Eosinophilic esophagitis (EoE) is a chronic, immune-mediated disease characterized by the infiltration of eosinophils into the esophageal mucosa. It is the most frequent cause of dysphagia and food impaction in adults. Due to its similar pathophysiology to allergic rhinitis, asthma, and atopic dermatitis, it has been considered the esophageal manifestation of allergy. It is more frequently seen in the United States, Europe, and Australia. Incidence and prevalence have increased significantly in those countries over the past three decades, to such a degree that some consider it an epidemic. The disease is infrequently diagnosed in Mexico and Latin America, and so little information on this disease is produced in our region of the world. The precise factors explaining this low incidence are unknown.
On the other hand, there has been intense research on EoE in other parts of the world in recent years. Its pathophysiology has been better understood and endoscopic and clinical procedures have been refined for making the diagnosis. In addition, new drugs and special formulations of existing ones have been introduced for treating the disease. Simpler and more effective dietary treatment strategies have also been evaluated.
The aim of the present work was to review the current status of EoE globally and in Mexico, emphasizing the probable factors (environmental and technical) that intervene in the low incidence recorded in our country. In addition, we conducted a review of the advances in research on the different aspects of EoE carried out in recent years.
La esofagitis eosinofílica (EEo) es una enfermedad crónica, inmunomediada caracterizada por infiltración de eosinófilos en la mucosa esofágica. Es la causa más frecuente de disfagia e impactación alimentaria en adultos. Por su fisiopatología similar a la rinitis alérgica, asma y dermatitis atópica se le ha considerado como la manifestación esofágica de la alergia. Se observa con mayor frecuencia en Estados Unidos, Europa y Australia. En estos países la incidencia y prevalencia se han incrementado significativamente en las últimas 3 décadas al grado que algunos la consideran una epidemia. En México y Latinoamérica es una enfermedad poco diagnosticada por lo que existe escasa información generada en nuestro país relacionada con esta patología. Se desconoce con precisión cuales son los factores que pudieran explicar esta baja incidencia.
Por otra parte, globalmente ha habido una intensa investigación sobre la EEo en los últimos años, su fisiopatología ha sido mejor comprendida y se han afinado los procedimientos clínicos y endoscópicos para el diagnóstico. Además, se han introducido nuevos fármacos y formulaciones especiales de los ya existentes para el tratamiento, también se han evaluado estrategias más efectivas y simplificadas en el tratamiento dietético de estos pacientes.
El objetivo del presente trabajo es revisar el estado actual de la EEo en el mundo y en México poniendo énfasis en los probables factores que intervienen en la baja incidencia registrada (medioambientales y técnicos) en nuestro país y, por otra parte, revisar los avances realizados en los últimos años de investigación sobre los diversos aspectos de la EEo.
Eosinophilic esophagitis (EoE) is a chronic immune-mediated disease characterized by the infiltration of eosinophils into the esophageal mucosa.1 Since its first description in 1970, there has been an exponential increase in incidence and prevalence in Western European countries, Australia, Canada, and the United States.2,3 In Mexico and Latin America, it is an uncommon disease, and most of the available information comes from small studies and case series.
Research on EoE has increased worldwide in recent years, enabling a better understanding of the pathophysiologic processes, noninvasive diagnosis, and treatment and follow-up of patients. The aim of the present review is to describe the advances that have been made, in general, in the different aspects of the disease in recent years and analyze the factors most likely involved in its low prevalence in Mexico, based on the Mexican and Latin American experience in EoE.
DefinitionThe definition of EoE has been modified, as knowledge of the disease has increased. The first formal definition appeared in 2007, in the first international consensus on the diagnosis and treatment of EoE, describing the disease as a disorder characterized by esophageal symptoms associated with the infiltration of ≥15 eosinophils/high power field (HPF) in the esophageal mucosa. In that definition, EoE and gastroesophageal reflux disease (GERD) were considered mutually exclusive, thus, requiring normal 24-h esophageal pH measurement or the absence of treatment response with proton pump inhibitors (PPIs), as diagnostic criteria.4
In the 2011 international consensus, a different EoE phenotype with a favorable response to PPIs was recognized. It was given the name PPI-responsive esophageal eosinophilia (PPI-REE), to differentiate it from EoE.5 However, years later, PPI-REE and EoE were found to have similar pathophysiologic and clinical characteristics and that PPIs had an anti-inflammatory effect on the Th2 inflammatory pathway, similar to that conferred by steroids.6,7 Therefore, PPI-REE was accepted as part of the EoE spectrum. On that basis, the 2018 international consensus removed the lack of response to PPIs as a diagnostic criterion.1,8
EpidemiologyIn the global context, EoE is more frequent in European countries, the United States, Canada, and Australia,3 affecting both children and adults. In a recent meta-analysis that included 40 population studies conducted between 1976 and 2022, a worldwide incidence of 5.3 cases/100,000 inhabitants and a prevalence of 40 cases/100,000 inhabitants were reported. A sub-analysis revealed that prevalence has increased considerably in recent years; between 1976 and 2001, it was at 8.1 cases/100,000 inhabitants and between 2017 and 2022, it was at 74.4 cases/100,000 inhabitants.2 This increase in incidence and prevalence has been considered an epidemic,9 albeit whether due to an increased search for the disease or to a change in its biologic behavior, or both, has not been determined.
Prevalence varies in relation to the clinical characteristics of the population studied. In patients with esophageal symptoms that underwent esophagogastroduodenoscopy (EGD), prevalence was 6.5%, in patients with dysphagia it was 12-15%, and in patients with impaction it reached up to 50%.10–12
Prevalence in Mexico and Latin America is lower than that described in the abovementioned regions. In addition, studies conducted in the United States reported a significantly lower prevalence of EoE in Hispanics than in Whites.13–15 In Mexico and Brazil, the reported prevalence in patients with upper endoscopy due to esophageal symptoms was 1 and 1.7%, respectively.16,17 In other studies conducted in Mexico, prevalence was 4% in patients with refractory GERD symptoms and 11% in patients with food impaction, figures significantly lower than those reported in other countries.18,19
The factors involved in the low prevalence of EoE in Mexico and Latin America are not known. Genetic, environmental, sociocultural, and economic factors have been proposed and are discussed herein.20
Probable factors involved in the low detection of eosinophilic esophagitis in MexicoEoE is an entity caused by the interaction of different risk factors: genetic, environmental, and nonbiologic.
Genetic and environmental factorsGeneticSome of the genes related to the increase in risk for developing EoE are TSLP, CCL26, and CAPN14, which play an important role in the regulation of the immune response and the migration of eosinophils to the esophageal epithelium.21 The risk of developing EoE is greater in first-degree relatives than in the general population, but in family studies and cohorts of twins, the weight of heredity has been lower, compared with environmental factors.22,23
Environmental and sociocultural factorsBecause EoE is an allergic disease whose incidence in developed countries has been on the rise, the same as other atopic diseases (allergic rhinitis, asthma, and atopic dermatitis), environmental causes appear to have greater relevance. This is the basis for the “hygiene hypothesis” proposed by Okada et al., which suggests that the significant lifestyle and socioeconomic modifications in certain populations over the past few decades have brought about changes in immunoallergic tolerance in those individuals.
The mechanisms through which the “hygiene hypothesis” attempts to explain those phenomena are not well defined; it proposes that the first major one could be related to a redirection induced by exposure to certain factors of the Th1 and Th2 inflammatory reactions. The helper T lymphocytes in the Th1 inflammatory pathway produce inflammatory cytokines, such as interleukin (IL)-2, interferon-γ, and tumor necrosis factor-alpha (TNF-α), that act on cell-mediated immunity. In contrast, the helper T lymphocytes of the Th2 reaction that produce IL-4, IL-5, and IL-13 contribute to immunoglobulin type E (IgE) production and eosinophil-mediated allergic responses.24
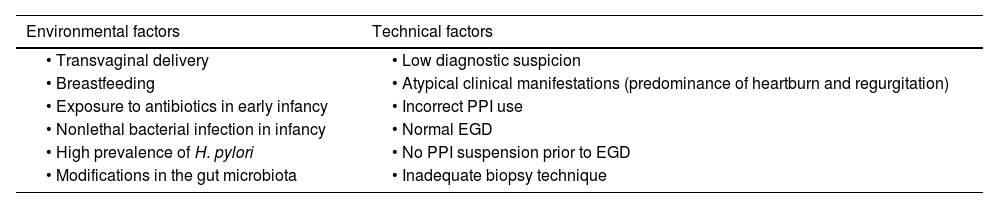
In Latin American countries, vaginal childbirth and breastfeeding are more frequent due to sociocultural and economic reasons, which could contribute to the lower incidence of EoE.25 On the other hand, nonlethal bacterial infections in infancy (including Helicobacter pylori) have also been proposed as a protective factor against EoE.26 In a meta-analysis of 11 studies, an OR of 0.63, with a reduction of 37% in the risk for EoE in patients with exposure to H. pylori, was reported.27 This inverse association has also been described in a Mexican population, but whether it is a cause/effect relation or is an epiphenomenon has yet to be clarified28 (Table 1).
Probable causes of the low prevalence of eosinophilic esophagitis in Mexico
| Environmental factors | Technical factors |
|---|---|
| • Transvaginal delivery | • Low diagnostic suspicion |
| • Breastfeeding | • Atypical clinical manifestations (predominance of heartburn and regurgitation) |
| • Exposure to antibiotics in early infancy | • Incorrect PPI use |
| • Nonlethal bacterial infection in infancy | • Normal EGD |
| • High prevalence of H. pylori | • No PPI suspension prior to EGD |
| • Modifications in the gut microbiota | • Inadequate biopsy technique |
EGD: esophagogastroduodenoscopy; PPI: proton pump inhibitor.
Regarding the low prevalence of EoE in Mexico, it is reasonable to state that, in addition to the environmental factors discussed above, other factors existing in medical practice may be involved. They include low diagnostic suspicion on the part of physicians (due to insufficient knowledge of the disease), atypical manifestations of the disease, lower frequency of endoscopic alterations, deficiencies in taking esophageal biopsies, and the masking of EoE due to inadequate PPI use, especially due to diagnostic testing with PPIs29,30 (Table 1). In support of this, a study conducted in Mexico found that of 186 patients with dysphagia referred for EGD, 45.7% had received PPIs before the study and biopsies had been taken in only 23.7% of the cases (the American Gastroenterological Association [AGA] Abstracts, Gastroenterology S-358 DDW Chicago 2023). The low number of patients in whom biopsies were taken could be due to the low frequency of typical endoscopic abnormalities of the disease. Up to 32% of patients with EoE in a Mexican population had normal endoscopy results, compared with 10% in White patients.31 In addition, due to the patchy mucosal inflammation in EoE, at least 6 biopsy specimens of the proximal and distal esophagus are needed to increase the possibility of diagnosis.8
PathophysiologyEpithelial barrier dysfunction, inflammation mediated by Th2 lymphocytes and different cytokines, and tissue remodeling are the distinctive pathophysiologic characteristics of EoE.32,33
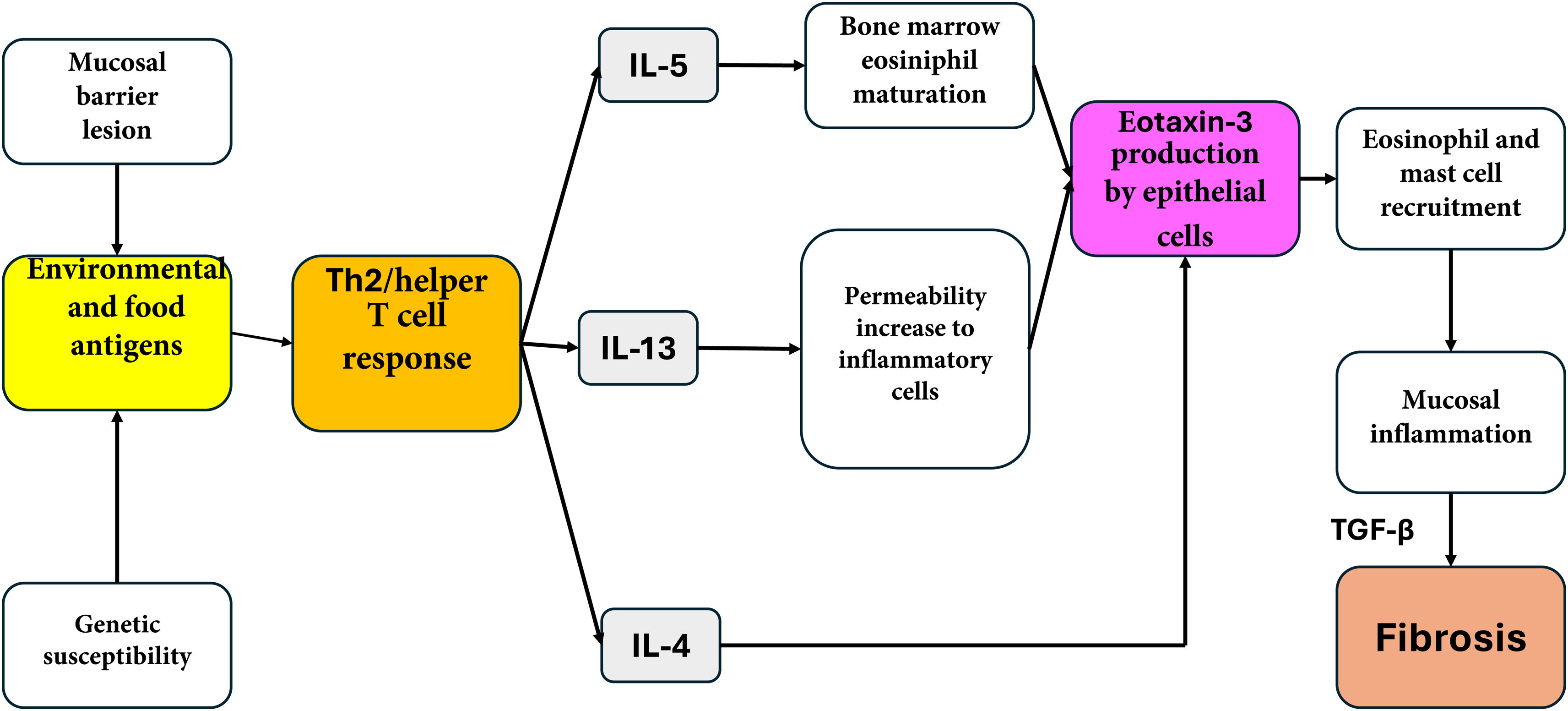
Under normal conditions, there are no eosinophils in the esophageal mucosa. In EoE, the exposure to environmental allergens increases the endogenous activity of the proteases in the esophagus. This results in the disruption of the epithelial intercellular junctions, facilitating the main access of food allergens into the deep layers of the mucosa.33 As a consequence, a Th2 lymphocyte-mediated inflammatory immune response is triggered. Those lymphocytes are sensitized, releasing proinflammatory cytokines, such as IL-4, IL-5, and IL-13 that induce the eosinophilic infiltration into the esophageal mucosa.34,35 IL-5 promotes the differentiation, maturation, and release of eosinophils from the bone marrow and facilitates the transport of eosinophils to the esophagus. IL-4 promotes the proliferation and differentiation of the Th2 lymphocytes and also has an inhibitory effect on apoptosis.36 On the other hand, IL-13 induces eotaxin 1 and 3 secretion.37 Said agent is a potent recruiter of eosinophils and mast cells, whose presence causes mucosal inflammation. The persistence of inflammation stimulates the production of fibrosis due to the activity of TGF-β in a process known as esophageal remodeling37 (Fig. 1).
Clinical characteristicsEoE affects both children and adults. The clinical manifestations in infants are feeding difficulties and failure to thrive. In school-age children, vomiting and retrosternal pain predominate, and dysphasia is most prominent in adolescents. Dysphagia and food impaction predominate in adults and are described as capital symptoms.8,21,38 In developed countries, EoE behaves like a chronic disease with elevated recurrence rates that often progresses from the inflammatory phenotype (with a predominance of esophageal mucosal inflammation, more frequent in children and in the initial phases in adults) to the fibrostenotic phenotype (in which esophageal stricture predominates and is more frequent in adults), resulting in food impaction.12 Esophageal stricture with food impaction is more frequent as disease duration increases; 70% of cases have had symptom activity for more than 2 decades.
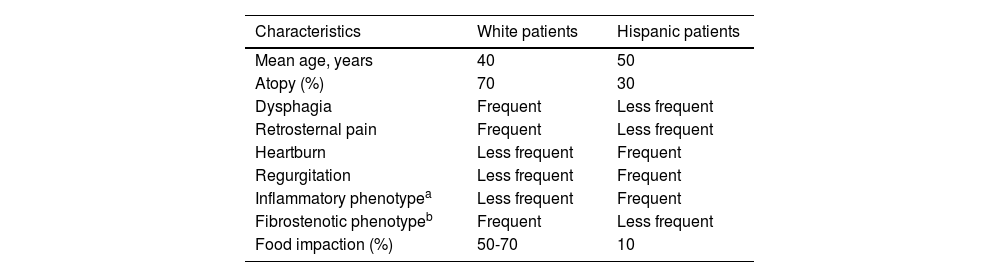
Adult patients in Latin America have shown a clinical profile similar to that reported in other countries (males, youths, with dysphagia and atopy), albeit with a higher proportion of heartburn and regurgitation, evoking GERD. In a series of adult Mexican patients with EoE, up to 71% presented with heartburn and/or regurgitation, 61% with dysphagia, and only 18% had had an episode of food impaction.31 In another study conducted in the United States on 64 White, African American, and Hispanic patients with EoE and a 10-year follow-up, the African Americans and Hispanics presented with a clinically atypical form of the disease characterized by older age, greater frequency of GERD symptoms, less dysphagia for solid foods, and fewer esophageal rings on endoscopy than Whites.39 Those results were later confirmed in a multicenter study conducted in the United States on 793 patients with EoE. The same as in the previously described study, African American and Hispanic patients had an atypical form of the disease, given that they presented with less dysphagia (56 and 53% vs 74%) and esophageal food impaction (13 and 13% vs 35%, respectively) than the White population15 (Table 2). The prevalence of EoE observed in Mexican patients with food impaction described by our group was also very low, at 11%, compared with 48% in developed countries.19
Clinical and endoscopic differences of eosinophilic esophagitis between White and Hispanic patients
| Characteristics | White patients | Hispanic patients |
|---|---|---|
| Mean age, years | 40 | 50 |
| Atopy (%) | 70 | 30 |
| Dysphagia | Frequent | Less frequent |
| Retrosternal pain | Frequent | Less frequent |
| Heartburn | Less frequent | Frequent |
| Regurgitation | Less frequent | Frequent |
| Inflammatory phenotypea | Less frequent | Frequent |
| Fibrostenotic phenotypeb | Frequent | Less frequent |
| Food impaction (%) | 50-70 | 10 |
The evidence provided in the abovementioned studies suggests that persons of Latin American and Mexican origin have differences in both the type and severity of the clinical manifestations of EoE, compared with Whites. Whether there are differences in the natural history of the disease is still to be determined. These data should be taken into account for suspecting the disease, planning the diagnostic approach, and establishing follow-up strategies in the Mexican population.
DiagnosisEoE should be suspected when there are esophageal symptoms (mainly dysphagia) in young male patients with a history of allergies. However, the demonstration of eosinophilic intraepithelial infiltration (≥15 eosinophils/HPF) into the mucosa of the esophagus, ruling out secondary causes of esophageal eosinophilia, is obligatory.1 The most important differential diagnosis for EoE is GERD, which can also cause eosinophilic infiltration. However, in most cases, the infiltration seen in GERD does not surpass 10 eosinophils/HPF but if there is doubt in the diagnosis, carrying out 24h pH monitoring is recommended. Nevertheless, due to the high prevalence of GERD, both entities can coexist and act synergically. EoE can favor reflux due to esophageal motility disorders and GERD can facilitate EoE, damaging the epithelial barrier, thus enabling exposure to exogenous antigens, in the mucosa.40,41
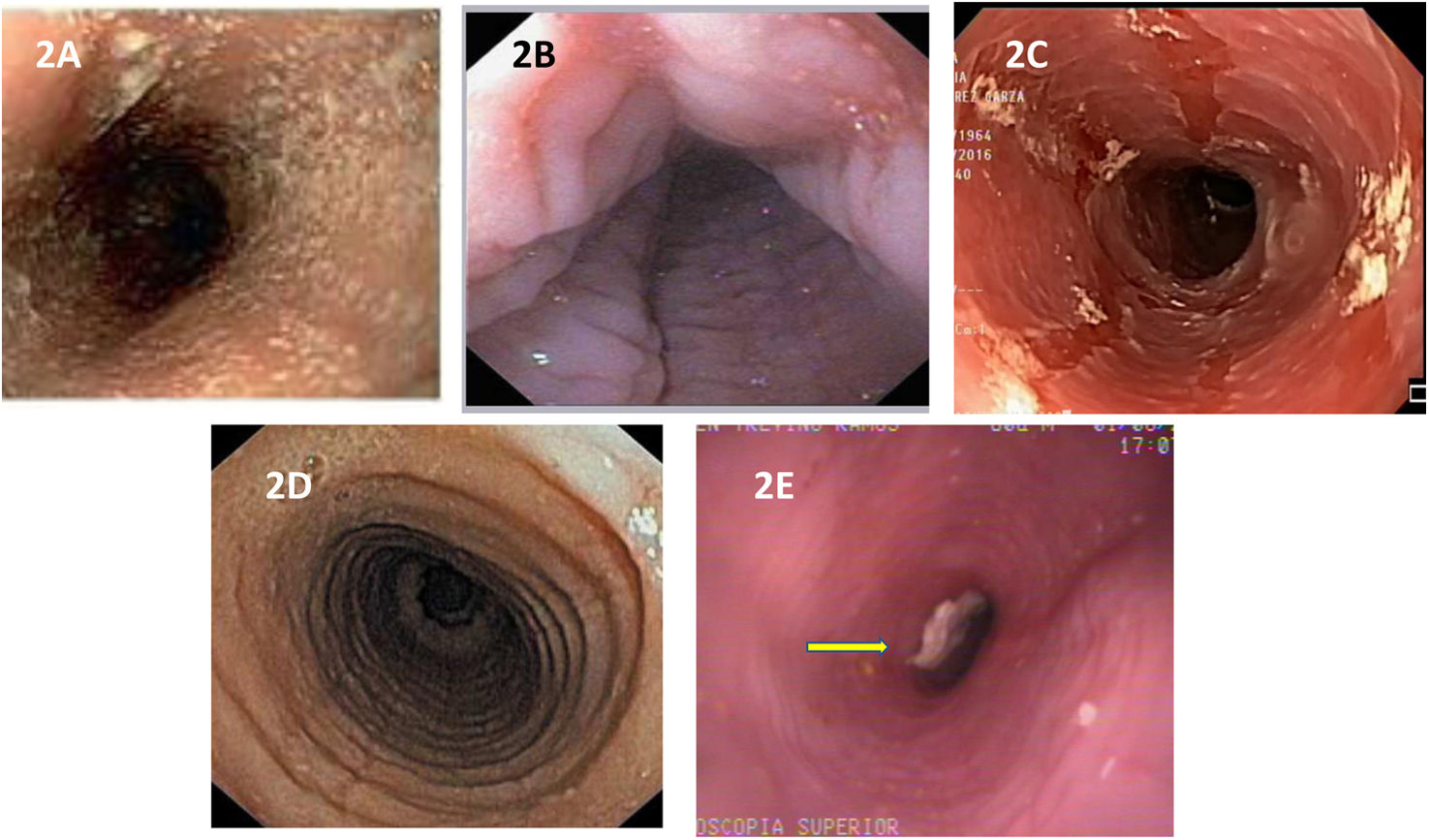
Endoscopic characteristicsTypical endoscopic abnormalities of EoE present in 70 to 90% of cases and include edema, linear furrows, esophageal rings, whitish exudates, and strictures (Fig. 2A-E).42 The edema, rings, exudates, furrows, stricture endoscopic reference score (EREFS), is utilized to describe and classify said alterations and has taken on an important role in clinical practice since its description in 2013 by Hirano et al.43 It is currently the most widely accepted system for evaluating endoscopic activity in EoE and has shown good interobserver concordance and correlation with treatment response and histologic activity.44–46 However, the absence of endoscopic abnormalities does not rule out the disease, thus a high degree of suspicion is needed on the part of the endoscopist, for deciding whether or not to take esophageal biopsies.
Endoscopic images of the esophagus in patients with EoE. Inflammatory phenotype: A) edema of the mucosa and whitish exudates on the circumference of the esophagus. B) Transverse and longitudinal furrows in the mucosa, or “feline esophagus”. C) mucosal sloughing, “crêpe paper mucosa”. Fibrostenotic phenotype: D) severe grade circumferential rings. E) Stricture of the esophageal lumen with food impaction (arrow).
Due to the patchy distribution of the disease, biopsies should be taken from the proximal/middle and distal esophagus, ideally from the inflammation zones, taking at least 6 samples.47 It is essential to suspend PPIs at least 3 weeks before the endoscopy because they can mask the inflammatory findings in the endoscopy exam and biopsies.29 Taking biopsy specimens from the stomach and duodenum to rule out celiac disease or gastrointestinal eosinophilic syndromes is also recommended.48–50
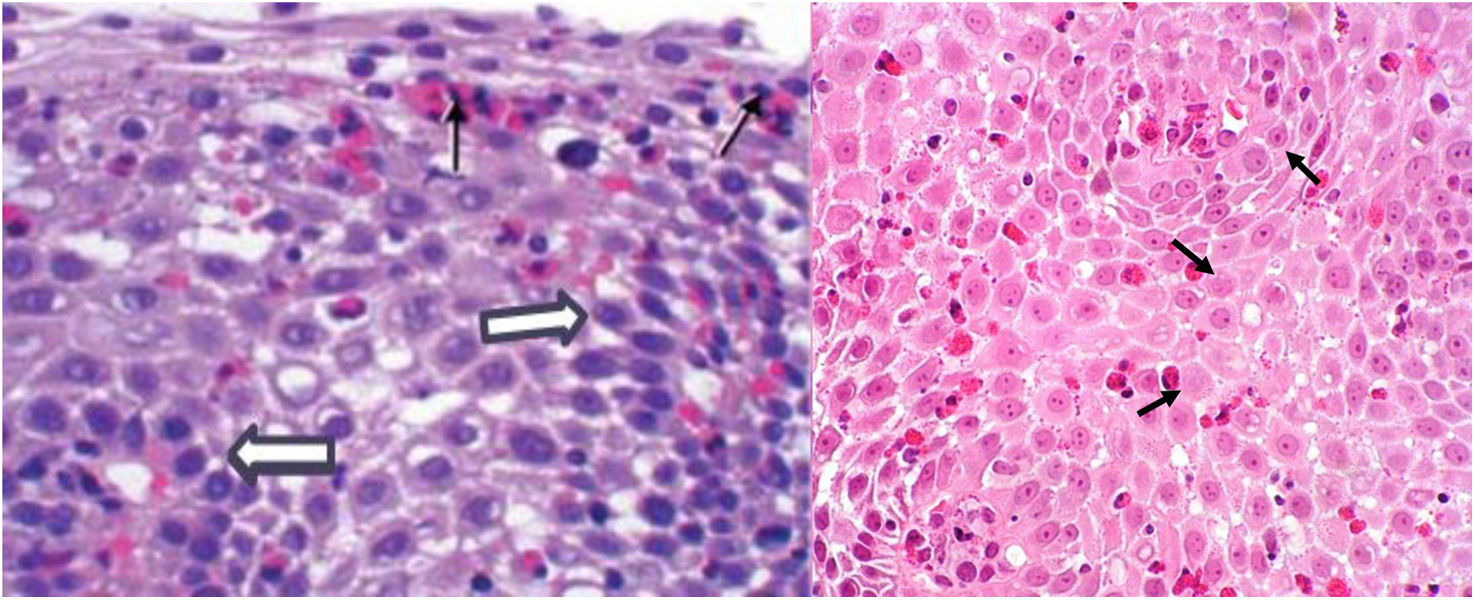
Histopathologic characteristicsIn addition to the infiltrate with a total peak eosinophil count of ≥15 eosinophils/HPF in the esophageal mucosa, other histologic abnormalities described in this disease are the superficial grouping of eosinophils, the formation of eosinophilic microabscesses, eosinophilic degranulation, spongiosis, epithelial basal hyperplasia, and subepithelial fibrosis (Fig. 3).51 To refine the diagnostic accuracy through histopathology, the eosinophilic esophagitis histological scoring system (EoEHSS) has been developed, and takes into account other characteristics besides the total peak eosinophil count to evaluate disease activity.52 It has been shown to be a good tool for making the diagnosis, as well as evaluating treatment response, and has good concordance with endoscopic abnormalities and better symptom correlation than the total peak eosinophil count.53–55
Even though histologic activity is considered a fundamental element in disease progression and the development of strictures and food impaction, therapeutic studies have shown there is a disassociation between histologic activity grade and symptoms, especially dysphagia.56,57 Dysphagia more clearly reflects the grade of esophageal fibrostenosis than inflammation grade. This datum is important for a more rational evaluation of the short and long-term therapeutic effect of medical treatments.
Emerging/adjuvant diagnostic methodsThe Functional Lumen Imaging Probe, or EndoFLIP, has been introduced as a novel adjuvant modality in the evaluation of patients with EoE. It enables the measuring of esophageal distensibility, which can be reduced in patients with EoE.58,59 The EndoFLIP can identify partial strictures, even in patients with no endoscopically visible narrowing. Therefore, the distensibility plateau calculated through this procedure has a greater independent predictive capacity for food impaction than inflammatory activity. This procedure can be useful in planning endoscopic dilatations in patients with persistent dysphagia.60–62
Because the diagnosis and evaluation of disease activity mandatorily require EGD, noninvasive techniques have been implemented for obtaining esophageal samples. The cytosponge consists of a capsule connected to a string, which the patient swallows. The capsule dissolves and releases a sponge that, when pulled back up by the string, takes samples of the esophageal mucosa. Tissue samples from patients with EoE are collected by this method; 95% are adequate, with 80% concordance with biopsy specimens.63 Another method that has been evaluated is the esophageal Enterotest or Esophageal String Test, which when swallowed obtains eosinophil-derived proteins, including eosinophil secondary granule proteins. It has shown good correlation with eosinophilic infiltration revealed through biopsy.64
Biomarkers for EoE diagnosis and follow-up have also been evaluated. The strongest candidates are the proteins contained in the secondary eosinophil granules, including eosinophil peroxidase, major basic protein, eosinophil-derived neurotoxin, and eosinophil cationic protein.65–67 However, the role of biomarkers in this disease has not yet been defined.
The abovementioned methods continue to be developed, and further research is warranted to validate their definitive clinical use. Most likely, they will play a complementary role in EoE diagnosis and follow-up in the future, together with endoscopy and biopsy.
TreatmentIn general terms, the treatment of EoE is medical and endoscopic, and the latter is based on esophageal dilatations.
Three medical treatment modalities have proven effectiveness: PPIs, topical steroids, and elimination diet therapy.68 Topical steroids are more effective than PPIs and restrictive diets. Nevertheless, each one has advantages and disadvantages, which is why, in choosing the initial treatment, not only efficacy, but also ease of administration, expected adherence, treatment cost, and patient preference should be taken into account.69 In general, the goal is histologic remission induction, through 8 to 12 weeks of treatment. Once remission is achieved, the need for maintenance treatment based on disease severity should be individually evaluated.
The main treatment goal is histologic remission at a level of <15 eosinophils/HPF (preferably under 5) in esophageal biopsy samples, given that this factor is more strongly associated with disease progression and the development of stricture and food impaction. Nevertheless, symptom disappearance, quality of life improvement, and the disappearance of endoscopic abnormalities should also be included as treatment goals.70 Unfortunately, there is a lack of agreement between histologic remission and esophageal symptoms, making control endoscopy with biopsy a necessity.
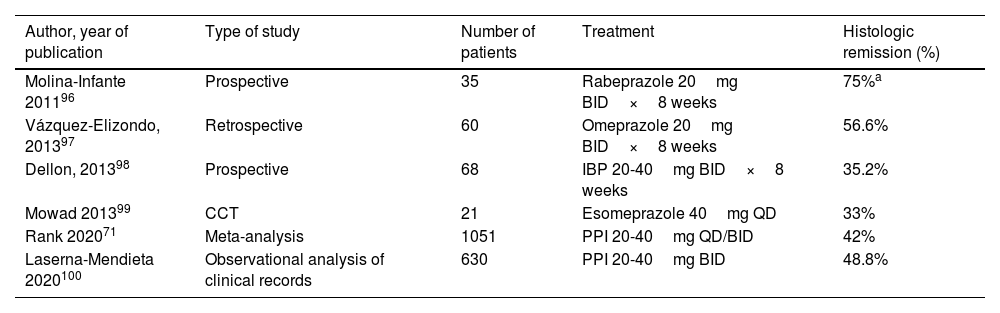
Proton pump inhibitors (Table 3)PPIs are considered first-line treatment for EoE in the Mexican population. In a meta-analysis with 23 observational studies, there was a 42% histologic remission rate in patients treated with PPIs compared with 13% of patients treated with placebo.71 In another meta-analysis of 33 observational studies (11 prospective analyses), the histologic response rate with PPIs was 50.5% (95% CI 42.2-55.7) and the symptom response rate was 60.8% (95% CI 48.38-72.2). However, high heterogeneity and publication bias were factors to consider in interpreting the results.72
Studies on PPI efficacy as treatment for inducing remission in eosinophilic esophagitis
| Author, year of publication | Type of study | Number of patients | Treatment | Histologic remission (%) |
|---|---|---|---|---|
| Molina-Infante 201196 | Prospective | 35 | Rabeprazole 20mg BID×8 weeks | 75%a |
| Vázquez-Elizondo, 201397 | Retrospective | 60 | Omeprazole 20mg BID×8 weeks | 56.6% |
| Dellon, 201398 | Prospective | 68 | IBP 20-40mg BID×8 weeks | 35.2% |
| Mowad 201399 | CCT | 21 | Esomeprazole 40mg QD | 33% |
| Rank 202071 | Meta-analysis | 1051 | PPI 20-40mg QD/BID | 42% |
| Laserna-Mendieta 2020100 | Observational analysis of clinical records | 630 | PPI 20-40mg BID | 48.8% |
BID: two times a day; CCT: controlled clinical trial; PPI: proton pump inhibitor; QD: once a day.
Anti-inflammatory properties of PPIs were recently discovered. They include inhibition of eosinophil chemotaxis, decrease in the production of free radicals by inflammatory cells, and decrease in the expression of cytokines typical of the Th2 response, such as IL-13.1 In addition, their inhibitory effect on gastric acid contributes to re-establishing esophageal mucosal integrity and less exposure to food antigens linked to the Th2 response.73–75
In conclusion, PPIs are a very attractive treatment option due to their well-established long-term safety, good tolerance, low cost, oral administration, and wide availability. A therapeutic dose every 12h is sufficient for producing a histologic and clinical response and there appear to be no therapeutic differences between the different PPIs in EoE.6 PPIs offer a better response in patients with the inflammatory phenotype and are not effective in patients with the fibrostenotic phenotype or in patients with failed steroid and/or dietary restriction therapies.76 Response is also better in patients with EoE who also present with GERD that has been documented by 24h esophageal pH monitoring. Upon completing 8 weeks of treatment, endoscopy with biopsy should be repeated to corroborate the histologic response.
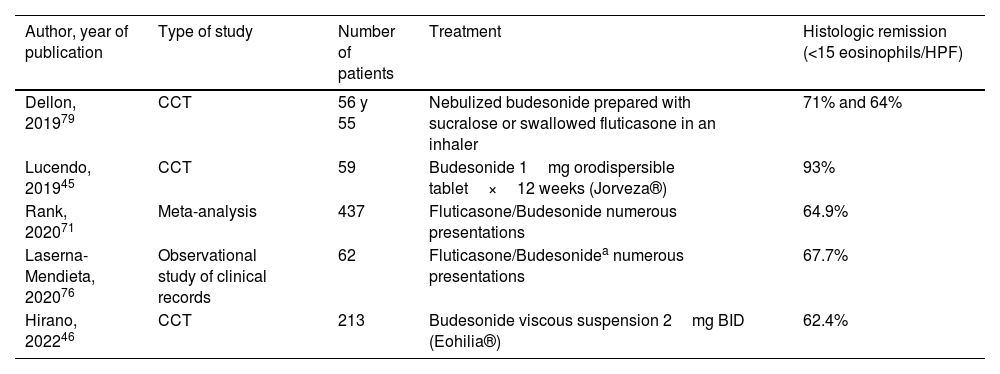
Topical steroids (Table 4)Topical steroids are the most frequently used medications in the treatment of EoE since the disease was first described. Their potent anti-inflammatory effect suppresses the Th2 response in the esophagus. The most widely studied topical steroids are budesonide and fluticasone. Numerous studies show their superiority, compared with placebo, for inducing symptomatic, endoscopic, and histologic responses.77–79 A meta-analysis of studies with budesonide and fluticasone reported histologic response rates of 64.9%, with a mean 8 weeks of follow-up.71 The recommended dose for budesonide is 1 to 2mg every 12h orally and is 880 to 1,760 mcg orally per day for fluticasone.
Studies on the efficacy of topical steroids as treatment for inducing remission in eosinophilic esophagitis
| Author, year of publication | Type of study | Number of patients | Treatment | Histologic remission (<15 eosinophils/HPF) |
|---|---|---|---|---|
| Dellon, 201979 | CCT | 56 y 55 | Nebulized budesonide prepared with sucralose or swallowed fluticasone in an inhaler | 71% and 64% |
| Lucendo, 201945 | CCT | 59 | Budesonide 1mg orodispersible tablet×12 weeks (Jorveza®) | 93% |
| Rank, 202071 | Meta-analysis | 437 | Fluticasone/Budesonide numerous presentations | 64.9% |
| Laserna-Mendieta, 202076 | Observational study of clinical records | 62 | Fluticasone/Budesonidea numerous presentations | 67.7% |
| Hirano, 202246 | CCT | 213 | Budesonide viscous suspension 2mg BID (Eohilia®) | 62.4% |
BID: two times a day; CCT: controlled clinical trial.
Optimum treatment duration for achieving remission appears to be 12 weeks, compared with 6 weeks, according to a randomized study (84.7% vs 57.6%, respectively).45 The most common adverse effect with topical steroids is asymptomatic esophageal candidiasis, reported in up to 12-15% of patients. Suppression of adrenal gland function is rare.79 The fact that topical steroids could be more effective than PPIs in patients with a higher grade of esophageal inflammation and with fibrostenosis has been pointed out.
Unfortunately, in Mexico, there are no specific galenic formulations of topical steroids for EoE, making their artisanal preparation necessary. Budesonide in solution for nebulization indicated for treating asthma can be mixed with sucralose to give it a viscous consistency for oral administration. Fluticasone can be administered in swallowed nebulizations at a dose of 880 to 1,760 mcg a day. It is important to avoid the ingestion of liquids or solids 30min after drug administration.80
In the United States, the FDA recently authorized an oral budesonide suspension (Eohilia®),46 and an orodispersible tablet of budesonide (Jorveza®) was authorized in Europe.45 However, their high cost and scant availability presently hinders their use in Mexico.
Dietary therapyThere are 3 types of dietary therapy in the treatment of EoE: elemental diet, allergy testing-based elimination diet, and empiric food elimination diet.
Elemental dietThe elemental diet consists of the exclusive administration of allergen-free formulations. It has the highest response rates (90%) but is expensive, difficult to administer, and not well-tolerated; in addition, it can lead to de novo sensitization and produce acute IgE-mediated allergic reactions.68 Thus, it is used very little and mainly as a last therapeutic resort.
Testing-based elimination dietThis type of diet is based on the results of allergy tests (the patch test, prick test, or serum IgE-mediated food allergy tests). It should be pointed out that IgE is not a mediator in the pathogenesis of EoE. Regarding the diet’s effectiveness, a meta-analysis of 11 studies described a histologic response rate of 50.8%.71 Another meta-analysis reported a histologic response rate of 45.7%.81 Due to the difficulty in implementing a testing-based diet and the low histologic response rate, it is not widely used.
Empiric elimination dietsThis diet is based on the elimination of allergenic foods frequently implicated in EoE (dairy products, wheat, eggs, soy, dried fruit, and seafood). There are several types of restrictive diets: those involving 1, 2, 4, and 6 foods; the most widely studied is the 6-food elimination diet. After 8 weeks on the diet, the histologic and clinical response should be corroborated, and if necessary, food groups should be sequentially reintroduced to find the dietary culprit. Each time a food group is reintroduced, the histologic response should be evaluated through endoscopy. A meta-analysis of studies with a 6-food elimination diet described histologic response rates of 67.9%, and the most implicated foods were dairy products, wheat, and eggs.71 The 6-food elimination diet is difficult to implement due to low adherence, as well as possibly requiring at least 7 endoscopies at 8-week intervals, for evaluating the effect of the food reintroduction. Thus, the duration of the process is about one year, which is costly and bothersome. Given those disadvantages, simpler restrictive diets of 4, 2, and even one food have been evaluated. A recent meta-analysis compared a 6-food elimination diet with 4-food (dairy products, wheat, eggs, and soy) and one-food (dairy products) elimination diets in 1,762 children and adults with EoE. The histologic and clinical response rates were 61.3% and 92.8% with the 6-food elimination diet, 49.4% and 74.1% with the 4-food elimination diet, and 51.4% and 87.1% with the one-food elimination diet, respectively.81,82
In Mexico, there is no documented experience with the use of empiric food elimination diets in the treatment of EoE. The pragmatic recommendation based on the results of the abovementioned meta-analysis would be to start by eliminating only dairy products, and in patients with an unfavorable histologic response, escalate to more restrictive diets.
Maintenance therapyStudies show that patients with untreated EoE (whether because they never received it or because they stopped taking it after achieving histologic remission) have elevated endoscopic, histologic, and symptomatic recurrence, a reduced quality of life, and a high risk for stricture and food impaction.83–86 Thus, maintenance therapy after achieving remission has been suggested. Nevertheless, maintenance therapy does not appear to be indicated in all patients, and those that can actually be more benefitted by it, is a subject of debate. In addition, the drug that confers the best long-term effect has not been determined. The AGA guidelines recommend maintenance therapy with low doses of topical steroids, based on a randomized trial that compared the administration of 0.25mg of budesonide twice a day for one year versus placebo, in patients with an initial response to steroids. There was a lower eosinophilic tissue load in the budesonide group, compared with placebo, but only 36% of patients maintained histologic remission at one year.87 In their recent study on patients with EoE treated with orodispersible budesonide (the new drug presentation) at doses of 1 mg and 0.5 mg daily versus placebo, Straumman et al. reported remission maintenance at 48 weeks in 80, 80.4, and 5.3% of patients, respectively.88
For patients taking PPIs or on restrictive diets, the decision to maintain long-term treatment is more difficult, given the absence of robust evidence on the topic. In those cases, the decision to do so should be made together with the patient and his/her relatives, understanding that it will probably be beneficial. If the decision is made to suspend maintenance treatment of any kind, strict clinical and endoscopic observation should be maintained, to be on the lookout for disease reactivation.
Biologic treatmentsDupilumab is an IgG4 human monoclonal antibody directed against IL-4 and IL-13, the cytokines involved in the Th2 inflammatory response related to the pathogenesis of EoE. For some time, it has been used for treating atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyps; it has recently been approved by the FDA for EoE. In a phase 3 controlled clinical trial, there was histologic remission (defined as a decrease of <6 eosinophils/HPF) after 24 weeks of treatment with a subcutaneous dose of 300mg every week or 300mg every 2 weeks, in 60% of patients with EoE that was resistant to other therapies. The most common adverse effects were local reactions at the injection site and upper respiratory tract infections.89 A recent publication evaluated the real-world experience of the use of dupilumab in patients with treatment-refractory EoE and fibrostenotic disease. A substantial improvement in the histologic response (<15 eosinophils/HPF) was found, compared with pre-treatment values (80 vs 11% p≤0.001), as well as in the EREFS (4.62±1.84 vs 1.89±1.31, p≤0.001) and the post-dilatation diameter of the esophagus (16.0±2.3 vs 17.0±2.0, p≤0.001).90 Dupilumab is the most expensive treatment option, and so is should be reserved for cases that are refractory to conventional therapies or for patients with severe concomitant allergic conditions.91
Other biologic treatments that have been analyzed, or are currently being studied, are the antibodies that target IL-5 (mepolizumab and reslizumab), IL-13 (cendakimab), and the IL-5 receptor (benralizumab).92 In a phase 3 study, benralizumab had a histologic response of 87.4%, compared with the 6.5% response with placebo, but no symptomatic or endoscopic improvement was shown.93
Esophageal dilatationEndoscopic esophageal dilatation with bougies or balloons may be necessary in EoE patients with esophageal stricture and/or persistent dysphagia, despite medical therapy, and has been shown to be safe and effective.47
A meta-analysis of retrospective studies reported clinical improvement in 95% of patients with low perforation rates (0.38%) and need for hospitalization (0.67%). The most common adverse effect was chest pain in 23.6% of cases and the median duration of symptom improvement was 12 months.94 The esophageal diameter necessary for achieving relief from dysphagia is 16mm. Several sessions, with sequential 2mm increases each, are needed to meet that goal. A mucosal tear is an acceptable outcome for ending the dilatation session.47 For better results, dilatation should always be performed in conjunction with medical therapy, and histologic active inflammation is not a contraindication for dilatation.
Bougie dilators have not been shown to be better than balloon dilators.95 Therefore, the technique employed depends on the experience of the endoscopist and the characteristics of the stricture.
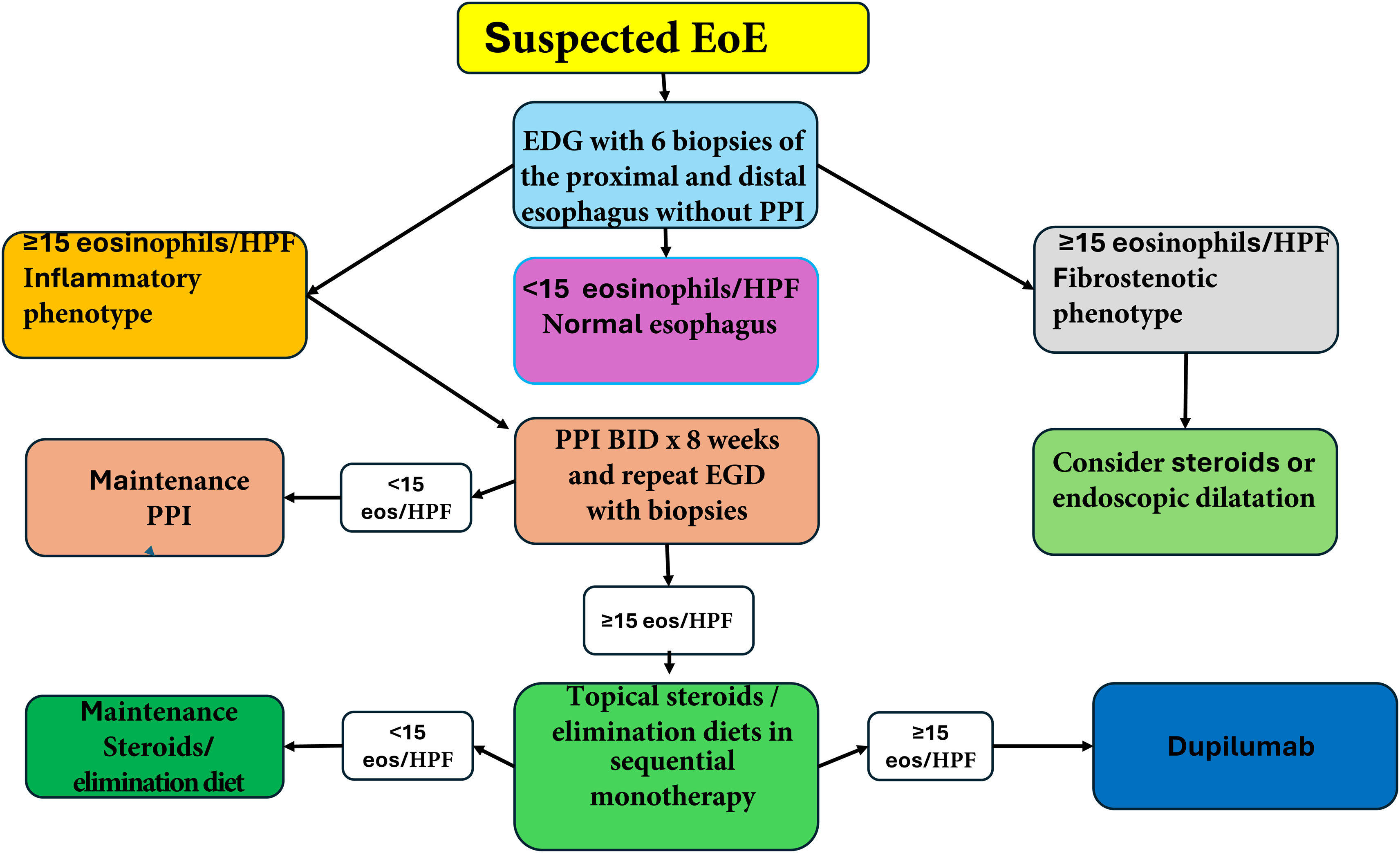
ConclusionsThe reported incidence and prevalence of EoE in Mexico and Latin America are low, compared with those described in developed countries. The explanation for the low prevalence is probably more dependent on environmental factors than on genetic ones. Different degrees of immunologic reactivity to environmental allergens, as a result of the modification of the inflammatory response pathways (Th1 and Th2), facilitated by socioeconomic, cultural, and ethnic aspects typical of the Mexican population, could also intervene. Poor diagnostic suspicion, inadequate PPI use, not suspending PPIs before endoscopy, and not taking biopsies in patients with a clinical profile of EoE are also important factors that should be considered. The correction of those technical deficiencies requires greater awareness of the disease, achieved through the continuous education of general physicians, family physicians, and specialists, on the nature of EoE and the keys to suspecting it. When there is a reasonable suspicion of the disease, the most adequate conduct is to avoid the PPI test and perform endoscopy with biopsy; when the diagnosis is confirmed, treatment should be started according to the EoE phenotype. Fig. 4 describes the simplified algorithm for the diagnosis and treatment of EoE in the Mexican environment.
In conclusion, more studies, preferably multicenter or multinational analyses, on Mexican and Latin American populations are needed to better characterize the clinical profile of the patients with EoE, as well as to define the risk factors. As a consequence, more adequate diagnostic strategies can be applied and therapeutic management guided, taking into account medication availability and the relation between risk and cost benefits in our countries.
Despite not having precise knowledge of the factors involved in the emergence and progression of EoE in Mexico, we have no doubt that its incidence will increase in the future, as has occurred with other initially rare autoimmune diseases (such as inflammatory bowel disease), as a consequence of the modification of environmental and sociocultural factors in the Mexican population.
Ethical considerationsBecause this article is a literature review, ethical considerations do not apply.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.