Clostridioides difficile (C. difficile) infection is the main cause of nosocomial diarrhea. First-line treatment is oral vancomycin, but that presentation is not commercially available in Latin America. Our aim was to determine the fecal concentration of the oral administration of the conventional dose of an intravenous vancomycin preparation (VCM), in an experimental model.
MethodsA preclinical trial was conducted on 18 male mice (Balb/c strain), in three batches. The following doses of VCM were administered: 125 mg in batch A; 500 mg in batch B; and VCM-placebo in batch C. After receiving the doses, the mice were placed in metabolic cages, by batch. Feces were collected and the fecal concentration of VCM was analyzed through high pressure liquid chromatography 2, 4 and 6 h after drug administration.
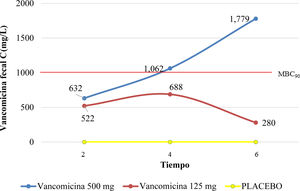
ResultsThe 125 mg dose of VCM reached the minimum inhibitory concentration (MIC) for C. difficile, without reaching the minimum bactericidal concentration (MBC90), at 2, 4, and 6 h (521, 688, and 280 mg/L, respectively). Likewise, the 500 mg dose of VCM reached the MIC at 2 h, increased gradually, and reached MBC90 between 4 and 6 h, in feces (1,062 and 1,779 mg/L, respectively), ANOVA, p = 0.0005.
ConclusionThe fecal concentration of vancomycin was dependent on the intragastric dose administered. Only the 500 mg dose of VCM reached therapeutic concentration for C. difficile (MIC and MBC90), in the mice. We suggest starting a dose of 500 mg QID for achieving therapeutic concentration against C. difficile, as soon as 4 h after the first dose.
La infección porClostridioides difficile (C. difficile) es la principal causa de diarrea nosocomial, el tratamiento de primera línea es vancomicina oral; sin embargo, esta presentación no está disponible comercialmente en Latinoamérica. Nuestro objetivo fue determinar la concentración fecal de la preparación de vancomicina intravenosa (VCM) administrada por vía oral, a dosis convencional, en un modelo experimental.
MétodosEnsayo preclínico, con 18 ratones macho (cepa Balb-C), en tres grupos. Se administraron las siguientes dosis de VCM: en grupo A) 125 mg; grupo B) 500 mg; y grupo C) VCM-placebo. Después de recibir la dosis de VCM, los ratones se colocaron en jaulas metabólicas por grupo. Se recolectaron las heces y se analizó la concentración fecal de VCM mediante cromatografía líquida de alta presión 2, 4 y 6 h después de la administración.
ResultadosLa dosis de 125 mg de VCM alcanzó la concentración mínima inhibitoria (CMI) para C. difficile, sin alcanzar la concentración bactericida mínima (CBM90) a las 2, 4 y 6 h (521, 688 y 280 mg/L, respectivamente). Asimismo, la dosis de VCM 500 mg alcanzó la CMI a las 2 h, aumentó gradualmente y alcanzó CBM90 entre las 4 y 6 h, en heces (1,062 y 1,779 mg/L, respectivamente), ANOVA, p = 0.0005.
ConclusiónLa concentración fecal de vancomicina depende de la dosis intragástrica administrada, solo la dosis de VCM 500 mg alcanzó concentración terapéutica paraC. difficile (CMI y CBM90), en ratones. Sugerimos comenzar con una dosis de 500 mg QID para lograr la concentración terapéutica contra C. difficile, tan pronto como 4 h después de la primera dosis.
Clostridioides difficile infection (CDI) is the main cause of nosocomial diarrhea in industrialized countries. A recent increase in its incidence has been observed in Europe and North America. There are few studies on the prevalence or incidence of CDI in Latin America, so the actual impact of the infection in our population is unknown.1–3 In Argentina and Mexico, the incidence rate reported in hospitalized patients is 3.1 cases/1,000 patient-days, and 1.1 cases/1,000 patient-days during the 30-day follow-up period, respectively.4 In Mexico, the crude 30-day mortality rate was 8.4%.5 International guidelines for the treatment of CDI propose the use of oral antibiotics (metronidazole, vancomycin, and fidaxomicin), according to the severity of the clinical profile, as well as other treatment modalities, such as fecal microbiota transplantation.6,7 For many years, metronidazole was recommended as first-line treatment, but since 2017, international guidelines have recommended oral vancomycin and fidaxomicin as first-line drugs due to CDI recurrence after treatment with metronidazole.8 The recommended oral dosage of vancomycin is 125 mg to 500 mg QID for 10-14 days.1,6–8 However, the optimum dose for CDI is not well established, whereas the minimum inhibitory concentration (MIC) is 0.5-8 mg/L and its minimum bactericidal concentration (MBC90) is above 1,000 mg/L.9,10
Currently, the oral presentation of vancomycin is commercially unavailable in Mexico, obliging us to extrapolate the oral presentation information to the oral administration of the intravenous preparation. Diarrhea associated with CDI is a highly prevalent nosocomial infection, and in Mexico, only the intravenous presentation of vancomycin (referred to hereafter as VCM) is available. In addition, there are few studies assessing fecal vancomycin concentrations. Therefore, our primary aim was to determine, in an experimental model, the fecal vancomycin concentration at 3 different time intervals, after the oral administration of single 125 mg and 500 mg doses of VCM, using water as a vehicle.
Material and methodsPreclinical trial.
Materials and animalsThe intravenous formulation of vancomycin hydrochloride was obtained from PISA Laboratories under the name, Vanaurus (Guadalajara, Jalisco, Mexico; Reg. No. 487M96 SSA. IPP-A: GEAR-108175/RM 2002). Balb/c strain male mice (weight 27.8 ± 0.5 g; 18 weeks of age) were used. The present study was approved by the Research and Ethics Committee of our hospital (R-2016-3601-199). The mice were kept in an environment with a temperature of 18 to 26 °C, relative humidity 40-70%, a ventilation system with 15 to 18 replacements per hour for 24 h, a light/darkness cycle of 12 h with artificial daylight from fluorescent lamps, and noise intensity below 85 dB. The mice had free access to water and received 3 to 6 g of commercial rodent food daily, containing 17-24% raw protein, 4-11% raw fat, 3-6% raw fiber, 5-7% ash, and no vitamin C. The mice were handled according to the International Guiding Principles for Biomedical Research Involving Animals developed by the Council for International Organizations of Medical Sciences (CIOMS)11 and the Official Mexican Norm on techniques for the production, care, and use of laboratory animals.12
Preparation of vancomycin for administration to the miceThe vancomycin hydrochloride powder was weighed on an analytical balance (Ohaus, Analytical Plus), to obtain the necessary dose for each mouse, and was reconstituted in its diluent (water for injection), for the present investigation, at a rate of 1.0 mL per dose. The dose of vancomycin hydrochloride administered was 125 mg (equivalent to 1.78 mg/kg of mouse body weight) and 500 mg (equivalent to 7.14 mg/kg of mouse body weight). Subsequently, the reconstituted VCM was administered as follows:
Batch A: VCM 125 mg in water for injection.
Batch B: VCM 500 mg in water for injection.
Batch C: VCM-placebo (vehicle).
Oral administration of vancomycinThree batches of 6 mice each were used in the assay. The dose for batch A was 125 mg VCM in water for injection (WFI); for batch B, 500 mg of VCM in WFI; and for batch C, 1 mL of the vehicle. The doses were given intragastrically via orogastric cannula.
After receiving the dose, the mice were placed in separate metabolic cages, by batch, and stool samples were taken 2, 4, and 6 h post-VCM administration. Stool samples were suspended in 4 mL of WFI and mixed by a Thomas® vortex mixer (Thomas Scientific Analog Vortex Mixer, model 945700). The resulting suspension was allowed to settle, and the supernatant was placed in an Eppendorf microtube (1.5 mL, MTC-150-C Maxiclear™ homopolymer boil-proof 311-08-051) and later centrifuged in a minispin (Eppendorf) at 12,500 rpm for 10 min. The entire supernatant was filtered (PALL 0.45 µm filter, Nylon Acrodisc 13 µm), and 20 µL of the filtrate was injected into the high-pressure liquid chromatographer (HPLC) to quantify the vancomycin.
Vancomycin assayVancomycin was analyzed by liquid chromatography in a Waters 2795 module equipped with a Waters 996 photodiode array, automatic simple injector, and the Millenium 3.1 program. We used a Waters Spherisorb 4.6x250 mm column and monobasic potassium phosphate 0.025 M phase at pH 3.0, acetonitrile ratio 92:8, and 0.8 mL/min flow. Detection occurred at 230 nm.
The vancomycin concentration in the samples was quantified by interpolation of the peak area of the drug in a calibration curve, obtained with vancomycin solutions at concentrations of 21.5, 43, and 86 µg/mL, with an R² of 0.9933.
Pharmacokinetic analysisMeasured parameters included the run time (RT) and area under the curve (AUC) of fecal vancomycin concentration multiplied by the quantification in fecal sample time. The VCM concentration was calculated with the following equation:
Statistical analysisAll values were expressed as mean ± standard deviation (SD), median (intervals), and ratios. The time/vancomycin concentration was graphed using the Graph-Pad statistics program. ANOVA was used to assess the differences in the mean values between the 3 groups, and multiple comparisons were made, utilizing the Bonferroni correction. Statistical significance was set at a p < 0.05.
ResultsA total of 18 male Balb/c strain mice were included in the study, divided into 3 batches. The average body weight was 27.8 g (± 0.5) and the average weight of feces in all 3 batches was greater than 0.200 g.
Two hours after intragastric administration of 125 mg of VCM, we observed a fecal vancomycin concentration of 522 mg/L, considered therapeutic against C. difficile, but the MBC90 was not reached. The maximum peak of 688 mg/L was reached at 4 h and then fell to 280 mg/L at 6 h.
Regarding the intragastric administration of 500 mg of VCM, 2 h after administration, a stool concentration of 632 mg/L was reached. It continued to rise over 4 h and 6 h, reaching the MBC90, with a maximum peak of 1,779 mg/L at 6 h, as shown in Fig. 1.
After intragastric administration, the AUC for 500 mg of VCM was approximately 1.9-times greater than the dose of 125 mg at 4 h, and approximately 6-times greater at 6 h. When the vancomycin doses (125 mg and 500 mg) were compared, there was a significant difference in the fecal vancomycin concentration at 4 h and at 6 h after administration (ANOVA, p = 0.0005; Bonferroni test, 95% CI, –1.11-0.089, and −2.194 to −0.803, respectively) (Fig. 2).
The average fecal VCM concentration for the 125 mg batch was 522 mg/L (± 26.3), 688 mg/L (± 145.7), and 280 mg/L (± 31.5), at 2, 4, and 6 h, respectively. For the 500 mg batch, it was 632 mg/L (± 70.5), 1,062 mg/L (± 261) and 1,779 mg/L (± 181.2), at 2, 4, and 6 h, respectively. For batch A, there was no significant difference between 125 mg and 500 mg (p = 0.7), whereas there was a significant difference at 4 h and 6 h between the 125 mg and 500 mg doses, for batches B and C (p = 0.005).
CDI is currently one of the main causes of nosocomial infection across the globe, and there has been an increase in the prevalence of community-acquired CDI, over the past decade. Likewise, there has been a worldwide growth of hypervirulent strains.1,2,5 The recommended treatment depends on the severity of the clinical profile. In the 1970s, treatment was based on oral metronidazole and oral vancomycin. Current first-line treatment is oral vancomycin and oral fidaxomicin.8 Treatment guidelines for CDI from North American and European gastroenterology groups recommend oral vancomycin at doses ranging from 125 mg to 500 mg every 6 h.6,7 However, oral vancomycin is not commercially available in Mexico and other Latin American countries, so oral administration of VCM has been used instead, with varying clinical results.4,5 In our study, we indirectly assessed fecal vancomycin concentration using fecal samples from mice receiving a single dose of VCM by orogastric cannula, and the results varied by dose. Based on those results, we suggest evaluating the use of orally administered VCM at a dose of 500 mg every 6 h, for achieving a therapeutic concentration (MIC and MBC90) against C. difficile, as soon as 4 h after the first administration.
An assay was performed to estimate the MIC of vancomycin in 6 isolates of C. difficile in a brain-heart infusion that was incubated anaerobically, whereas the MBC90 was obtained by subculture of the broths in blood agar, after 24 h and 96 h. The geometric mean of the vancomycin MIC for the C. difficile isolate was 1-2 mg/L. However, the MBC90 or vancomycin concentration needed to reduce the original bacterial inoculate by a percentage equal to or greater than 99.9%, was higher than 1,000 mg/L, presumably due to spore survival.10
At present, oral vancomycin is commercially unavailable in Mexico, making it necessary to extrapolate information available on the use of oral vancomycin in the treatment of CDI to the oral administration of its intravenous preparation. Considering that ethical and legal issues arise whenever healthcare personnel decide to use medications under conditions that differ from the corresponding fact sheet, we decided to carry out the trial on a murine model, using Balb/c strain mice because the physiology and immune response of their gastrointestinal tract is similar to that of humans, and it is also a model that has previously been used for the preclinical study of other antibiotics.13 We recognized that a proper bioavailability study would require a different focus, such as the use of an intestinal biomarker and the collection of all fecal matter over a sufficient period of time, to confirm 95-100% elimination of the dose, so we decided to carry out the study on an experimental model. Although pharmacokinetics is the point of interest, the results are limited for clinical application in humans. Nevertheless, the mice were not exposed to other compounds and the samples were collected in a homogeneous manner over short, regular periods, which simplified the analysis.
Ours is the first study in Mexico to assess the bioavailability of VCM, administered orally, at doses of 125 mg and 500 mg, as recommended by international guidelines for the treatment of CDI. There was higher fecal concentration of vancomycin with the higher dose, and the two doses analyzed reached the MIC, starting at 2 h after administration. However, only VCM 500 mg reached the MBC90 (>1,000 mg/L) against C. difficile between 2-4 h and was maintained at 6 h. A Canadian group assessed fecal levels of vancomycin, after administration of 125 mg, 250 mg, and 500 mg of generic VCM for an average of 6 days, in patients with suspected CDI.14 They concluded that fecal vancomycin concentration increased proportionally with the dose and found that patients receiving the standard dose of 125 mg every 6 h might have low levels in fecal matter during the first day of treatment. Thus, a priming dose of 250 mg or 500 mg QID in the first 24-48 h, followed by the standard dose, should be assessed in clinical studies, because it could be less harmful to the colonic flora and reduce costs.14
Our results, based on a single oral administration of VCM, at two different doses, are similar to those reported by other authors that have assessed various oral presentations of vancomycin in humans. A vancomycin liquid preparation of 125 mg every 6 h resulted in a mean average concentration in fecal matter of 399 mg/L (range 152-880).15 Over the same year, those authors found a mean concentration of vancomycin of 3,100 mg/L in fecal matter at a 500 mg dose every 6 h, although the days when samples were taken and analyzed were not specified in the study.16 Another study compared doses of 125 mg and 250 mg of oral vancomycin in patients with mild to moderate diarrhea and found that both produced equivalent fecal concentrations, with no increase in absorption or toxicity.10
Based on the above results, we can state that the oral administration of 125 mg and 500 mg of VCM had a bioavailability profile in our murine model that was similar to findings in studies on humans with various oral preparations that are commercially available in the United States and Europe.6,7,17 A 500 mg dose of VCM resulted in a safe and stable performance, reaching and maintaining MIC and MBC90 for C. difficile, from the first 4 h after oral administration. In contrast, the 125 mg dose achieved the MIC, but not the MBC90, possibly favoring recurrence of infection due to a limited elimination of bacterial spores and a consequent therapeutic failure.
Regarding the limitations of our study, feces were not collected over a long enough period of time, to confirm dose excretion at 95-100%, and thus evaluate whether the doses remained at therapeutic concentrations beyond 6 h. Likewise, we did not use an intestinal biomarker. In addition, we suggest performing an experimental assay with a model that includes C. difficile infection, to assess vancomycin in the presence of inflammation and motility conditions typical of the infection. We also believe it is necessary to determine the impact of the administration vehicle on the bioavailability and absorption of the drug.
ConclusionOur study assessed fecal vancomycin concentration after the intragastric administration of VCM at a single dose. We found that fecal vancomycin concentration was dose dependent. Based on those results, we suggest evaluating the use of orally administered VCM at a dose of 500 mg every 6 h, for reaching therapeutic concentrations (MIC and MBC90) against C. difficile, within the first 2-4 h of administration.
Ethical considerationsThe protocol of the present study conformed to the Declaration of Helsinki and was approved by the hospital’s Research and Ethics Committee (R-2016-3601-199). This study followed the ARRIVE guidelines for preclinical trials. Male mice were included and were handled according to the International Guiding Principles for Biomedical Research Involving Animals developed by the Council for International Organizations of Medical Sciences (CIOMS); the Official Mexican Norm on techniques for the production, care, and use of laboratory animals (NOM-062-ZOO-1999); and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
This trial included no human experiments.
Ethics committee approvalThis study was approved by our hospital’s Research and Ethics Committee (R-2016-3601-199).
Financial disclosureThe present study received financial support from the Fondo de Investigación en Salud FIS/IMSS/PROT/G17/1665.
Conflict of interestThe group declares that they have no conflict of interest.
Please cite this article as: Ramos-García J, Robles-Rivera F, Chávez-Soto M, Valdés M, Calzada F, Ortiz-Olvera N. Concentración fecal de la preparación de vancomicina intravenosa posterior a administración oral en un modelo experimental: ensayo preclínico. Rev Gastroenterol Méx. 2023;88:85–90.
See related content at https://doi.org/10.1016/j.rgmxen.2022.04.004, Asbun-Bojalil J. Fecal concentration of intravenous vancomycinpreparation after oral administration: Preclinical data supporting unmet clinical needs. Rev Gastroenterol Mex. 2023;88:83–84.