Gastric cancer is one of the most frequent neoplasias in the digestive tract and is the result of premalignant lesion progression in the majority of cases. Opportune detection of those lesions is relevant, given that timely treatment offers the possibility of cure. There is no consensus in Mexico on the early detection of gastric cancer, and therefore, the Asociación Mexicana de Gastroenterología brought together a group of experts and produced the “Mexican consensus on the detection and treatment of early gastric cancer” to establish useful recommendations for the medical community. The Delphi methodology was employed, and 38 recommendations related to early gastric cancer were formulated. The consensus defines early gastric cancer as that which at diagnosis is limited to the mucosa and submucosa, irrespective of lymph node metastasis. In Mexico, as in other parts of the world, factors associated with early gastric cancer include Helicobacter pylori infection, a family history of the disease, smoking, and diet. Chromoendoscopy, magnification endoscopy, and equipment-based image-enhanced endoscopy are recommended for making the diagnosis, and accurate histopathologic diagnosis is invaluable for making therapeutic decisions. The endoscopic treatment of early gastric cancer, whether dissection or resection of the mucosa, should be preferred to surgical management, when similar oncologic cure results can be obtained. Endoscopic surveillance should be individualized.
El cáncer gástrico representa una de las neoplasias más frecuentes en el aparato digestivo y en la mayoría de los casos es el resultado de la progresión de lesiones premalignas. La detección oportuna de estas lesiones es relevante ya que un tratamiento oportuno, brinda la posibilidad de curación. En nuestro país no existía un consenso respecto a la detección temprana del cáncer gástrico, por lo que la Asociación Mexicana de Gastroenterología reunió a un grupo de expertos y realizó el Consenso sobre detección y tratamiento del cáncer gástrico incipiente (CGI) para establecer recomendaciones de utilidad para la comunidad médica. En este consenso se utilizó la metodología Delphi y se emitieron 38 recomendaciones al respecto del CGI. El consenso define al CGI como aquel que al momento del diagnóstico se encuentra limitado a la mucosa y a la submucosa, independientemente de metástasis en ganglios linfáticos. En México como otras partes del mundo los factores asociados al CGI incluyen la infección por Helicobacter pylori, los antecedentes familiares, el tabaquismo y los factores dietéticos. Para el diagnóstico se recomienda utilizar cromoendoscopia, magnificación y equipos con luz mejorada. Un diagnóstico histopatológico preciso es invaluable para tomar de decisiones terapéuticas. El tratamiento endoscópico del CGI, ya sea disección o resección de la mucosa, debe ser preferido al manejo quirúrgico cuando se puedan obtener resultados semejantes en términos de curación oncológica. La vigilancia endoscópica se deberá de individualizar.
Gastric cancer is one of the most frequent neoplasias in the digestive tract, and in the majority of cases, is the result of premalignant lesion progression. Opportune detection of those premalignant or early lesions is relevant, given that timely treatment offers the possibility of cure and an increase in life expectancy. Despite the fact that Mexico has an intermediate prevalence of gastric cancer, there are no guidelines or consensuses on the detection of the tumor in its early stages. Therefore, in 2018, the Asociación Mexicana de Gastroenterología brought together a multidisciplinary group of health professionals made up of gastroenterologists, endoscopists, oncologists, pathologists, radiologists, surgeons, and a biomedical scientist to produce the Mexican consensus on the detection and treatment of early gastric cancer (EGC), establishing useful recommendations for the medical community.
The specific aim of the present consensus was to prepare an up-to-date document on the epidemiology, diagnosis, and treatment of EGC to be applied in the Mexican medical practice. Its recommendations are based on an extensive review of the literature and on the consensus of opinion of the participating specialists.
MethodsThe consensus was developed utilizing the Delphi process.1 Three coordinators (MEICh, FHI, MAT) were designated and 17 experts in the specialties related to the diagnosis and treatment of EGC were invited to participate. The coordinators carried out a detailed search in the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (from PubMed), EMBASE (from Ovid), LILACS, CINAHL, BioMed Central, and the World Health Organization International Clinical Trials Registry Platform (ICTRP). The search included articles published within the time frame of January 1, 2008 to March 31, 2018. The search criteria included the term: “gastric cancer” combined with the following terms: “early”, “epidemiology”, “incidence”, “prevalence”, “Mexico”, “pathophysiology”, “Helicobacter pylori”, “metaplasia”, “diagnosis”, “differential diagnosis”, “treatment”, “endoscopy”, “therapy”, “management”, “review”, “guidelines”, and “meta-analysis” and their Spanish equivalents. The entire bibliography was made available to the consensus participants.
The coordinators then formulated 60 statements that underwent a first round of anonymous, electronic voting (March 1 to 5, 2018) to evaluate the drafting and content of the statements. The votes were cast utilizing the following responses: a) in complete agreement, b) in partial agreement, c) uncertain, d) in partial disagreement, and e) in complete disagreement.
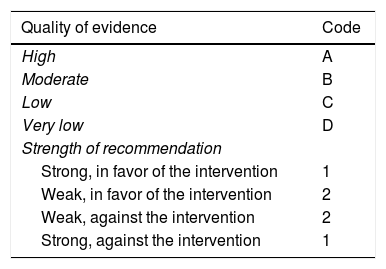
Once the first round of voting was carried out, the coordinators made the corresponding modifications. The statements that reached > 75% complete agreement were kept and the ones that had > 75% complete disagreement were eliminated. The statements that reached ≤ 75% complete agreement and ≤ 75% complete disagreement were reviewed and re-structured. The revised statements underwent a second round of anonymous, electronic voting (April 18 to 27, 2018). As part of the second round of voting, in addition to the drafting and content, each statement was evaluated according to its sustaining quality of evidence and strength of recommendation, utilizing the “Grading of Recommendations Assessment, Development, and Evaluation” (GRADE) system.2 In the GRADE system, the quality of evidence is not determined solely by the research design or methodology, but also by the function of a clearly posed question related to a clearly formulated outcome variable.3 Thus, evidence can be high, moderate, low, or very low. The GRADE system also establishes the strength of the recommendations as strong or weak, in favor of or against, the intervention or statement. Importantly, the GRADE system was used in the case of diagnostic tests and therapeutic interventions. Table 1 shows the codes employed in the GRADE system: upper case letters classify the quality of evidence, followed by a number indicating the strength of recommendation in favor of or against the intervention or statement.
GRADE system code.
| Quality of evidence | Code |
|---|---|
| High | A |
| Moderate | B |
| Low | C |
| Very low | D |
| Strength of recommendation | |
| Strong, in favor of the intervention | 1 |
| Weak, in favor of the intervention | 2 |
| Weak, against the intervention | 2 |
| Strong, against the intervention | 1 |
Adapted from Oñate-Ocaña et al.3
The results of the third round of voting were presented on May 23, 2018, at a face-to-face meeting conducted in the offices of the Asociación Mexicana de Gastroenterología in Mexico City. The statements that reached >75% agreement were ratified. Those that did not reach 75% were discussed and voted on again. Once all the consensus statements were established, the coordinators formulated the present manuscript, which was reviewed and approved by all the members of the consensus.
ResultsThe coordinators initially proposed 60 statements. In the first round of voting, 2 statements were eliminated because they did not reach agreement, and according to the recommendations, 16 were synthesized, leaving 42 statements for the second round of voting. The 42 statements were evaluated at the face-to-face meeting. Thirty-seven (88%) were ratified and 5 (12%) were voted on again. After the meeting, 4 statements were synthetized, resulting in a total of 38 recommendations for the consensus. Those final recommendations and the voting results follow below.
Definition and incidence of early gastric cancer1. Early gastric cancer (EGC) is that which at diagnosis is limited to the mucosa and submucosa, irrespective of lymph node metastasis.
Agreement reached: 96% in complete agreement, 4% in partial disagreement.
The original concept of EGC was established in Japan at the meeting of the Japanese Endoscopy Society in 1962 and defined as a gastric neoplasia that could be successfully treated through surgery.2 Said definition was later modified, describing an adenocarcinoma restricted to the mucosa or to the mucosa and submucosa. That definition was adopted by the National Cancer Center Japan, the Japanese Gastric Cancer Association, and the Japan Gastroenterological Endoscopy Society.2 The TNM stage of EGC is T1 and any N1.4 Those tumors have a better outcome, compared with other stages, with a 90% disease-free survival rate. A small number of those patients have lymph node involvement, disease is limited to the mucosa in 2 to 3% of patients, and 20 to 30% present with submucosal invasion.5 The risk factors for lymph node metastases are age > 60 years, macroscopically depressed type, ulceration, size greater than 2 to 3 cm, nondifferentiated histologic type, lymph node invasion, deep submucosal invasion (> 500 µm), and the mucinous gastric phenotype.5
2. The incidence of EGC ranges from 15 to 57% of the cases of gastric adenocarcinoma, depending on geographic area and screening programs. The incidence of EGC in Mexico is unknown.
Agreement reached: 82% in complete agreement, 6% in partial agreement, 6% uncertain, 6% in partial disagreement.
In different case series worldwide, an estimated 15 to 57% of cases of gastric adenocarcinoma can be detected at an early stage.2–11 Those percentages are dependent on the population studied, being higher in Asian countries, as well as on the performance of screening studies in the general population.
There are no early detection statistics or programs in Mexico that enable the incidence of EGC to be established. In a case series from the Hospital General de México on 63 surgical cases, none had a TNM T1 classification12 and in a report from the Instituto Nacional de Cancerología on 863 cases, only 3.1% of the patients had stage 1 disease at diagnosis.13 Of 588 cases operated on at the Centro Médico Nacional Siglo XXI, only 1.5% had stage IA and 3.7% had stage IB.14
Epidemiology and risk factors for gastric cancer3. Mexico is a country with an intermediate risk for the development of gastric adenocarcinoma. Said tumor is the fourth cause of cancer worldwide and the third cause of cancer deaths in Mexico, in individuals above 20 years of age.
Agreement reached: 93% in complete agreement, 7% in partial agreement.
According to the GLOBOCAN project, gastric adenocarcinoma is the fourth cause of cancer worldwide and the third cause of cancer death in Mexico, in individuals above 20 years of age.15 Epidemiologic evidence shows that Mexico has an intermediate incidence of gastric cancer (between 10 and 20 cases per 100,000 inhabitants),16 albeit with differences between the different regions of the country. For example, Sánchez-Barriga et al.17 reported a higher mortality rate for gastric cancer in Chiapas within the period of 2000-2012 and Gómez-Dantés et al.18 found that gastric cancer held the highest place in incidence and mortality in the states of Guerrero, Oaxaca, and Chiapas, on the list of the first 10 malignant tumors.
The incidence of gastric adenocarcinoma in Mexico in 2013 was 13.5/100,000 inhabitants and has been decreasing in recent years.
Agreement reached: 82% in complete agreement, 18% in partial agreement.
Colorectal cancer has replaced gastric cancer as the main gastrointestinal neoplasia in patients at referral hospitals in Mexico City, given that the incidence of gastric cancer has decreased within the time frame of 1978 to 2012.19 Even though there are no solid systems in Mexico that enable the exact incidence of the different tumors to be known, data from 2013 show that the incidence of gastric cancer standardized by age was 13.5/100,000 inhabitants.19 The age-adjusted mortality rates for gastric cancer in Mexico decreased from 7.5 to 5.6 per 100,000 inhabitants between 2000 and 2012.18
5. The histologic types of gastric adenocarcinoma based on the Lauren classification are intestinal, diffuse, and mixed. The intestinal type is more frequent in areas of high incidence and in older individuals, whereas the diffuse type occurs in areas of low incidence and in younger individuals.
Agreement reached: 88% in complete agreement, 6% in partial agreement, 6% uncertain.
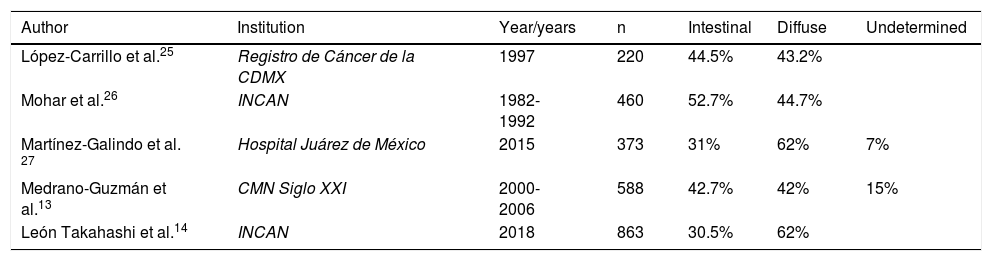
There are currently several histologic gastric cancer classifications, and the most important are the Lauren,20 Nakamura et al.,21 Ming,22 Goseki et al.,23 and World Health Organization (WHO)24 classifications. According to those classifications and epidemiologic studies, intestinal-type gastric cancer is the most frequent in areas of high incidence and in older individuals, whereas the diffuse type occurs in areas of low incidence and in younger individuals. Distribution is similar in Mexico, as in other parts of the world. Table 2 shows the distribution of the different types of gastric adenocarcinoma at different healthcare institutions in Mexico.13,14,25–27
Types of adenocarcinoma at different Mexican institutions.
| Author | Institution | Year/years | n | Intestinal | Diffuse | Undetermined |
|---|---|---|---|---|---|---|
| López-Carrillo et al.25 | Registro de Cáncer de la CDMX | 1997 | 220 | 44.5% | 43.2% | |
| Mohar et al.26 | INCAN | 1982-1992 | 460 | 52.7% | 44.7% | |
| Martínez-Galindo et al. 27 | Hospital Juárez de México | 2015 | 373 | 31% | 62% | 7% |
| Medrano-Guzmán et al.13 | CMN Siglo XXI | 2000-2006 | 588 | 42.7% | 42% | 15% |
| León Takahashi et al.14 | INCAN | 2018 | 863 | 30.5% | 62% |
Centro Médico Nacional (CMN) Siglo XXI: XXI Century National Medical Center; Instituto Nacional de Cancerología (INCAN): National Cancerology Institute; Registro de Cáncer de la Ciudad de México (CDMX): Cancer Registry of Mexico City.
First-degree relatives of individuals with gastric cancer are at an increased risk for presenting with the neoplasia.
Agreement reached: 88% in complete agreement, 6% in partial agreement, 6% uncertain.
The majority of gastric adenocarcinomas are sporadic but 10% have familial aggregation.28 A family history of gastric cancer is 2 to 3-times more frequent in patients with gastric cancer.29 The familial association of gastric cancer can be secondary to a shared environmental exposure or a true genetic susceptibility.30 Compared with individuals not infected with Helicobacter pylori (H. pylori) and no family history of gastric cancer, individuals with a family history of the disease and H. pylori CagA + infection had an odds ratio (OR) of 16 (95% CI: 3.9-66.4) for gastric cancer, except of the cardia, but H. pylori infection and a family history of gastric cancer were independently correlated with the risk for cancer.31
7. Certain genetic syndromes are associated with the development of gastric cancer, such as diffuse hereditary gastric cancer, Lynch syndrome, hereditary breast and ovarian cancer, and familial adenomatous polyposis.
Agreement reached: 93% in complete agreement, 7% in partial agreement.
Hereditary gastric cancer is a rare tumor and is the cause of 2% of all gastric cancers. It is due to germline mutations of the E-cadherin gene, and has an autosomal dominant pattern of inheritance with high penetrance.32 Lynch syndrome is an autosomal dominant alteration caused by germline mutations of the mismatch repair genes, such as MSH2, MLH1, MSH6, and PMS2, with a 13% risk for gastric cancer.33 Patients in the United States with familial adenomatous polyposis have a risk of 0.5% and the risk in Japanese individuals is up to 15.5%.33,34
8. Genetic single-nucleotide polymorphisms associated with gastric cancer have been detected that include: IL-1B, IL-1RN, TNF-α, methylenetetrahydrofolate reductase, and Cyp2e1, among others.
Agreement reached: 93% in complete agreement, 7% in partial agreement.
Polymorphisms in specific genes have been related to the development of gastric cancer. The TNF-α polymorphism is associated with a significant risk for gastric cancer.35 The IL1RN*22 genotype consistently increases the risk for precancerous gastric lesions.36 The methylenetetrahydrofolate reductase and Cyp2e1 polymorphisms have been described, as well.37–39 The prevalence of those genotypes in the Mexican population is unknown.
9. H. pylori infection in the gastric mucosa is considered the main risk factor for intestinal-type gastric adenocarcinoma. Infection reduction is one of the factors associated with the decrease in the incidence of intestinal-type gastric cancer in several regions of the world.
Agreement reached: 88% in complete agreement, 6% in partial agreement, 6% uncertain.
The decrease in H. pylori infection is one of the factors thought to be associated with the reduced incidence of gastric cancer in several regions of the world. Even though the prevalence of H. pylori is high in rural areas of Mexico, Costa Rica, Honduras, Nicaragua, Chile, and Colombia (between 70.1 and 84.7%, both figures in Mexico), the prevalence of cancer in those regions is quite variable,40 that is to say, there are areas with a high prevalence of H. pylori whose prevalence of gastric cancer is not so high. The genetic variation of the virulence factors of H. pylori can explain the differences in the pathogenic properties of the strains, contributing to the explanation of the discrepancy between the number of infected individuals in a population and the percentage of those that develop gastric cancer.41
10. Other well-known risk factors for gastric cancer are: smoking, the consumption of alcohol, processed meat and nitrosamines, Epstein-Barr virus infection, and high levels of salt consumption. Body mass index is elevated, specifically in adenocarcinoma of the cardia.
Agreement reached: 82% in complete agreement, 18% in partial agreement.
Smoking, as in other neoplasias, is considered a predominant risk factor in gastric cancer. The risk for gastric cancer in smokers is 1.62 in men (95% CI: 1.50-1.75) and 1.20 in women (95% CI: 1.01-1.43).42 In Latin America, a 1.47 (95% CI: 1.19-1.81) risk for gastric cancer in smokers has been established.43 In a meta-analysis of studies from several countries, the risk of alcohol consumption for the development of gastric cancer was determined, with an odds ratio of 1.39 (95% CI: 1.20-1.61).44
Processed meat consumption has been associated with the development of gastric cancer, with an estimated risk of 1.67 (1.36-2.05) and 1.76 (1.51-2.05) in case-control studies for red meat and processed meat, respectively, albeit with no statistical significance in cohort studies.45 In Latin America, the risk has been calculated at 1.64 (95% CI: 1.08-2.48).43 In a meta-analysis of 49 studies (19 for nitrates, 19 for nitrites, and 11 for N-Nitrosodimethylamine), the ingestion of high quantities of nitrates showed a weak statistical significance but was positive for gastric cancer reduction, whereas nitrites and N-Nitrosodimethylamine appeared to increase the risk. However, the results were highly heterogeneous and more evidence is needed to affirm the role of those substances in gastric cancer.46 In Mexican patients, Epstein-Barr virus was detected in 10.67% of gastric cancer samples, 1.36% of control samples with no tumor, and 8% of non-atrophic gastritis samples.47 Elevated salt consumption has been associated with the development of gastric cancer. In Latin America, the risk has been estimated at 2.24 (95% CI: 1.53-3.29).43 In a meta-analysis including 41,791 individuals, weight was found to be unassociated with the risk for gastric cancer, with the exception of gastric cancer located in the cardia, in which overweight had a risk of 1.21 (95% CI: 1.03-1.42) and obesity of 1.82 (95% CI: 1.32-2.49).48
11. Exercise, low-dose consumption of capsaicin, consumption of fresh vegetables, refrigeration of food, and high levels of selenium are considered protective factors against the development of gastric cancer.
Agreement reached: 82% in complete agreement, 18% in partial agreement.
The risk for gastric cancer is approximately 13% less in individuals that are more physically active, compared with those that are less active.49 In a meta-analysis of 10 studies with 2,452 cases and 3,996 controls, consumption of low-to-moderate doses of capsaicin was found to be protective against gastric cancer (OR: 0.55, p = 0.003), whereas high doses promoted its development (OR: 1.94, p = 0.0004).50 A study conducted in Mexico suggests that the consumption of moderate-to-high quantities of capsaicin could interact synergically, increasing the risk for gastric cancer in genetically susceptible subjects (carriers of the IL1B-31C allele) infected with the more virulent strains of H. pylori (CagA+).51 A meta-analysis showed that the elevated consumption of fresh vegetables reduced the risk for gastric cancer (OR: 0.62, 95% CI: 0.46-0.85) but pickled vegetables increased the risk (OR: 1.28, 95% CI: 1.06-1.53).52 The dietary intake of citrus fruit reduced the risk for gastric cancer by 13%, including cancer of the gastric cardia.53 In the multivariate analysis of a cross-sectional case-control study on risk factors, conducted in Veracruz, Mexico, the refrigeration of food was a protective factor against gastric cancer (OR: 6.58, 95% CI: 1.78-24.32, p = 0.005).54 High levels of selenium have also been associated with a reduced risk for gastric cancer.55
DiagnosisEven though EGC is an endoscopic finding detected in patients with risk factors, such as those mentioned in statements 6 through 10, it is important to emphasize that it can also be frequently found in patients undergoing an endoscopic examination for dyspeptic symptoms. In that context, and in accordance with the Mexican consensus on dyspepsia, endoscopy should be performed on all patients with uninvestigated dyspepsia that present with alarm symptoms or signs (anemia, weight loss, signs of gastrointestinal bleeding) or failed initial treatment directed at the predominant symptom. Even though the diagnostic yield of endoscopy in patients with persistent or refractory symptoms has not been established in Mexico, performing a diagnostic examination in that context is currently the most recommendable course of action.56
12. Conventional white light endoscopy cannot detect or accurately characterize EGC, making the use of chromoendoscopy (e.g., with indigo carmine) or image-enhanced endoscopic technologies (e.g., NBI) necessary.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 82% in complete agreement, 18% in partial agreement.
There is evidence showing that endoscopic findings utilizing white light (conventional endoscopy) to characterize premalignant lesions, such as atrophic gastritis and metaplasia, including the follicular pattern, have a poor correlation with histologic findings.57,58 Thus, the use of new endoscopic techniques, such as chromoendoscopy and image-enhanced endoscopy (narrow band imaging [NBI]), has shown better histologic correlation. For example, stains with acetic acid or indigo carmine have a greater capacity for diagnosing lesions that are suspicious for metaplasia or dysplasia.59,60 The use of magnification endoscopy (with or without chromoendoscopy) also increases diagnostic suspicion (see statements 12 and 15).61 Importantly, the use of those techniques requires adequate training, takes longer, and their availability is limited in many hospital centers.
13. After cleansing away saliva and other artifacts with dimethicone and N-acetylcysteine, the use of high-resolution endoscopic equipment, with or without magnification, is preferred for optimal detection of the early-stage lesion.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 75% in complete agreement, 15% in partial agreement, 15% uncertain.
For the endoscopic diagnosis of early-stage gastric lesions, the patient should undergo adequate preparation, consisting of drinking a solution that contains water, a mucolytic agent, and a defoaming agent 30 min before the procedure. Said preparation enables better gastric mucosa observation, better lesion identification, and better biopsy sample guidance, as well as favoring adequate resection or dissection of the mucosa.62
In Japan, the formula is: 100 ml of water with 20,000 U Pronase (Streptomyces griseus proteases), 1 g of sodium bicarbonate, and 10 ml of polydimethylsiloxane (20 mg/ml). If Pronase is not available, an alternative solution is: 100 ml of water, 2 ml of acetylcysteine (200 mg/ml), and 0.5 ml (40 mg/ml) of dimethicone. In Mexico, 7 effervescent tablets of 600 mg of acetylcysteine and 80 drops of dimethicone in a 100 mg/ml (4 ml) suspension in one liter of water for injection can be used. One hundred milliliters are administered to the patient 20 min before the examination and the rest is used for cleaning the residue during the study. It is indispensable for the endoscopist to make the differential diagnosis between benign and malignant lesions. Magnification endoscopy is a powerful tool for that purpose, enabling the correct diagnosis of superficial neoplasias in the mucosa of the esophagus, stomach, and intestine (see statement 15).
The morphologic characteristics of EGC and their combined forms should be reported in accordance with the Paris classification criteria.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 82% in complete agreement, 18% in partial agreement.
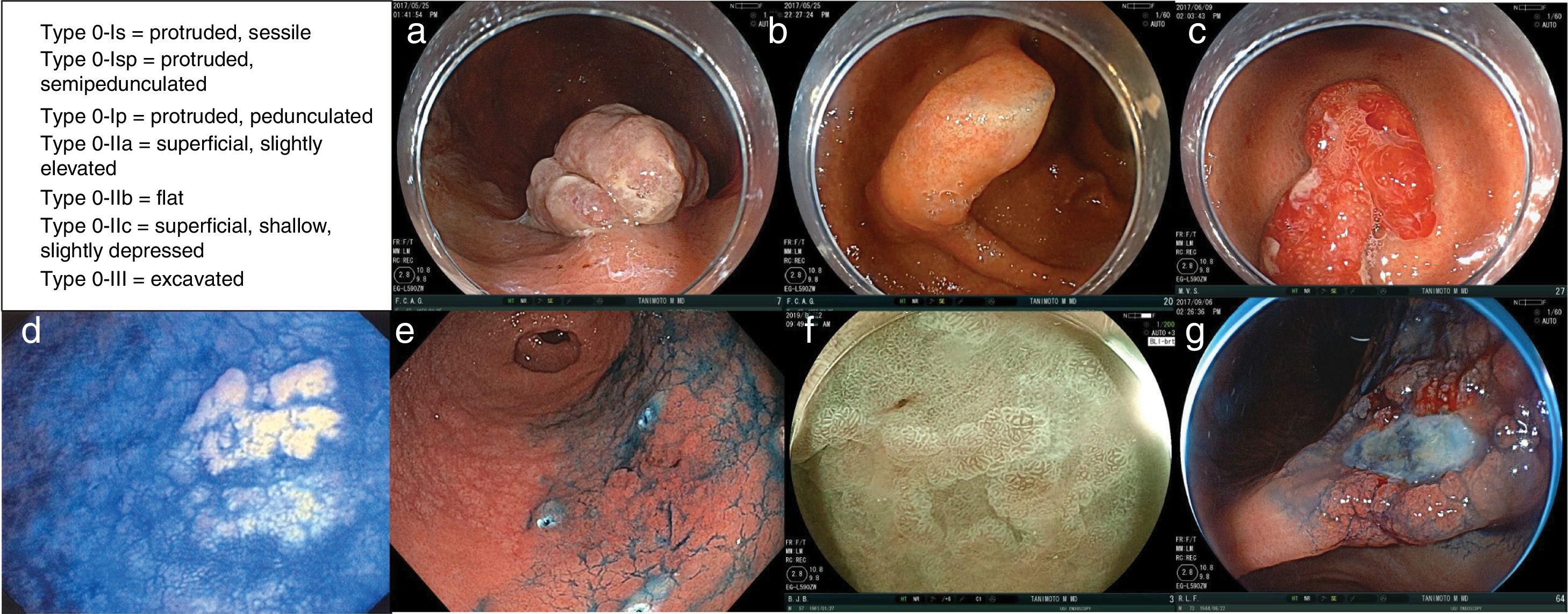
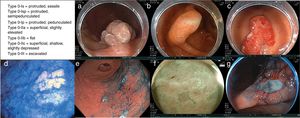
In Paris, an international group of endoscopists, surgeons, and pathologists reached agreements upon, revised, and validated the usefulness and clinical relevance of the Japanese endoscopic classification of superficial neoplastic lesions of the gastrointestinal tract.63 The resulting macroscopic description agreed upon for the variants of type 0 superficial or early-stage neoplastic lesions of the digestive tract are divided into (Fig. 1):
Examples of the different type 0 superficial or early-stage neoplastic lesions. a) Type 0-Is lesion (protruded, sessile). b) Type 0-Isp lesion (protruded, semipedunculated). c) Type 0-Ip lesion (protruded, pedunculated). d) Type 0-IIa lesion (superficial, elevated). e) Type 0-IIb lesion (flat). f) Type 0-IIc lesion (superficial, shallow, depressed). g) Type 0-III lesion (non-polypoid and excavated). Images courtesy of Dr. Miguel Ángel Tanimoto Licona.
–type 0-Is = protruded, sessile
–type 0-Isp = protruded, semipedunculated
–type 0-Ip = protruded, pedunculated
–type 0-IIa = superficial, slightly elevated
–type 0-IIb = flat
–type 0-IIc = superficial, shallow, slightly depressed
–type 0-III = excavated.
15. Ideally, EGC margin determination is achieved through the use of image-enhanced endoscopes (e.g., blue laser imaging or narrow band imaging digital chromoendoscopy), followed by chromoendoscopy (e.g., with 0.2% indigo carmine with/without 1.5% acetic acid).
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 82% in complete agreement, 18% in partial agreement.
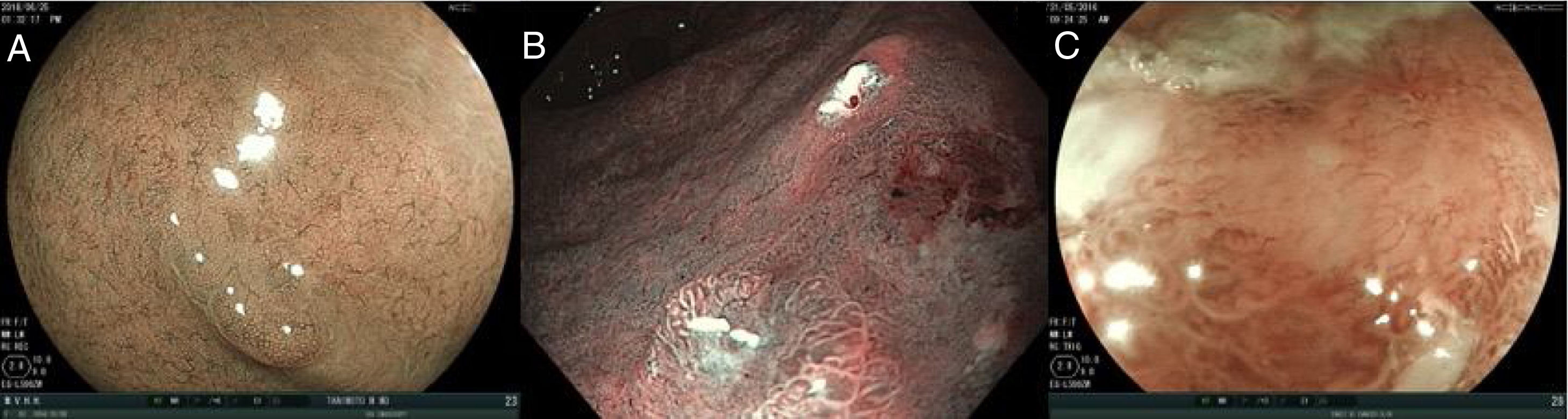
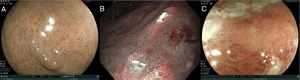
The use of image-enhanced endoscopic technologies (e.g., blue laser imaging and narrow band imaging digital chromoendoscopy), enables better characterization of suspicious lesions.58–62 NBI can improve the observation of the microvascular pattern and the micro-superficial structure of the gastrointestinal mucosa. The combination of NBI with magnification endoscopy is a useful tool for clearly visualizing the mucosa and delimiting the lesions more accurately.64
In some cases, after complete inspection with image-enhanced technologies, it may be necessary to use chromoendoscopy. As mentioned before, chromoendoscopy involves the uniform application of a dye on the gastric mucosa to characterize any subtle mucosal irregularity.65 Contrast dyes, such as indigo carmine, accumulate in the depressions of the lesion and accentuate its edges and surface topography.
To detect and characterize EGC with image-enhanced endoscopic technologies, 3 main characteristics must be evaluated, in both the mucosa and the microcapillaries:
a. If the surface pattern of the mucosa is: 1) regular, 2) irregular, and 3) aberrant or absent.
b. If the microcapillaries are: 1) regular, 2) irregular, and 3) aberrant or absent.
Fig. 2 shows the examples of the abovementioned patterns.
16. Atrophy, intestinal metaplasia, and low-grade dysplasia in the gastric mucosa are risk factors for EGC, whereas high-grade dysplasia is a precursor lesion for the disease.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 88% in complete agreement, 12% in partial agreement.
A risk factor consists of the association of the presence of a characteristic and the progression into a determined result. On the other hand, a precursor lesion is a pathologic state that directly progresses into a disease, with no intermediate step.66 Atrophy is a loss of gastric epithelial glands that presents as 2 main variants: a) the disappearance of glands that are replaced by fibrosis of the lamina propria and b) replacement of the native glands by metaplasia. Intestinal metaplasia is the reversible conversion of a mature epithelial cell type into another mature cell type, as an adaptive response.67 In the stomach, columnar cells are replaced by cells of intestinal morphology and are characterized by having goblet cells that contain mucus, Paneth cells, and absorptive cells. Those characteristics are not present in healthy gastric mucosa, and so they are easy to distinguish. On the other hand, gastric dysplasia is a precursor lesion of gastric cancer. The Padua, Vienna, and WHO classifications were developed to overcome the discrepancies between the Western and Japanese pathologic diagnoses and to provide a universally accepted classification of the gastric epithelial neoplasia.68 The evidence suggests that patients with high-grade dysplasia are at high risk for progression to carcinoma or synchronous carcinoma and patients with low-grade dysplasia have also been reported to have a lower risk for progression to carcinoma.65,66 However, due to the marked histologic discrepancies between the endoscopic biopsies obtained using biopsy forceps, corroboration by a second expert pathologist is recommended before those lesions are endoscopically resected, particularly when there are other risk factors, such as size, depressed morphology, superficial erythema, irregularity, ulcer or erosion, and tubulovillous or villous histology.69 Likewise, H. pylori eradication after endoscopic resection in patients with dysplasia appears to reduce the incidence of metachronous lesions.61,65,66
17. To adequately evaluate gastritis, atrophy, or intestinal metaplasia due to H. pylori, endoscopic biopsy samples should be obtained according to the updated Sydney protocol.
Quality of evidence: A2.
Strength of recommendation: weak, in favor of.
Agreement reached: 88% in complete agreement, 12% in partial agreement.
This statement is similar to the recommendations given in the “Fourth Mexican consensus on the diagnosis and treatment of Helicobacter pylori”.70 The recommendation is to follow the updated Sydney protocol to obtain efficient sampling that includes the taking of 5 biopsy samples: from the greater curvature (2) and from the lower curvature (2) at the level of the gastric corpus and gastric antrum 3 cm from the pylorus, as well as a sample from the incisura angularis (1). Biopsy at the incisura maximizes the identification of premalignant lesions.71 The severity and extension of those lesions determine which patients require endoscopic surveillance, utilizing the Operative Link on Gastritis Assessment (OLGA) and Operative Link on Gastric Intestinal Metaplasia (OLGIM) staging systems that stratify the risk for gastric adenocarcinoma, based on the severity and topographic extension of atrophic gastritis and intestinal metaplasia, respectively.72,73 The OLGA and OLGIM systems are endorsed by the European Society of Gastrointestinal Endoscopy (ESGE) and the European Helicobacter pylori Study Group (EHSG).61 Atrophy in the OLGA system is classified on a 4-level scale (0-3), in accordance with the Sydney system scale.69 The OLGIM scale has the same classification levels but evaluates intestinal metaplasia, rather than atrophy. The OLGIM scale tends to replace the OLGA system for detecting patients with gastric alterations with a premalignant potential and basically replaces the atrophy score with an evaluation solely of intestinal metaplasia.68,71Gastritis staging organizes the histologic phenotypes along a progressive scale of risk for gastric cancer, from the lowest (stage 0) to the highest (stage IV).69,71
18. The OLGA and OLGIM systems for diagnosing gastritis, mainly at stages III and IV, can be useful for categorizing the risk for progression to gastric cancer.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 75% in complete agreement, 12% in partial agreement, 12.5% uncertain.
Even though there are numerous publications on the use of the OLGA and OLGIM gastritis classification systems, there is no consensus on their usefulness. Those systems are not well known in Mexico and are more popular in Europe and the United States. Studies have shown that the kappa correlation coefficient for OLGA, between experienced pathologists and general pathologists, is low for the diagnosis of atrophic gastritis (k = 0.04 to 0.12), making its evaluation unreliable.74,75 In contrast, the OLGIM evaluation system is more reproducible because the kappa correlation coefficient varies between 0.68-0.70. A recent meta-analysis supports the importance of utilizing the OLGA/OLGIM systems, mainly in stage III/IV, as expressed in statement 17.76
19. The diagnosis of the histologic strain of EGC should be reported according to the Lauren classification
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 88% in complete agreement, 12% in partial agreement.
The traditional histopathologic Lauren classification (intestinal or diffuse types) more clearly reflects the epidemiologic and clinical characteristics of gastric cancer. Said classification is useful in clinical practice and aids in deciding the extension of gastric resection for gastric cancer.20 Whether the new classifications that divide gastric cancer into distinct molecular subtypes can contribute to defining a more personalized onco-surgical therapeutic strategy has yet to be demonstrated.77
20.The diagnosis of dysplasia grade in early gastric lesions should be reported according to the modified Vienna classification
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 88% in complete agreement, 12% in partial agreement.
The WHO recently reiterated the dysplasia/intraepithelial neoplasia classification.78 Recognizing the generalized use of the two terms, it utilizes “dysplasia” and “intraepithelial neoplasia” as synonyms. The modified Vienna classification, resulting from the meeting of 31 pathologists, in 2000, from 12 different countries,79 states that epithelial gastrointestinal neoplasias should be classified as follows:
- -
C1. Negative for intraepithelial neoplasia/dysplasia
- -
C2. Undefined for intraepithelial neoplasia/dysplasia
- -
C3. Low-grade intraepithelial neoplasia/dysplasia
- -
C4. High-grade intraepithelial neoplasia/dysplasia
- -
C5. Intramucosal invasive neoplasia/intramucosal carcinoma
The Vienna classification system for dysplasia was developed to standardize the definition of gastric dysplasia and gastric neoplasia between Japanese and Western pathologists. In Japan, carcinoma is diagnosed based on changes in cytology and tissue architecture, without taking invasion into account. Contrastingly, in the West, diagnosis is based on invasion into the lamina propria, emphasizing invasion as an indicator of metastatic potential.
21. Patients diagnosed with neoplasia/low-grade dysplasia in the absence of an endoscopic lesion should undergo follow-up endoscopy one year after diagnosis.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 82% in complete agreement, 18% in partial agreement.
Gastric cancer risk in patients with low-grade dysplasia is similar to, or even considerably higher, than the risk for cancer in patients after colonic adenoma extirpation, in patients with Barrett’s esophagus, or in patients with long-lasting inflammatory bowel disease.80–82 Compared with patients with high-grade dysplasia, those with low-grade dysplasia appear to have a lower risk (of 7%) for progression to invasive carcinoma (95% CI: 6-8%).83–85 Therefore, endoscopic surveillance at regular intervals appears to be indicated, even though the cost-effectiveness requires additional evaluation.86,87 When repeat surveillance endoscopy with biopsy confirms the presence of low-grade dysplasia, continued surveillance is justified. The time intervals are debatable, but the majority of case series recommend at least one follow-up study per year after diagnosis.81,84,85 When low-grade dysplasia cannot be confirmed at endoscopic re-evaluation, the amount of time that surveillance should be continued has not been defined.
The fact that low-grade and high-grade dysplasias can present as endoscopically visible depressed or elevated lesions, as well as small or flat lesions, and can be isolated or multifocal, is of great importance.80,88,89 Thus, the disappearance, or supposed disappearance, of dysplasia, through successive biopsies taken utilizing videoendoscopic techniques during follow-up procedures, does not rule out possible progression to invasive cancer.90–92 With respect to obvious endoscopic lesions, complete resection is recommended, as stated in the Treatment section below.
22. The diagnosis of high-grade dysplasia in the absence of endoscopic lesion should be corroborated by a second expert pathologist, and if there is agreement, the patient should be referred to a specialized center.
Quality of evidence: C2.
Strength of recommendation: weak, in favor of.
Agreement reached: 92% in complete agreement, 8% in partial agreement.
The evidence suggests that patients with high-grade dysplasia are at high risk for progression to carcinoma or synchronous carcinoma.61,72 However, due to the marked histologic discrepancies between endoscopic biopsies taken with biopsy forceps, corroboration by a second expert pathologist is recommended before the endoscopic resection of those lesions, especially in the presence of other risk factors, such as size, depressed morphology, superficial erythema, irregularity, ulcer or erosion, and tubulovillous or villous histology.66,72 Because the treatment of those lesions requires special training and equipment (see the Treatment section), and the availability of experts is limited in Mexico, the recommendation is to refer those patients to specialized centers. Likewise, there is a recognized need for training programs in the techniques described further below.
23. There is no scientific evidence for the surveillance of patients with mild-to-moderate atrophy/intestinal metaplasia limited to the gastric antrum.
Quality of evidence: D2.
Strength of recommendation: weak, in favor of.
Agreement reached: 82% in complete agreement, 18% in partial agreement.
Because the risk for annual progression in patients with atrophy or metaplasia limited to the gastric antrum is not known, other international consensuses do not recommend surveillance in those patients.61 Nevertheless, the risk for gastric cancer is greater in patients with the incomplete type of intestinal metaplasia, in patients with involvement of the gastric antrum or the gastric corpus. Control endoscopy could be justified in patients with intestinal metaplasia with at least one of the following conditions: 1) intestinal metaplasia extension > 20% of the surface of the stomach; 2) incomplete-type intestinal metaplasia; 3) a first-degree relative with gastric cancer; and 4) smoking.93 According to the consensus of the European group, surveillance should be carried out 3 years after the initial diagnosis was made in patients with extensive lesions in the gastric antrum.61
24. In Mexico, screening is suggested in subjects with a first-degree relative with a history of gastric cancer.
Quality of evidence: D2.
Strength of recommendation: weak, in favor of.
Agreement reached: 82% in complete agreement, 18% in partial agreement.
As described in statement 6, a history of gastric cancer can have a familial aggregation. Thus, it is reasonable to affirm that first-degree relatives of patients with gastric cancer should undergo a screening program. However, there are no recommendations or guidelines with respect to how and when to begin screening. The use of noninvasive diagnostic methods, such as the serologic diagnosis of H. pylori associated with low serum levels of pepsinogen (PG) has been proposed in some populations. That test can predict the extension of gastric atrophy and be useful for determining which individuals could be at risk for EGC. The best evidence on the risk associated with premalignant gastric lesions and those noninvasive methods comes from well-designed cohort studies, preferably with long follow-up periods, that include a large number of patients and a small number of patients lost to follow-up. For example, Zullo et al.94 conducted a cohort study on 6,983 patients for 4.7 years and found that patients with positive H. pylori, with a PG-I < 70 ng/ml and a PGI/II ratio < 3.0, had a hazard risk (HR) for gastric cancer of 6.0 (95% CI: 2.4-14.5). In patients with negative H. pylori, with the same PG profile, the HR increased to 8.2 (3.2-21.5). In a cohort of 100 patients followed for 3 years, the combination of incomplete intestinal metaplasia and a PGI/PGII ratio < 3 was significantly associated with progression to dysplasia, with a HR of 13.9 (1.6-122.1), compared with patients with chronic atrophic gastritis or complete intestinal metaplasia.95 Despite the aforementioned results, we have no evidence in Mexico supporting the use of endoscopy or serologic tests, except in the study and treatment of H. pylori infection in first-degree relatives of patients with a history of gastric cancer.96
Treatment25. Once EGC diagnosis is confirmed, each patient should be offered individualized curative treatment, whether through endoscopy or surgery.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 88% in complete agreement, 12% in partial agreement.
EGC should be treated as soon as it is diagnosed, to prevent progression and achieve its cure. Patients should be indistinctly offered endoscopic or surgical treatment, given that there are no studies that objectively demonstrate that prognosis or quality of life in patients with EGC is different with one treatment or the other.97
In a recent meta-analysis of 19 studies that grouped together 6,118 patients with EGC that were treated either with endoscopic mucosal resection (EMR) or surgery, endoscopic treatment was found to be associated with progression and survival similar to surgical management, albeit offering the advantages of shorter procedure duration, shorter hospital stay, fewer complications, and lower cost. However, there was also a higher incidence of local recurrence and metachronous lesions, favored by the organ having remained in the patient.98
26. Endoscopic treatment should be performed in patients with lesions whose size permits complete resection in a single specimen and in those with lesions that have a very low probability of lymph node metastasis.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 82% in complete agreement, 18% in partial agreement.
Endoscopic treatment of any of the variants of EGC should be preferred to surgical management, when similar results can be obtained in terms of oncologic cure. In addition to the abovementioned advantages, endoscopic management also preserves the entire organ, affording the patient with adequate quality of life.93 Endoscopic treatment should be performed in patients at low risk for lymph node metastasis, given that lymph node extirpation is not possible because the procedure is a local therapy. The risk of adenopathies is 1% for pT1a lesions and lower than 3% for pT1b lesions.99 Ideally, endoscopic treatment of EGC should be performed only in referral centers with a high level of experience in those techniques.
27. The endoscopic techniques suggested for EGC extirpation are EMR and endoscopic submucosal dissection (ESD).
Agreement reached: 88% in complete agreement, 12% in partial agreement
EMR involves the separation of the lesion from the underlying submucosa through the injection of a liquid or through suction, or a combination of both, and its later resection, utilizing a polypectomy snare.93 On the other hand, ESD is the technique by which the mucosa that surrounds the lesion is resected, utilizing an electro-surgical knife, followed by the dissection of the submucosa underneath the lesion.100 The endoscopic technique should be chosen, based on the general condition of the patient, lesion characteristics, the experience of the endoscopist, and the resources of the endoscopic unit of each hospital center.
28. ESD is superior to EMR in the endoscopic treatment of EGC.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 92% in complete agreement, 8% in partial agreement.
In different meta-analyses of retrospective studies, ESD was shown to be significantly more effective than EMR for achieving single-specimen lesion resection (92% vs. 52%, RR of 9.69, 95% CI: 7.74-12.13), as well as for complete resection (82% vs. 42%, RR of 5.66, 95% CI: 2.92-10.96) and curative resection (RR of 3.28, 95% CI: 1.95-5.54).95,101,102 Even though complications have been reported during the procedure, such as bleeding (RR of 2.16, 95% CI: 1.14-4.09) and perforation of 4% for ESD vs. 1% for EMR (RR of 4.67, 95% CI: 2.77-7.87), bleeding was similar in the two groups, with an incidence of 9%, and a similar all-cause mortality rate (RR of 0.65, 95% CI: 0.08-5.38). The authors of the study concluded that ESD was superior to EMR, when performed by experienced endoscopists.93–98
29. Endoscopic treatment is indicated in differentiated intramucosal tumors (cT1a) with a diameter smaller than 2 cm.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 82% in complete agreement, 18% in partial agreement.
The macroscopic type of tumor is not as important as the finding of ulceration in the lesion.93 There can be some exceptions to that statement, based on the so-called extended indications for ESD. When in the absence of vascular infiltration, they suggest that the probability of lymph node metastasis is very low.93 The extended indications are:
- –
Non-ulcerated intestinal-type adenocarcinomas larger than 2 cm in diameter (cT1a UL−)
- –
Ulcerated intestinal-type adenocarcinomas smaller than 3 cm in diameter (pT1a UL+)
- –
Non-ulcerated diffuse-type adenocarcinomas smaller than 2 cm in diameter (pT1a UL−).
30. The evaluation of cure is based on the analysis of the histologic strain of the resected specimen, its lateral margins, the depth of invasion expressed in µs if it has reached the submucosa, and the presence or absence of lymphovascular infiltration.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 88% in complete agreement, 12% in partial agreement.
The resected specimen study should be evaluated by an expert pathologist and should report the depth, margins, and vascular invasion, which as described beforehand, indicates the reduced possibility of lymph node metastasis. They are recommended to be reported as follows:
- –
Intestinal-type intramucosal adenocarcinoma, larger than 2 cm in diameter, with no ulceration.
- –
Intestinal-type intramucosal adenocarcinoma, smaller than 3 cm in diameter, with ulceration.
- –
Diffuse intramucosal adenocarcinoma, smaller than 2 cm in diameter, with no ulceration.
- –
Intestinal-type submucosal adenocarcinoma (sm1, < 500 μm under the muscularis mucosae), smaller than 3 cm in diameter.
31. If endoscopic treatment is considered noncurative histopathologically, additional surgical resection is indicated, given the risk for lymph node metastasis.
Quality of evidence: C2.
Strength of recommendation: weak, in favor of.
Agreement reached: 94% in complete agreement, 6% in partial agreement.
When the en bloc resected lesion is a nonulcerated diffuse-type adenocarcinoma, smaller than 2 cm in diameter, with no lymphatic and vascular permeation, and with negative surgical margins, the resection is considered curative.93
When endoscopic treatment is considered noncurative in the histopathologic study, additional surgical resection is indicated, given the risk for lymph node metastasis. Nevertheless, in some cases of noncurative resection of differentiated lesions, whose only criterion for non-cure is partial resection or positive lateral margins, a new attempt at endoscopic resection based on the criteria of each hospital can be made, ESD can be repeated, diathermia can be utilized, or endoscopic therapy can be disregarded. In a study on 102 patients with residual tumor in the lateral or vertical margins after EMR (n = 10) or ESD (n = 92), a residual or recurrent tumor rate of 33.3% (34/102) was reported.103 Of those, 17 residual lesions in 46 patients were immediately newly resected through endoscopy or surgery, and another 17 recurrent lesions in 56 patients were monitored through endoscopy for 17 months (2-70). In a univariate analysis, the presence of ulcer, the direction of the lesion in the resected margin, and the length of the lesion in the lateral margin were associated with the incidence of residual or recurrent tumor. In a multivariate analysis, the total length in centimeters of the lesion in the lateral margin was the only independent factor for that risk (RR 2.05, 95% CI: 1.22-3.44, p = 0.006).99
32. When EGC cure is achieved through ESD, the reported risk for local recurrence is from 0.2 to 1.1%, with a 5-year survival rate of 92 to 99.9%.
Agreement reached: 94% in complete agreement, 6% in partial agreement.
In a study on 472 patients with 570 EGC lesions, a single-specimen resection rate of 97.7% was found for lesions treated with ESD, with a mean procedure duration of 47 min (8-345). The incidence of positive lateral margins was 3.7% and was 3.4% for vertical margins. Local recurrence was 1.1% and metachronous tumors were observed in 7.8% of the patients.104 In another study on patients with extirpated lesions, based on extended indications (described in statement 29), there was a tumor recurrence rate of 0.2% and a cancer-related mortality rate of 0.2% in the 5-year follow-up of 1,205 patients with curative resections.105 Finally, in another study on 1,956 patients, in which 2,210 EGC lesions were extirpated through ESD, complete cure with no positive margins was achieved. The patients were followed for a mean 83.3 months, with only 2 recurrences, a general 5-year survival rate of 92.6%, and a disease-specific survival rate of 99.9%.106
33. The main complications of ESD are bleeding and perforation.
Agreement reached: 82% in complete agreement, 18% in partial agreement.
Bleeding and perforation are the 2 major complications in ESD. ESD-related bleeding is divided into 2 types, according to the time of onset: intraoperative, at the time of resection; and delayed, in the form of melena or hematochezia after resection.107 Achieving hemostasis during intraoperative bleeding is very important to ensure procedure success. The same electrocautery or endoscopic staples can be used, albeit they can interfere with the resection process. Delayed bleeding, which presents in 1.8-15% of patients can be treated in different manners, based on the experience of the surgeon, the resources available, and the corresponding work center.103,108 The risk factors for that type of bleeding are age above 80 years, antiplatelet drug intake, resection site in the gastric corpus or antrum, and the size of the resected lesion (> 40 mm).
Perforation is reported to occur in 1.2 to 4.5% of ESDs.104 Its associated factors are lesion size, resection extension, the presence of ulcers, and lesion location in the gastric corpus and gastric fundus.104,109
When a perforation occurs, it should initially be closed with an endoscopically applied staple. If closure is successful, a nasogastric tube is placed, the patient is left in a fasting state, and a broad-spectrum antibiotic is administered. If closure is not achieved, or peritonitis is suspected, a surgical evaluation must be ordered.93
34. To reduce the risk for complications after endoscopic treatment of EGC, the use of standard doses of proton pump inhibitors (PPIs) is recommended.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 88% in complete agreement, 12% in partial agreement.
Even though it seems logical to use PPIs to reduce the risk for bleeding and to favor healing, evidence to that respect is scarce. For example, in a randomized prospective study that included 143 patients with EGC that underwent ESD, they were divided into 2 groups. One received 20 mg daily of rabeprazole (PPI) and the other received 800 mg daily of cimetidine (H2RA) for 8 weeks.110 The primary aim of the study was to determine the incidence of gastrointestinal bleeding that would require endoscopic therapy and that had a decrease in hemoglobin greater than 2 g/dl. Bleeding presented in 4 patients in the PPI group and in 11 patients in the H2AR group. The multivariate analysis showed that the use of a PPI reduced the risk for bleeding, with a RR of 0.47 (95% CI: 0.22-0.92, p = 0.028).
35. Once the EGC is resected, patients with H. pylori infection should be given eradication therapy, based on the current guidelines of each region and country.
Quality of evidence: A1.
Strength of recommendation: strong, in favor of.
Agreement reached: 88% in complete agreement, 12% in partial agreement.
The recently published “Fourth Mexican consensus on the diagnosis and treatment of Helicobacter pylori” contains the most up-to-date recommendations on the theme.68 We recommend that our readers review that document.
H. pylori eradication is controversial, given that some retrospective studies and case reports have described that said eradication does not affect the development of metachronous gastric cancer.111,112 In contrast, a randomized study demonstrated that eradication of the bacterium after the resection of a gastric cancer reduced the annual incidence of metachronous gastric cancer from 2-3% to a 1% incidence.113
36. After endoscopic treatment of EGC, endoscopic surveillance is recommended every 6 to 12 months, even if there was histopathologic evidence of curative resection, to detect any metachronous gastric cancer.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 82% in complete agreement, 18% in partial agreement.
Metachronous gastric cancer is defined as a neoplastic lesion located at a distance from the original EGC site, within a one-year surveillance period. After surgical treatment of gastric cancer, the accumulated incidence rate for metachronous gastric cancer ranges from 0.9 to 3.0%.114–116 In endoscopic resection, incidence is higher due to the presence of the stomach. There are no studies that directly compare the results between surveillance at 6 months and surveillance at 12 months. One study reports that annual surveillance enables up to 95% of metachronous gastric cancers to be treated.110–112 However, regardless of whether the lesion is new, is a nondetectable previous lesion in the preclinical phase, or is a lesion that already existed and went undetected, the corresponding consensuses conclude that all such lesions must be considered an undetected synchronous cancer.117 Therefore, after the resection of an EGC, endoscopic surveillance at intervals that can vary from 6 to 12 months is recommended.110,114
37. Adjuvant chemotherapy is not recommended in cases in which curative EGC criteria have been achieved.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 94% in complete agreement, 6% in partial agreement.
In some cases of EGC extirpated through EMR, the combination of adjuvant chemotherapy can reduce recurrence and improve patient quality of life. In a study conducted on 112 patients with EGC (IA-IB) that were randomized to receive either EMR alone or EMR plus chemotherapy (5-fluorouracil + oxaliplatin), even though there was no difference in the incidence of complications, there was a statistically significant difference regarding EGC recurrence in favor of the group with the combined therapy, as well as better mental and emotional health (p < 0.05). However, when the ESD technique is employed, studies have shown that once the curative criteria are achieved, there is no need to implement management with chemotherapy.118
38. Open or laparoscopic surgical resection is indicated in the majority of cases in which curative endoscopic resection has not been achieved, given the high risk for lymph node metastasis.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 94% in complete agreement, 6% in partial agreement.
When an extirpated lesion does not meet the absolute or extended curative resection criteria, the resection should be considered noncurative.93 The criteria for curative resection based on extended criteria are:
- –
Intestinal-type intramucosal adenocarcinoma, larger than 2 cm in diameter, with no ulceration.
- –
Intestinal-type intramucosal adenocarcinoma, smaller than 3 cm in diameter, with ulceration.
- –
Diffuse intramucosal adenocarcinoma, smaller than 2 cm in diameter, with no ulceration.
- –
Intestinal-type submucosal adenocarcinoma (sm1, < 500 μm underneath the muscularis mucosae), smaller than 3 cm in diameter.
In such cases, the patient should be offered open or laparoscopic surgical management because of the high risk for lymph node metastasis.93 Nevertheless, in some cases of noncurative resection of adenocarcinomas, especially due to lateral margin positivity, the ESD can be repeated, and diathermia can be used. No treatment is also an option, in cases in which the morbidity and mortality of the surgical act is greater than the quality of life and survival benefits for the patient.93
ConclusionsThis first Mexican consensus on the detection and treatment of EGC produced 38 recommendations on the theme. The consensus defined EGC as that which is limited to the mucosa and submucosa at diagnosis, regardless of lymph node metastasis. Even though there are no epidemiologic data in Mexico, the factors associated with EGC include H. pylori infection, family history, smoking, and diet, as in other parts of the world. Chromoendoscopy, magnification endoscopy, and equipment-based image-enhanced endoscopy are recommended for the diagnosis of EGC. Histopathologic diagnosis is of the utmost importance, given that it aids in determining treatment. Endoscopic treatment of EGC, whether through EMR or ESD, should be preferred to surgical management, when similar curative oncologic results can be achieved. Endoscopic surveillance should be individualized.
Financial disclosureThe present consensus was carried out with the support of the Asociación Mexicana de Gastroenterología, which made the participation, transportation, and lodging possible during the face-to-face vote. The authors received no professional fees.
Conflicts of interestDra. María Eugenia Icaza-Chávez is or has been a speaker for Takeda, Asofarma, and Mayoly Spindler.
Dr. Miguel Ángel Tanimoto-Licona is or has been a speaker for Takeda.
Dr. Francisco Huerta-Iga is a member of the advisory board of Asofarma and is or has been a speaker for Asofarma, Takeda, and Astra-Zeneca.
Dr. José María Remes-Troche is a member of the advisory board of Takeda and Asofarma. He received research funding from Sanfer and Asofarma. He is a speaker for Takeda, Asofarma, Alfa-Wassermann, Carnot, Menarini, and Astra-Zeneca.
Dr. Ramón Isaías Carmona-Sánchez is or has been a speaker for Asofarma, Astra-Zeneca, and Chinoin.
Dr. Arturo Ángeles Ángeles declares that he has no conflict of interest.
Dr. Francisco Bosques-Padilla is or has been a speaker for Takeda, Abbvie, and UCB.
Dr. Juan Manuel Blancas Valencia is or has been a speaker for Chinoin.
Dr. Guido Grajales Figueroa declares that he has no conflict of interest.
Dr. Oscar Víctor Hernández Mondragón declares that he has no conflict of interest.
Dr. Angélica I. Hernández-Guerrero declares that she has no conflict of interest.
Dr. Miguel Ángel Herrera Servín declares that he has no conflict of interest.
Dr. Fidel David Huitzil Meléndez declares that he has no conflict of interest.
Dr. Kenji Kimura Fujikami declares that he has no conflict of interest.
Dr. Eucario León-Rodríguez declares that he has no conflict of interest.
Dr. Heriberto Medina-Franco declares that he has no conflict of interest.
Dr. Miguel Ángel Ramírez-Luna declares that he has no conflict of interest.
Dr. Clara Luz Sampieri declares that she has no conflict of interest.
Dr. Beatriz Vega-Ramos declares that she has no conflict of interest.
Dr. Alejandro Zentella-Dehesa declares that he has no conflict of interest.
Please cite this article as: Icaza-Chávez ME, Tanimoto MA, Huerta-Iga FM, Remes-Troche JM, Carmona-Sánchez R, Ángeles-Ángeles A, et al. Consenso mexicano sobre detección y tratamiento del cáncer gástrico incipiente. Revista de Gastroenterología de México. 2020;85:69–859.