Hepatic encephalopathy (EH) is a common complication among patients with liver cirrhosis associated with a high mortality rate. The costs attributed to the management of patients with cirrhosis are especially high due to complications such as EH, since it increases the days of hospitalization. Currently for the treatment of EH, there are different drugs of which the standout lactulose, L-ornithine, L-aspartate (LOLA) and certain antibiotics, especially rifaximin-α (RFX). Although many of them have been shown to be as effective as the others, in order to that it is important to have information that allows us to discern about each other. Furthermore, that can help to individualize the patient's treatment and choose the best options in different scenarios. Therefore, the objective of this study was to analyze the evidence on the advantages and disadvantages of individual use or in combination of the 3 main treatments of EH, specifically its effectiveness in different grades of EH, its impact on quality of life, prophylaxis and costs reduction.
La encefalopatía hepática (EH) es una complicación frecuente en pacientes con cirrosis hepática y se asocia a una alta tasa de mortalidad. Los costos atribuidos al manejo de pacientes con cirrosis son especialmente elevados debido a complicaciones como la EH puesto que prolongan los días de estancia hospitalaria. Actualmente para el tratamiento de la EH, existen diferentes fármacos de los cuales los principales son la lactulosa, L-ornitina, L-aspartato (LOLA) y ciertos antibióticos, especialmente la rifaximina-α (RFX). Aunque muchos de ellos han demostrado ser efectivos en mayor o menor medida, es importante contar con información que permite discernir sobre uno y otro con el objetivo de individualizar el tratamiento al paciente y elegir la mejor opción en diferentes escenarios. Por lo anterior el objetivo de este estudio fue analizar la evidencia sobre las ventajas y desventajas del uso individual o en combinación de los 3 principales tratamientos de la EH, específicamente su eficacia en los diferentes grados, su impacto en la calidad de vida, profilaxis y reducción de costos.
Cirrhosis of the liver is the fourth cause of death in the Mexican population and is mainly caused by hepatitis C virus infection and alcoholic liver disease.1 Approximately 1.5 million cases of chronic liver disease have been projected for the year 2020, which will be susceptible to the development of serious complications, such as portal hypertension, ascites, and hepatic encephalopathy (HE).2
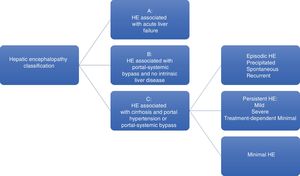
HE is a severe complication in patients with decompensated cirrhosis of the liver. It is a consequence of liver failure or portal-systemic bypass, or both, and manifests as neuropsychiatric alterations (Fig. 1).3–4 Said complication importantly affects the quality of life of those that have it and is associated with a high mortality rate.
Hepatic encephalopathy (HE) classification.
Persistent hepatic encephalopathy (PHE) starts from grade II of the West Haven classification, and its severity depends on a higher grade of said classification. Minimal hepatic encephalopathy (MHE) is characterized by mild cognitive alterations that are only detectable through psychometric tests and is considered as such when the patient presents with grade I of the West Haven classification.
From a 2011 Italian database that included 2,678,462 patients hospitalized in different regions of the country, 381 (0.014%) of the hospitalizations were due to HE. Twenty-one percent of those patients died during their first hospitalization and another 5.8% died during the remainder of 2011. Of the patients that did not die, 42% were hospitalized again in the same year, and the majority because of HE recurrence.5 Similar findings were reported in a review of 8,766 adults hospitalized in the United States due to HE. The mortality rate ranged from 5 to 17%, but increased to 40% in the cases of severe HE, whereas another 40% were rehospitalized due to HE over the course of one year.6 HE can also be an important mortality factor, independent of other complications related to cirrhosis or even to extrahepatic organ failure. That was observed more recently by Bajaj et al. in a study on more than 1,500 patients hospitalized because of grade III or grade IV HE that had a greater in-hospital mortality rate at 30 days, regardless of extrahepatic organ failure.7
For the study of HE and a better clinical approach, the pathology is classified into 3 main categories, depending on its cause, according to the 2002 Vienna consensus (Fig. 1).8,9 Category C includes the subcategories of episodic HE, which is divided according to its first clinical appearance; minimal HE (MHE), which includes subclinical neurocognitive disorders identified only by neuropsychologic testing; and persistent HE (PHE), which is frankly manifest and subdivided as severe, if there are West Haven classification criteria, or as treatment-dependent, when the symptoms begin as soon as treatment for HE is suspended.9,10 The term recurrent HE is reserved for those patients that present with 2 events of episodic HE in one year, regardless of whether or not there are triggering factors. The costs attributed to HE can also be differentiated, depending on its severity.
According to the European and American clinical practice guidelines on HE management, the therapeutic options that have shown better results are lactulose, which is considered first-line treatment, rifaximin-α (RFX), and L-ornithine L-aspartate (LOLA), the stable salt composed of the amino acids, ornithine and aspartate.4 In contrast, the Mexican guidelines are less illustrative, with respect to the different therapeutic strategies. For example, since 2009, the Asociación Mexicana de Gastroenterología distinguishes lactulose, applied enterally or through enemas, as the first-line treatment for any grade of HE, followed by RFX, LOLA, or metronidazole, in its treatment algorithm,11 in addition to presenting evidence on other medications, but without comparing the effectiveness between one and another. The 2013 clinical practice guidelines from the Secretaria de Salud continue to recommend the use of neomycin and metronidazole, as second-line treatment for patients that do not respond to lactulose or lactitol, despite the evidence on the effectiveness of RFX and LOLA in PHE and the disuse that neomycin has fallen into, as well as the conditions in which metronidazole should be used.12
Therefore, the aim of the present work was to carry out a review of the most up-to-date evidence on the efficacy of RFX, compared with lactulose and LOLA, in improving the different aspects of HE. We also included the reduction of costs associated with treatment and hospitalization, their efficacy in the different grades of HE, their efficacy in relapse prevention, the decrease in accidents due to falls, cognitive status improvement, and quality of life improvement in the patients with MHE, as well as treatment adherence, and finally, their usefulness regarding other complications of chronic liver disease (Table 1).
Comparison of the efficacy of rifaximin in different PHE variables.
| Comparison of meta-analyses | Therapeutic intervention | Clinical improvement/PHE resolution | Decrease inmortality | Primary prophylaxis | Maintenance therapy Impact on quality of life | Tolerability/Safetyprofile | Cost-effectiveness | |
|---|---|---|---|---|---|---|---|---|
| Jiang et al. 200849 | RFX vs. lactulose | RFX=lactulose in the treatment of acute and chronic symptoms | Not measured | Not measured | Not measured | Not measured | RFX>lactulose (especially when the main side effect of lactulose was abdominal pain) | Not measured |
| Eltawil et al. 201248 | RFX vs. lactuloseRFX vs. antibiotics(neomycin and paromomycin) | RFX=lactuloseRFX=other antibiotics | Not measured | Not measured | Not measured | RFX>lactulose and antibiotics in the performance on psychometric tests | RFX>lactulose and antibiotics | Not measured |
| Wu et al. 201387 | RFX vs. lactulose | RFX=lactulose | Not measured | Not measured | Not measured | RFX>lactulose in the performance on psychometric tests | RFX>lactulose and antibiotics | Not measured |
| Kimer et al. 201488 | RFX vs. placeboRFX vs. lactuloseRFX vs. otherantibiotics | RFX>placeboRFX>lactuloseRFX>other antibiotics | RFX>placeboRFX>lactuloseRFX>other antibiotics | Not measured | RFX> placeboRFX>lactuloseRFX>other antibiotics | Not measured | RFX>placeboRFX>lactuloseRFX>other antibiotics | RFX>placeboRFX>lactuloseRFX>other antibioticsRFX>placebo/no interventionLactulosemonotherapy>RFX(monotherapy) |
| Zhu et al. 201589 | Comparison of amino acids, RFX, lactulose, LOLA | All interventions>none | LOLA>amino acids, lactulose, RFX | Not measured | Not measured | Not measured | Same tolerability for all interventions | Not measured |
| Thumpuru et al. 201790 | Comparison of lactulose, LOLA, RFX, probiotics, and amino acids in individual and/or combined treatment | Not measured | Not measured | LOLA>placeboLactulose>placeboProbiotic>placeboRFX=placeboNo intervention was superior to another | Not measured | Not measured | Not measured | Not measured |
| Wang et al. 201950 | Lactulose+RFX vs. lactulose(monotherapy) | Lactulose+RFX>lactulose | Lactulose+ RFX> lactulose | Not measured | Not measured | Lactulose+RFX> lactulose | Lactulose+RFX=lactulose | Lactulose+RFX>lactuloseDecrease of 10 hospitalizations |
=: equal to; >: superior to; LOLA: L-ornithine L-aspartate; PHE: persistent hepatic encephalopathy; RFX: rifaximin; RFX/lactulose: combined therapy of rifaximin and lactulose.
Despite the fact that there has been steady progress over the last 20-30 years in the understanding of the pathophysiologic bases of HE, the lack of a direct correlation between the pathogenic factors and severity of HE makes selecting the adequate therapy difficult. Nevertheless, the goal of the majority of current treatments is to reduce the levels of ammonia (NH3).13,14
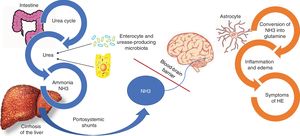
Even though other factors are involved in the pathophysiology of HE, such as inflammatory cytokines and manganese deposits in the basal lymph nodes, NH3 is still considered the main factor for developing HE.15 NH3 is produced from nitrogen, especially by the action of the glutaminase enzyme located in the enterocytes of the small bowel and colon, as well as the from the action of a large quantity of urease-producing bacteria that form part of the gut microbiota. Under normal circumstances, NH3 is metabolized in the liver and then eliminated by the kidney, and to a lesser degree, by the muscles (Fig. 2).16
Pathophysiology of hepatic encephalopathy.
Ammonia (NH3) is composed of nitrogen and hydrogen and is principally derived from the metabolism of amino acids. Under normal circumstances, NH3 is converted into urea, to be eliminated by the kidneys and skeletal muscle. In parallel, one-fourth of the urea produced in the urea cycle is sent to the intestine, where it is converted into NH3, especially by the action of the glutaminase enzyme located in the enterocytes of the small bowel and the colon, as well as by the action of a large quantity of urease-producing bacteria that form part of the gut microbiota. In the context of liver failure, such as the case of patients with cirrhosis of the liver, the process of NH3 metabolism is interrupted, resulting in increased NH3. In addition, portosystemic shunts can aid in the circulating increase of NH3, which crosses the blood-brain barrier and is metabolized in the astrocytes by glutamine synthetase, converting the NH3 and glutamate into glutamine. The accumulation of glutamine in the astrocytes creates an osmotic gradient, resulting in inflammation of the astrocytes and production of reactive oxygen species. Imaging studies have demonstrated edema of the brain, even in patients with MHE, and said edema is related to cognitive decline.
In patients with cirrhosis, liver dysfunction alters the hepatic metabolism of NH3, and portal hypertension causes the diversion of NH3-rich portal blood into the circulatory system. In the brain, NH3 crosses over the blood-brain barrier and is metabolized in the astrocytes by glutamine synthetase, which converts the NH3 and glutamate into glutamine.15 The accumulation of glutamine in the astrocytes creates an osmotic gradient, resulting in inflammation of the astrocytes and the production of reactive oxygen species, which contributes to the brain dysfunction observed in HE (Fig. 2).17,18
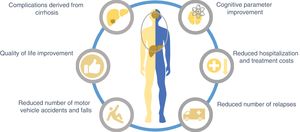
The treatment goals for HE in general are to resolve the acute symptoms, reduce mortality, and shorten hospital stay. Afterwards, relapses and rehospitalizations must be prevented. Treatment is expensive, and therefore an attempt must be made to reduce the cost of medical attention, while maintaining the highest degree of efficacy. In addition, treatments that improve quality of life of the patients with HE, as well as those that act on the other complications of cirrhosis of the liver, must be considered (Fig. 3).
Hepatic encephalopathy (HE) treatment goals.
The treatment of cirrhosis of the liver should include the integral management of HE, thus it is important to consider that a large percentage of patients with cirrhosis will develop some grade of HE. Therefore, primary prevention treatment can be begun to improve quality of life, along with preventing accidents, caretaker dependency, and hospitalizations. In the patients that have already developed HE and are hospitalized, treatment should be contemplated that reduces hospital stay.
MHE is characterized by neurocognitive decline associated with poor quality of life and increases the risk for developing PHE.10 In fact, those patients already have significant brain alterations that can be demonstrated through magnetic resonance imaging, before progressing into a more severe grade of HE. Those changes are correlated with the presence of edema of the brain in patients with MHE, who after treatment, have improved cognitive status, shown by better performance on different psychometric tests.19 Said information exemplifies the fact that MHE, which is still underestimated, should be treated.10,20
Despite the negative effects of MHE, lack of treatment also results from its lack of diagnosis, which is made through the application of psychometric tests. The psychometric hepatic encephalopathy score (PHES), inhibitory control test (ICT), and critical flicker frequency (CFF) stand out among them.21,22
Those tests not only enable MHE diagnosis, but also allow the lack of important cognitive functions that cause the patient to live in a disabled state regarding daily activities to be determined.23 Those patients with HE are susceptible to motor vehicle accidents and frequent falls when walking. The latter are due to the decline in neuromotor functions and they later become complicated with fractures secondary to osteoporosis from malnutrition, hypogonadism, or liver failure.24
In relation to motor vehicle accidents, a 2004 prospective study evaluated driving capacity through number-connection tests, complex choice reaction tests, and digit symbol tests in patients with MHE. Those authors concluded that said patients did not have the capacity to drive.25 A later study showed that a high percentage of MHE patients with a low performance on ICTs suffered more motor vehicle accidents than patients without MHE (17% vs. 0.0%, p=0.0004).26 Given the potential risk of death from those accidents and their costs for the patient, early treatment for patients with HE is recommended. To demonstrate the above, an 8-week study was conducted on 42 patients that were randomized to receive a placebo or RFX for the entire study to evaluate the performance of those patients in driving simulators after treatment. Patient quality of life and their cognitive abilities were also evaluated, utilizing the sickness impact profile (SIP). The conclusion was that the performance in the driving simulator improved significantly in the patients with MHE after treatment with RFX, compared with placebo.27
Treatment with lactulose has also benefitted that population. In the context of MHE, 3-month long treatment with lactulose improved both cognitive performance and quality of life, which were measured by repeat application of number and figure connection tests, block design tests, and the SIP questionnaire for evaluating quality of life.28
Contradictory results are found when comparing lactulose with RFX in MHE. Some authors support the use of one over the other, whereas other authors find no inferiority or superiority between them. A study conducted in India on 351 patients with MHE compared the effectiveness of RFX (tablet of 400mg, 3 times a day) versus lactulose (30-120ml/day) on quality of life, utilizing SIP questionnaires. At 3 months of treatment, MHE was reduced in 73.3% of the patients that received RFX and in 69.1% of the patients that were treated with lactulose. Both treatments improved quality of life, but the noninferiority of RFX over lactulose could not be established because the prespecified noninferiority margin (−5%) was within the two-sided 90% confidence interval of difference. Nevertheless, the group treated with lactulose presented with significantly more flatulence than the patients treated with RFX.29
Finally, from the pharmacogenomic perspective, there is a significant difference between lactulose and RFX in relation to MHE cost reduction. Current evidence suggests that lactulose is the better cost-effectiveness option. In fact, treatment with RFX in MHE did not reduce costs with any of four screening strategies analyzed and showed no cost-saving benefit when compared with lactulose.30
The combination of RFX/lactulose was superior to the individual management of either of the two options, reversing MHE in 3 months and preventing relapses for up to 6 months.31
Regarding LOLA, the conclusion of a meta-analysis that included 8 randomized clinical trials stated that its treatment efficacy was the same versus placebo in PHE and MHE and that it had the same effectiveness as lactulose.32 Two randomized clinical trials compared the 3 therapeutic options versus placebo, but not with each other. There are currently no studies comparing LOLA and RFX in MHE.33,34
In conclusion, the primary aim of treatment for MHE is the prevention of accidents and hospitalization, improving patient quality of life in relation to cognitive, economic, and functional aspects. From the evidence found, there appears to be no difference between RFX and lactulose for achieving that goal.29 However, even though lactulose apparently implies lower cost, RFX is better tolerated by patients, as shown by Bajaj et al. in 2010.35 Tolerance is an important factor for good treatment adherence, thus patient status should be considered in choosing one option over the other. Even though LOLA has not been shown to be superior to lactulose or RFX, its safety allows it to be considered in combination with either of the other 2 options. It can even be used as nutritional support, together with branched-chain amino acids, which have also been shown to improve psychometric test results in patients with MHE.36,37
Persistent hepatic encephalopathyPHE is associated with higher rates of hospitalization, mortality, and deficient quality of life.38 Its management must also be directed at educating the patient and his or her relatives, preventing disease progression to more severe grades (Table 1), and promoting treatment adherence.39
According to the West Haven classification, grade I HE is characterized by a trivial lack of awareness, shortened attention span, and mild asterixis or trembling. In grade II, which can be considered PHE, there is lethargy, disorientation, and obvious asterixis. Grade III consists of somnolence, disorientation with bizarre behavior, and muscular rigidity with clonus and hyperreflexia. Grade IV is coma, with or without response to painful stimuli.40
The first step in the treatment of PHE is to identify and treat the precipitating causes, which include the management of hypovolemia, gastrointestinal bleeding, infection, excessive diuretic use, diarrhea, vomiting, hyponatremia, hypokalemia or hypercalcemia, constipation, benzodiazepine use, and failure to carry out the indicated treatment.
A 2004 meta-analysis41 evaluated 22 clinical trials for the purpose of comparing the performance of nonabsorbable disaccharides versus placebo, no intervention, or antimicrobials in PHE management. Compared with placebo or no intervention, lactulose and lactitol appeared to reduce the risk for no HE improvement (relative risk 0.62, 95% CI: 0.46 to 0.84). However, they were not superior when compared with antibiotics that included neomycin, ribostamycin, vancomycin, and RFX. Unlike RFX, the other 3 antibiotics have fallen into disuse, mainly due to their side effects.37
In addition, treatment with lactulose also helped prevent recurrent HE and reduce the risk for severe adverse events due to liver failure, variceal bleeding, spontaneous bacterial peritonitis, hepatorenal syndrome, and ultimately, death.42
RFX use is also documented in the treatment of overt HE, especially for acute symptoms. The first multicenter study on RFX was conducted in 1993, in which, in addition to its effectiveness, it was confirmed as a well-tolerated drug,43 especially in the short-term comparison with lactulose, as well as with other antibiotics.44 There appears to be no difference between RFX and disaccharides in relation to long-term treatment and relapse prevention.45
A recent randomized, double-blind, placebo-controlled trial by Bass et al.46 included 299 patients in remission from recurrent HE that received either RFX at a dose of 550mg twice a day (140 patients) or placebo (159 patients) for 6 months. A total of 13.6% of the patients in the RFX group had a relapse of HE, compared with 22.6% of the patients in the placebo group, for a relative risk of 0.50 (95% CI: 0.29 to 0.87; P=0.01). However, more than 90% of the patients in both groups received concomitant treatment with lactulose.
As with MHE, when RFX was compared with lactulose, it appeared to have at least the same efficacy.47,48 Even though said effectiveness could depend on whether the treatment was short-term or long-term, RFX continues to be better tolerated, causes fewer side effects, and produces a higher reduction of NH3.45,49
In contrast, the combination of RFX/lactulose is more efficacious than lactulose, alone, in the treatment of overt HE. The combination, versus lactulose, alone, can reduce mortality (23.8% vs. 49.1%, respectively) and hospital stay (5.8 days vs. 8.2 days, respectively).50
RFX and/or lactulose appear to be the best options, whereas treatment with LOLA alone is not sufficiently supported to be considered first-line therapy. In fact, its effectiveness in overt HE has only been observed in less severe grades (grade II) of both PHE and MHE.51–53
Finally, in relation to other treatments, trials that were studied in a meta-analysis on the use of zinc had heterogeneous results. In addition, critical results, such as quality of life, could not be measured, making it difficult to reach conclusions about the value of oral supplements, such as zinc, in the treatment of hepatic encephalopathy. There was also little information available on the clinical importance of the different formulations of zinc utilized in the trials.54
Relapse prevention and prophylaxisProphylactic treatment of HE can be divided into primary and secondary treatment. There are few studies on primary prevention, which consists of preventing the appearance of the first episode of HE in patients with cirrhosis. The aim of secondary prevention is to prevent another episode of HE after a previous one.52
The use of lactulose, LOLA, and RFX, respectively, was recently compared as primary prevention for HE in patients with variceal bleeding. In fact, the probability of developing overt HE is particularly correlated with acute variceal bleeding. RFX and LOLA were superior for preventing overt HE, compared with lactulose, in that group of patients. In addition, both drugs were better tolerated than lactulose, given that 54.4% of the patients treated with lactulose presented with severe diarrhea.55
Regarding primary prevention, nonabsorbable disaccharides were effective, compared with no intervention.56 Lactulose and lactitol showed the same effectiveness, but had different results when compared with other options.57
In the context of secondary prevention, lactulose at 2 or 3 divided doses of 30-60ml per day were also effective, preventing relapse of overt HE over a 14-month period. It should be pointed out that the probability of relapse can be evaluated through psychometric tests, in which poor performance in 2 or more tests indicates the need for treatment change.58,59
Probiotics have also shown good results in primary prevention, even on a par with lactulose. They can prevent relapses of HE for up to 11 months and significantly reduce NH3 levels in 3 months.60
RFX has also been tested as maintenance therapy, preventing the recurrence of HE for 6 months at a dose of 550mg twice a day.46 Treatment with LOLA versus placebo as maintenance therapy was recently compared and demonstrated effectiveness in the prevention of relapses. Nevertheless, different studies state that the combination of lactulose/RFX is superior as prophylaxis.61
Impact on quality of life and cost reductionAs previously described, the quality of life of patients with MHE is deficient, but that situation is not limited to MHE. Patients with overt HE present with cognitive alterations, even after the acute episode is resolved. Indeed, the risk for presenting with MHE is greater in patients that previously presented with an episode of overt HE.62
As a matter of fact, cognitive decline appears to be permanent in patients with cirrhosis. In studies on MHE, in which quality of life changes are more subtle than those found in patients with previous overt HE, the quality of life questionnaires specific for liver disease or longer generic questionnaires, such as the SIP, can be better for finding differences. The changes in quality of life are important for predicting relevant clinical results, such as HE recurrence, hospitalizations, and death in ambulatory patients with cirrhosis.63
As well as cognitive alterations, patients with HE usually have sleep disorders64 and require caretakers for daily functions. In addition, patients become increasingly dependent with each episode of HE.65
With respect to lactulose, studies on MHE have shown improvement in quality of life, especially in relation to cognitive function, but there are few studies on the theme in PHE.28,66
Regarding RFX, the 2011 RIME study evaluated its impact on quality of life and cognitive function improvement. Ninety-eight patients were randomized to receive RFX (1,200mg day) or placebo for 8 weeks and then evaluated through psychometric tests and the SIP. In the RFX group, 75.5% of the patients had HE remission and better scores on the psychometric tests and SIP questionnaires, compared with 20% of the placebo group.66 In parallel, in another study conducted in the same year, there was improved quality of life with RFX at a dose of 550mg twice a day in patients with recurrent HE. Measurements were carried out using the chronic liver disease questionnaire (CLDQ), which was applied every 4 weeks for the 6 months of the study. Nevertheless, it should be kept in mind that concomitant lactulose administration was allowed in the patients.67
Likewise, in 2011, a study on the use of LOLA was conducted on 191 patients with HE. The CLDQ was also utilized to measure the effect on quality of life. The intervention consisted of 3 packets of 6g of LOLA daily for 8 weeks. There was notable improvement with the treatment in all the domains evaluated in the questionnaire, especially in regard to fatigue, and it was well tolerated in 97% of the patients.68
Up to 80% of the patients with cirrhosis experience HE and the majority are at high risk for recurrence. Several factors should be considered in developing a cost-effective management of HE, such as patient compliance, the adverse effects of medical therapies, and the relative cost-benefits of the therapy.
The main pharmacologic agents utilized for the treatment and prevention of HE are commonly associated with gastrointestinal adverse effects. Even though the adverse reactions are common, they can be bothersome and lead to patient noncompliance, thus increasing the risk for HE. In addition, the first-line agent, lactulose, requires self-dosing, which can be confusing for the patient and result in an increased risk for severe adverse effects.
HE imposes a significant economic burden on the patient, caretaker, health system, and society. Not only does it have a negative impact on the morbidity and mortality of the patient, but it also affects his or her psychologic and social functioning, as well as quality of life, in general. It can affect the patient’s ability to work, resulting in reduced productivity and a loss of income. A patient with HE may require hospitalization, which is a substantial proportion of the costs associated with HE. Early prevention is important for minimizing the associated social and economic costs.
Regardless of geographic location, patients with HE represent a considerable economic burden on the medical care infrastructure, and the annual hospitalizations for patients with HE can be very costly for health systems. The mean duration of HE-related hospital stay has been reported to vary from 5.9-9 days.69,70
In the United States, the direct costs of chronic liver disease were above 2 billion USD and the indirect costs were over 450 million USD. As mentioned before, hospitalization because of HE is the main contributor to that burden, at a cost of $30,000 USD/hospitalization. In addition, 22% of the patients that are released, are generally placed in assisted living facilities for the elderly or rehabilitation centers, increasing total costs. In that respect, even though RFX is more expensive than lactulose, it appears to be superior in reducing hospitalization rates, has a better adverse effect profile, and increases patient compliance, thus saving more than $3,000 USD in costs/patient/year, compared with lactulose therapy.71
Studies suggest that beginning therapy with RFX could lead to reduced hospital stay.72 In addition, the estimated cost savings for RFX versus lactulose, albeit based on published estimates of the numbers affected, support the idea of a reduced risk for hospitalization in patients with HE, which would significantly reduce the economic burden on health systems. Importantly, the higher cost of RFX is compensated by the potential savings in medical care costs.73
The literature suggests that RFX can provide better symptom control, and in turn, reduce not only costs related to hospitalization and hospital stay duration, but also other medical care-related costs, especially if RFX use results in less need for ambulatory follow-up care. For patients receiving RFX550mg twice a day versus 400mg 3 times a day, the former was shown to be more cost effective than the latter.74
Treatment with lactulose is associated with a greater number of hospitalizations, as well as longer hospital stay duration and higher hospitalization costs.75 A retrospective study at a single center in the United States indicated that the use of lactulose after initial hospitalization was associated with rehospitalization within the following 90 days, whereas RFX use after hospitalization was not significantly associated with the risk for hospital readmission within 90 days.76 In another study, the use of healthcare resources was significantly reduced after the commencement of RFX use, compared with before its use.77
A low incidence of HE has also been shown in clinical practice regarding long-term treatment, when RFX was added to lactulose therapy.78 As with all antibacterial agents, Clostridium difficile-associated diarrhea has been reported with treatment with RFX, albeit at a low frequency. Nevertheless, it has not become an important safety concern in long-term treatment. Therefore, there is a large amount of evidence showing that the addition of RFX to standard therapy with lactulose produces significant long-term reductions in the use of healthcare resources.
In their study, Stauch et al.79 demonstrated clear advantages in the use of LOLA, compared with placebo, for achieving improvement in encephalopathy associated with chronic liver injury. It is considered a pioneer study, given that there are no cost-effectiveness studies comparing those treatment strategies. A comparative study by Poo et al.80 evaluated the therapeutic response with lactulose and LOLA, assigning 10 patients to each group. The authors demonstrated that both medications were useful in the management of encephalopathy caused by the increase in ammonia. However, response in relation to mental health parameters, number-connection tests, asterixis score, and encephalographic activity were better in the group treated with LOLA.
In a retrospective review on 137 patients from a single center that received lactulose for a mean duration of 27±6 months after a first episode of HE, 75% of the patients experienced recurrence of HE after 9±1 months. Thirty-nine (28.5%) patients had recurrence of HE associated with lack of adherence to treatment with lactulose, mainly due to gastrointestinal adverse effects.35
On the other hand, it is important to mention the contradictory evidence on the superiority of one drug over another.81 Despite the elevated cost of RFX, it is the drug that is tolerated the best, with a higher reduction of hospitalization costs.82,83 In addition, that antibiotic can be used in other complications of cirrhosis of the liver, beyond HE, such as ascites with kidney function deterioration upon the increase in the glomerular filtration rate and the urinary excretion of sodium,84 and in patients with ascites and spontaneous bacterial peritonitis.85,86
In addition to decreasing NH3, RFX also has anti-inflammatory effects and produces changes in the fecal microbiota, which other options do not. That is important, given that patients with cirrhosis have a high incidence of dysbiosis and altered bile acid composition, which are ultimately associated with disease severity.87
ConclusionsCharacterized by periods of cognitive decline of varying severity, HE is a frequent complication of chronic liver disease and a frequent cause of hospitalization, as well as morbidity and mortality, in that population of patients. Up to 70% of patients with cirrhosis can present with some grade of HE. All the current treatments have been shown to be efficacious in the different grades of HE, and their use is superior to no treatment at all in patients with MHE. Whether or not to treat patients with MHE has been the subject of discussions, and LOLA has been suggested to possibly play an important role by being the medication that produces fewer side effects, and therefore, is better tolerated.
On the other hand, current first-line treatment for HE patients is with nonabsorbable disaccharides, such as lactulose, even for preventing recurrent HE. However, its side effects, especially the gastrointestinal ones, make it difficult for many patients to tolerate. In contrast, RFX has shown at least the same effectiveness as lactulose, as well as the following advantageous attributes: 1) reduced hospital stay, 2) reduced mortality rate, 3) use as maintenance therapy, 4) a greater safety and tolerability profile, and 5) better psychometric test performance in patients with MHE and patients in whom acute episodes of overt HE were resolved. Although there are few meta-analyses that include the combination of RFX/lactulose, clinical trials comparing said combination versus RFX or lactulose as monotherapies, have shown it to be superior.
In conclusion, the treatment of HE should be considered in the integral management of the patient with cirrhosis. When MHE is suspected, adequate evaluation should be carried out and treatment begun as soon as possible, to improve patient quality of life. In general, the treatments described herein have been shown to effective, therefore their individualization for each patient is of the utmost importance. For example, if a patient does not tolerate lactulose, it would be better to begin treatment with RFX. Even though RFX is more expensive than lactulose, it shortens hospital stay and significantly improves the cognitive status of the patient, resulting in an option with greater cost-effectiveness than lactulose. In contrast, LOLA and lactulose can be better than RFX for primary prevention. The use of LOLA alone continues to be a viable option for patients with MHE, as long as they respond adequately, and treatment with lactulose has failed. Individually, it has also been shown to be superior to RFX for the prevention of PHE. Studies and meta-analyses have also supported the combination of LOLA and probiotics, finding it superior to the use of LOLA alone, but inferior to lactulose plus RFX, for the treatment of acute episodes.
Finally, the combination of RFX and lactulose has been shown to be the best treatment option in both the prophylaxis and treatment of PHE.
Ethical disclosuresInformed consentThe present study did not require any type of informed consent, given that it is a bibliographic review.
Protection of persons and animalsNo tests on persons or animals were conducted, given that the study is a bibliographic review.
Confidentiality of dataNo type of sensitive information was managed, given that the study is a review of published articles, and as such did not require access to patient information, such as clinical records.
Due to the above, the present study did not need to be evaluated by the ethics committee of the Fundación Clínica Médica Sur.
Financial disclosureNo financial support was received in relation to the present study.
AuthorshipN. Méndez-Sánchez is the lead researcher. He collaborated on the study design, reviewed the literature, and the content of the manuscript. A.C. Frati-Munari conceived the idea of the work, collaborated on the study design, and revised the content of the manuscript. J. Contreras-Carmona and C.E. Coronel-Castillo reviewed the literature, collaborated on the study design, and drafted the manuscript. M. Uribe collaborated on the study design and reviewed the literature and the quality of the manuscript.
Conflict of interestThe authors declare that there is no conflict of interest.
The present study was supported by the Fundación Clínica Médica Sur.
Please cite this article as: Coronel-Castillo CE, Contreras-Carmona J, Frati-Munari AC, Uribe M, Méndez-Sánchez N. Eficacia de la rifaximina en los diferentes escenarios clínicos de la encefalopatía hepática. Revista de Gastroenterología de México. 2020;85:56–68.