced stages of disease could be explained by the tendency toward late diagnosis seen in Mexico,15,21 a finding that could be secondary to a lower screening adherence rate documented in all Latin America.22,23 This concurs with descriptions by Sierra et al., who state that the high mortality rate of CRC in Latin America could be the result of the absence of screening and the lack of diagnoses made in early disease stages.24
Prevalence distribution for metastatic sites was similar to other distant metastasis patterns reported in other studies,14 with the liver being the most frequent site of distant tumor implants, at 42.3% of cases. Eighty percent of the metastases occurred in the liver and lung or as peritoneal carcinomatosis, which are the same sites described for distant spread in metastatic CRC.13,14
The estimated prevalence of mutations reported in the literature for metastatic cases is 35-38% for the KRAS gene, 4% for the BRAF gene, and 7% for the NRAS gene.25,26 The low mutation prevalence seen in our study could be explained by the fact that more than half of the cases with metastasis did not undergo testing to detect those mutations. Such under-reporting has been observed in other Latin American countries, resulting in a considerable number of cases with distant metastasis not receiving targeted chemotherapy that could be effective.27
In contrast to reports of an older patient age at PCC diagnosis, compared with DCC,5,10,28 we found a younger age for PCC, which when added to a higher clinical stage at diagnosis, could signify a more aggressive carcinogenic progression in our study population. This observation could be related to the high intake of foods catalogued as carcinogenic, according to the World Health Organization, that has been observed and described, particularly in the border states of Northern Mexico, as reported by Manzanares-Rivera,29 which could be the origin of or a contributing factor to the greater aggressiveness of PCC seen in our study.
In Mexico and Latin America, data on the differences in demographics and risk factors associated with the proximal and distal segments of the colon are scarce. Our results show a relation between sex and the anatomic distribution of CRC, predominating in women in PCC (55% vs. 44%) and men in DCC (61% vs. 39% ), supporting the notion that hormonal and genetic factors could participate in their development.5,30,31 Concerning other risk factors for CRC, the only significant difference between the two groups was cholecystectomy for PCC (25% PCC vs. 10% DCC, p = 0.001), which has recently been studied more thoroughly.32–35 Those findings support the probable influence of different factors that predispose to the development of CRC in these two segments of the colon; female sex, in particular, could present an increased risk for developing CRC after cholecystectomy. The clinical relevance of such observations should be investigated through studies with more solid designs and a higher number of cases or patients.
Even though there is a lack of information on and characterization of PCC in Latin America, our results were similar to those of other countries reported in the literature, for a greater trend toward PCC when diagnosed at elevated clinical and TNM stages,28,36,37 with our patients with PCC showing a greater prevalence of diagnosis in stages with regional and distant spread. Those results could be a consequence of accelerated local and regional tumor progression in our study population, or a delay in screening for the early and timely detection of PCC.23,38 Likewise, the PCC cases showed a greater prevalence for developing peritoneal carcinomatosis (M1c), a severe regional complication that has been previously documented in that segment of the colon,39,40 whereas the DCC cases showed a higher prevalence for distant metastasis to other organs, as individual metastasis (M1a), as well as multiple site metastasis (M1b).14,39
Similar to that reported in the literature, we found no considerable differences in prevalence for the KRAS, NRAS, or BRAF gene mutations, between the metastatic PCC and DCC cases.31,41 Nevertheless, the low gene mutation prevalence was probably biased due to a lack of genetic testing seen in Latin American and Caribbean populations, as stated above.27,42
Strengths of the present work were its multicenter population and the objective collection of relevant study data, such as clinical stage, TNM stage, and metastatic sites obtained through the definitive histopathologic reports. In contrast, its limitations were those inherent to the retrospective design, mainly the subjective data collected from the patient in the anamnesis. Future studies should include more centers, to increase the population base and obtain more concrete results. Likewise, developing prospective works could help strengthen a more accurate collection of relevant data for analyzing clinical and demographic characteristics. Lastly, a comparative analysis of the influence and difference that the distinct risk factors have on the proximal and distal segments of the colon would provide an opportunity for strengthening the data on their relation in the Mexican literature, especially regarding cholecystectomy as a risk factor for developing PCC.
In conclusion, there were significant differences between PCC and DCC for the relative frequencies of sex and a history of cholecystectomy. More than one-third of the cases presented with metastatic disease at diagnosis. Likewise, PCC appears to be more aggressive in the population in Chihuahua, due to the younger age at diagnosis and a more significant number of cases diagnosed in advanced stages. On the other hand, DCC was more prevalent in the study population, and the rectum was the most frequent CRC site. The Northern Mexican border states could benefit from stricter adherence to CRC screening programs, mainly due to the high intake of foods with carcinogenic properties in that region of the country and the lack of screening implementation in said population.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
The authors declare that there is no conflict of interest.