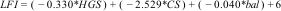Chronic hepatitis C virus (HCV) infection is one of the leading causes of cirrhosis. Frailty and malnutrition are comorbidities associated with cirrhosis, impacting patient quality of life and survival. The aim of this study was to evaluate frailty and food intake quality in patients with chronic HCV infection, with or without cirrhosis, and the association between demographic, clinical, and anthropometric variables.
Material and methodsA cross-sectional study was conducted at the hepatitis clinic of the Hospital Civil de Guadalajara Fray Antonio Alcalde. Each participant was evaluated using the Liver Frailty Index (LFI), the mini-survey for assessing dietary intake quality (Mini-ECCA v.2), and upper arm anthropometry.
ResultsOf the sample of 52 patients, nearly 40% presented with chronic HCV alone and close to 80% were classified as pre-frail on the LFI. The study patients had a mean handgrip strength of 25.5 ± 11.1 kg and under 10% had a healthy diet.
ConclusionsThere was a high prevalence of frailty in the patients with inadequate dietary intake. In addition, the arm circumference measurement was positively correlated with handgrip strength, highlighting the importance of considering arm anthropometry in those patients as part of their nutritional assessment.
La infección crónica por virus de hepatitis C (VHC) es una de las principales causas de cirrosis, algunas comorbilidades asociadas a la cirrosis son la fragilidad y la malnutrición y cuya presencia impacta en la calidad de vida y en la sobrevida de los pacientes; el objetivo de este estudio es evaluar la fragilidad y la calidad de consumo alimentario en pacientes con infección crónica de VHC con o sin cirrosis así como evaluar la asociación entre variables demográficas, clínicas y antropométricas.
Material y métodosSe realizó un estudio transversal en la clínica de hepatitis del Hospital Civil de Guadalajara Fray Antonio Alcalde. A cada participante se le evaluó con el Liver Frailty index (LFI) así como la miniencuesta para evaluar la calidad del consumo de alimentos (Mini-ECCA v.2) y por último se realizó antropometría braquial.
ResultadosDe una muestra de 52 pacientes cerca del 40 % presentaban solo infección crónica de VHC, en cuanto a la aplicación del LFI cerca del 80% se encontraba en la clasificación de prefragilidad; con una fuerza de agarre de mano promedio de 25.5 ± 11.1 kg; respecto a la calidad del consumo menos del 10% presentaba alimentación saludable.
ConclusionesSe encontró una alta prevalencia de fragilidad en los pacientes acompañada de una calidad de consumo alimentario inadecuada. Además, el MAMC tiene una correlación positiva con la fuerza de agarre de mano, lo cual demuestra la importancia de considerar la antropometría de brazo en estos pacientes como parte de su evaluación nutricional.
Close to 71 million persons across the globe are estimated to live with chronic hepatitis C virus (HCV) infection. Despite new treatments for its eradication, HCV continues to be one of the main causes of cirrhosis in Mexico and worldwide.1,2 As the disease progresses, the presence of frailty and sarcopenia becomes more common. This is clinically important because of reduced survival and a decrease in quality of life, as well as a greater predisposition to decompensations and poor post-transplantation prognosis.3 Sarcopenia can be defined as the loss of muscle mass, whereas frailty is considered a manifestation of the loss of muscle function.4 In the population with HCV, the prevalence of sarcopenia tends to vary from 45 to 88%5–7 and frailty is reported at up to 53%.8 One of the most widely utilized tools for evaluating frailty is the Liver Frailty Index (LFI). It evaluates physical frailty through 3 tests,8 and has also been found to help improve the prediction of mortality in patients on the waiting list for liver transplantation.9
Malnutrition is another common complication in patients with cirrhosis, with a prevalence over 50%.7,10 In addition, insufficient food intake has been observed to increase mortality and there is a higher risk of infections and complications.11 Likewise, malnutrition is one of the main causes of sarcopenia.12 Thus, dietary factors have an influence on the course of liver disease and are a cornerstone in its treatment approach.10,13 Even though there are different indexes and instruments that evaluate dietary quality, very few are specific for the Mexican population. One such tool is the mini survey for evaluating food intake quality (the Mini-ECCA v.2), which evaluates dietary intake quality rapidly.14
The importance of anthropometric parameters, such as the mid-arm muscle circumference (MAMC) and triceps skinfold thickness (TSFT), should be underlined because they dependably enable the nutritional status of patients with liver disease to be evaluated. Their reliability lies in the fact that they are not altered in the presence of edema, and they reflect muscle mass and fat. Additionally, both MAMC and TSFT are survival predictors in patients with cirrhosis.10,15
Few studies evaluate both food consumption quality and frailty. Therefore, the aim of the present study was to evaluate frailty and dietary intake quality in patients with chronic HCV infection, with or without cirrhosis, as well as the association between demographic, clinical, and anthropometric variables.
MethodologyA cross-sectional study was conducted that was structured according to the strengthening the reporting of observational studies in epidemiology (STROBE) checklist. The patient recruitment period was from March 2023 to July 2023 at the Gastroenterology Service’s Hepatitis Clinic of the Hospital Civil de Guadalajara Fray Antonio Alcalde. Patients diagnosed with HCV infection, with or without cirrhosis, who were regularly seen at the hepatitis clinic, were invited to participate. The diagnosis of HCV infection was made by the presence anti-HCV antibodies and a positive HCV viral load. The diagnosis of cirrhosis was based on the combination of the clinical history, biochemical data (APRI > 1.7 and FIB 4 > 3.25), and imaging studies (endoscopy revealing the presence of esophageal varices, liver ultrasound and/or computed tomography showing the presence of irregular edges, splenomegaly, and vena porta dilatation above 13 mm of diameter). Patients with decompensated cirrhosis or a physical or motor impediment for undergoing frailty testing were excluded.
After the participation of each patient was confirmed, arm anthropometry in the dominant arm was carried out. MAMC was measured from the midpoint between the subscapular acromion and the olecranon process of the elbow, using a Lufkin anthropometric metal tape measure, and TSFT was measured using a Slim Guide skinfold caliper (1 mm).
Arm anthropometry included calculating the total arm area (TAA), the arm muscle area (AMA), the arm fat area (AFA) and the MAMC. The MAMC result was compared with the patient’s position in the percentiles for age and sex, considering it to be “low” if under the 10th percentile and “very low” if under the 5th percentile.16
The LFI, which consists of 3 tests, was utilized as described below to evaluate frailty:
- –
Mean handgrip strength: the mean of 3 attempts using the CAMRY EH 101 hand dynamometer.
- –
Chair stands: the time it takes the patient to do 5 chair stands with arms crossed at the chest level.
- –
Balance: the total sum of the seconds each foot position was held (side-by-side, semi-tandem, and tandem foot positions), with a maximum of 10 seconds each.17
The LFI was calculated using the following equation:
*where HGS is handgrip strength, CS is chair stands, and bal is balance*According to the LFI cutoff points, a score < 3.2 is considered robust, from 3.2 to 4.4 is pre-frail, and ≥ 4.4 is frail.17 Reduced handgrip strength was a result < 27 kg in men and < 16 kg in women, and reduced strength measured by chair stands was a time above 15 seconds.18
To evaluate the quality of food intake the Mini-ECCA v.2 was applied. It consists of 14 questions accompanied by a reference image, with 3 or 4 response options on a Likert scale. The corresponding groups are evaluated according to the consumption frequency of each item, and in relation to bowel movements, as: healthy food intake, habits in need of improvement, and unhealthy food intake.14
To calculate the sample, we utilized the formula for comparing two proportions and a 95% confidence interval, considering an α of 0.05 and a β of 0.8,19 based on the prevalence of low handgrip strength in patients with cirrhosis,7 resulting in a total of 19 subjects per group.
Statistical analysisThe data were analyzed using SPSS version 25. Measures of central tendency were expressed as frequency, percentage, median, and quartiles, according to their normality. The Shapiro-Wilk test was applied to evaluate the normality of the variables, and the non-normal ones were transformed to logarithm base 10. The Student’s t-test or Mann-Whitney U test were carried out for the group comparisons, and the chi-square test or Fisher’s exact-test were performed for the nominal variables according to their normality. The parametric correlations were analyzed through the Pearson test and the Kendall’s Tau b was applied for the nominal variables. Statistical significance was set at a p < 0.05.
Ethical considerationsThe patients included in this study gave their informed consent. In addition, the study complies with the ethical guidelines of the 1975 Declaration of Helsinki (the 2013 revision) and was approved by the ethics committee of the Hospital Civil de Guadalajara Fray Antonio Alcalde, with registry number CEI 183/23.
ResultsDemographic dataOf the sample of 52 patients, more than 50% had cirrhosis classified as Child-Pugh class A or B, with a frequency and percentage of 23 (76.7%) and 7 (23.3%), respectively. In addition, they had a mean MELD-Na score of 10.22 ± 2.8. On the other hand, the patients with HCV infection alone had a median APRI of 0.35 (0.2−0.5) and FIB-4 of 1.21 (0.89–1.77) (Table 1).
Demographic and anthropometric data.
| Variable | Total | HCV | Cirrhosis | p | |
|---|---|---|---|---|---|
| n = 52 | n = 22 | n = 30 | |||
| Sex | Men | 21 (40.4) | 11 (50) | 10 (33.3) | |
| Women | 31 (59.6) | 11 (50) | 20 (66.7) | ||
| Age (years) | 57.3 ± 10.3 | 54.1 ± 9.5 | 59.6 ± 10.4 | 0.06 | |
| Anthropometric measurements | MAC | 30.9 ± 4.3 | 31.4 ± 4.2 | 30.6 ± 4.4 | 0.508 |
| TSFT | 1.8 ± 0.6 | 1.8 ± 0.7 | 1.8 ± 0.5 | 0.953 | |
| MAMC | 25.2 ± 3.5 | 25.7 ± 3.6 | 24.9 ± 3.4 | 0.435 |
MAC: mid-arm circumference; MAMC: mid-arm muscle circumference; TSFT: triceps skinfold thickness.
Values expressed as mean ± standard deviation (SD).
Student’s t-test for unrelated samples was used to compare groups. Statistical significance: p < 0.05.
Regarding percentile position, only 15% of the total study population was below the 10th percentile for TSFT (Table 2), whereas 29% were below the 10th percentile for MAMC (Table 3).
Triceps skinfold thickness (TSFT) percentiles.
| <5th percentile | 5th–10th percentiles | 10th–25th percentiles | 25th–75th percentiles | >75th percentile | Total | |
|---|---|---|---|---|---|---|
| HCV | 2 (9.1) | 0 (0) | 12 (54.5) | 6 (27.3) | 2 (9.1) | 22 |
| Cirrhosis | 2 (6.7) | 4 (13.3) | 19 (63.3) | 2 (6.7) | 3 (10.0) | 30 |
| Total | 4 (7.7) | 4 (7.7) | 31 (59.6) | 8 (15.4) | 5 (9.6) | 52 |
Values expressed in frequency and percentage (%).
HCV: hepatitis C virus.
Mid-arm muscle circumference (MAMC) percentiles.
| <5th percentile | 5th–10th percentile | 10th–25th percentiles | 25th–75th percentiles | >75th percentile | Total | |
|---|---|---|---|---|---|---|
| HCV | 3 (13.6) | 5 (22.7) | 2 (9.1) | 3 (13.6) | 9 (41.0) | 22 |
| Cirrhosis | 3 (10.0) | 4 (13.3) | 10 (33.3) | 7(23.4) | 6 (20.0) | 30 |
| Total | 6 (11.5) | 9 (17.3) | 12 (23.1) | 10 (19.2) | 15 (28.9) | 52 |
Values expressed as frequency and percentage (%).
HCV: hepatitis C virus.
According to the LFI, none of the patients were classified as robust, and more than 80% of the patients were in the pre-frail category. There were significant differences between the patients with HCV infection alone versus those with HCV and cirrhosis, regarding the total LFI score, number of chair stands per second, total time in performing the chair stands, and mean handgrip strength.
Likewise, 34% had reduced handgrip strength, whereas 50% had reduced strength measured by chair stands (Table 4).
Classification of frailty through the Liver Frailty Index (LFI), handgrip strength, and chair stands.
| HCV | Cirrhosis | Total | p | ||
|---|---|---|---|---|---|
| n = 22 | n = 30 | n = 52 | |||
| LFI components | Total LFI score | 4.07 ± 0.4 | 3.88 ± 0.4 | 4.2 ± 0.4 | 0.007a |
| Stands per second | 0.3 ± 0.07 | 0.36 ± 0.06 | 0.30.6 ± 4.4 | 0.015a | |
| Total time for standsd | 15.5 ± 3.7 | 14.0 ± 2.8 | 16.6 ± 3.93 | 0.011a | |
| Handgrip strength kgd | 29.13 ± 11.7 | 22.8 ± 10.0 | 25.5 ± 11.1 | 0.05a | |
| LFI categories | Pre-frail | 20 (90.9) | 22 (73.3) | 42 (80.8) | 0.161b |
| Frail | 2 (9.1) | 8 (26.7) | 10 (19.2) | ||
| Handgrip strength categories | Non-reduced strength | 17 (77.3) | 17 (56.7) | 34 (65.4) | 0.123c |
| Reduced strength | 5 (22.7) | 13 (43.3) | 18(34.6) | ||
| Chair stand time categories | Non-reduced strength | 14 (63.6) | 9 (30.0) | 23 (44.2) | 0.016c |
| Reduced strength | 8 (36.4) | 21 (70.0) | 29 (55.8) | ||
Values expressed as frequency and percentage (%).
Statistical significance: p < 0.05.
According to the results of the Mini-ECCA v.2, 67.3% of the patients were classified as having “habits in need of improvement”, 25.0% as having “unhealthy food intake”, and the remaining 7.7% as having “healthy food intake”. There were no significant differences in food consumption quality between the HCV patients with cirrhosis or those without cirrhosis (p = 0.751). The most frequently consumed foods evaluated through the Mini-ECCA v.2 were desserts, legumes, fruits, and water, and the foods consumed the least were fish and vegetables (Table 5).
Frequency of food intake according to the Mini-ECCA v.2.
| Item | Never | Sometimes | Almost always | Always |
|---|---|---|---|---|
| Legumes | 4 (7.7) | 9 (17.3) | 7 (13.5) | 32 (61.5) |
| Fruits | 2 (3.8) | 21 (40.4) | 7 (13.5) | 22 (42.3) |
| Water | 5 (9.6) | 12 (23.1) | 5 (9.6) | 30 (57.7) |
| Processed foods | 25 (48.1) | 21 (40.4) | 3 (5.8) | 3 (5.8) |
| Desserts | 8 (15.4) | 22 (42.3) | 7 (13.5) | 15 (28.8) |
| Seed oils | 11 (21.2) | 30 (57.7) | 8 (15.4) | 3 (5.8) |
| Vegetables | 6 (11.5) | 27 (51.9) | 11 (21.2) | 8 (15.4) |
| Fish | 31(59.6) | 16 (30.8) | 2 (3.8) | 3 (5.8) |
Values expressed as frequency and percentage (%).
Oils other than olive oil were the most widely consumed, at 75% of the population, whereas the most widely consumed cereal was in the “corn, oatmeal, and bran” group, at 80.8% (data not shown).
Correlation between frailty, dietary quality, and MAMCThere was a negative correlation between the MAMC and LFI score (r = –0.384, p = 0.005) and a positive correlation between the MAMC and handgrip strength (r = 0.485, p < 0.005).
Likewise, there was a significant positive result between the MAMC category and the Mini-ECCA category (T = 0.315, p < 0.005), as well as a positive correlation between the presence of HCV with or without cirrhosis and reduced strength determined by chair stands (T = 0.335, p < 0.005).
DiscussionThe prevalence of frailty in patients with HCV in this study was over 80%, results that are comparable to those reported in the literature.8,9,20,21 Wang et al. evaluated LFI reproducibility in patients with chronic liver disease, patients with cirrhosis, and healthy patients, and found that close to 40% of the patients with chronic liver disease presented with pre-frailty, whereas 70% of the patients with cirrhosis presented with pre-frailty or frailty.8
Our study population, despite being clinically stable, presented with a higher LFI score, compared with other studies. For example, the study by Lai et al. evaluated patients with cirrhosis who were on the waiting list for liver transplantation, with Child-Pugh class B or C and a MELD-Na score of 18, and reported a median LFI score of 3.8, with an interquartile range of 3.4 to 4.3.9 In our study, handgrip strength was similar to the 20 kg–28 kg reported in other studies.8,17,22 Likewise, the prevalence of reduced strength was similar to that observed in patients with cirrhosis in Brazil, where it was reduced in approximately 50%.23
Regarding the anthropometric evaluation, more than 25% of our patients were positioned at under the 10th percentile for MAMC and under the 10th percentile for TSFT, reflecting muscle wasting and a decrease in the fatty tissue reserve. Our findings coincide with the results of other studies that reported a prevalence of malnutrition from 30 to 50%, albeit those studies were conducted on decompensated patients.15,24 Our results showed nutritional deficiencies that, when contrasted with food consumption quality, revealed low intake of fish and vegetables, but more frequent intake of processed foods, sweets, and legumes. That consumption pattern is similar to those reported in the literature, such as the study by Georgiou et al. They evaluated dietary habits and energy intake in patients with cirrhosis and found that only 30% of the patients approached adequate protein and energy requirements, whereas there was low intake of vegetables and fruit, and almost no intake of fish. In contrast, there was high consumption of sweets and desserts that rose to 11 portions per week.25
Another study evaluated dietary habits, associating them with the presence or absence of sarcopenia in patients with cirrhosis. That analysis found that dietary quality was poor, with low protein intake. In addition, the intake of sweets was frequent, with 64% of the patients with sarcopenic obesity consuming them daily. Low intake of meat, dairy products, and fish was also observed.12 Such a selection and consumption of processed foods and sweets, followed by low intake of protein, fiber, and polyunsaturated and monounsaturated fatty acids, not only impacts the nutritional status of patients but also their physical frailty, and as a consequence, their survival and quality of life. Because said pattern of consumption and the presence of frailty have been shown to be similar in different populations, this has become a point of interest in the approach to liver disease.
Among the limitations of our study is its sample size and the fact that we did not apply tools for obtaining a deeper dietary evaluation, such as a daily food consumption diary. Nevertheless, despite its limitations, our study offers a first approximation to analyzing the quality of food consumption in Mexican patients with chronic HCV infection, with or without cirrhosis. It also underlines the importance of an early nutritional approach, given the high percentage of frailty in that population. Relevantly, despite having found a relation between food consumption quality and the MAMC, we believe further research is needed on eating patterns and frailty.
In conclusion, we found a high prevalence of frailty in our study patients that was accompanied by inadequate food intake quality. In addition, the MAMC had a positive correlation with handgrip strength, demonstrating the importance of considering arm anthropometry as part of the nutritional evaluation of patients presenting with chronic HCV infection, with or without cirrhosis.
FundingThis project was funded through the Ciencia al Mercado 2022 call by COECYTJAL and the Secretariat of Innovation, Science and Technology (SCIyT), grant number 10417-2023.
The authors declare no conflict of interest.