Glomus tumors originate from smooth muscle cells of the glomus bodies that help regulate arteriolar flow. They are mesenchymal tumors with malignant potential.1 Though infrequent, malignant gastric glomus tumors with metastasis to different organs have been reported.2
A 52-year-old man presented with intermittent abdominal pain in the epigastrium of several months’ progression, with partial response to medical treatment. He did not complain of fever, vomiting, constipation, diarrhea, weight loss, or any other systemic symptomatology. Physical examination revealed normal vital signs and no cardiopulmonary alterations. He had pain in the upper hemiabdomen upon deep palpation, no peritoneal irritation, and no other pathologic signs.
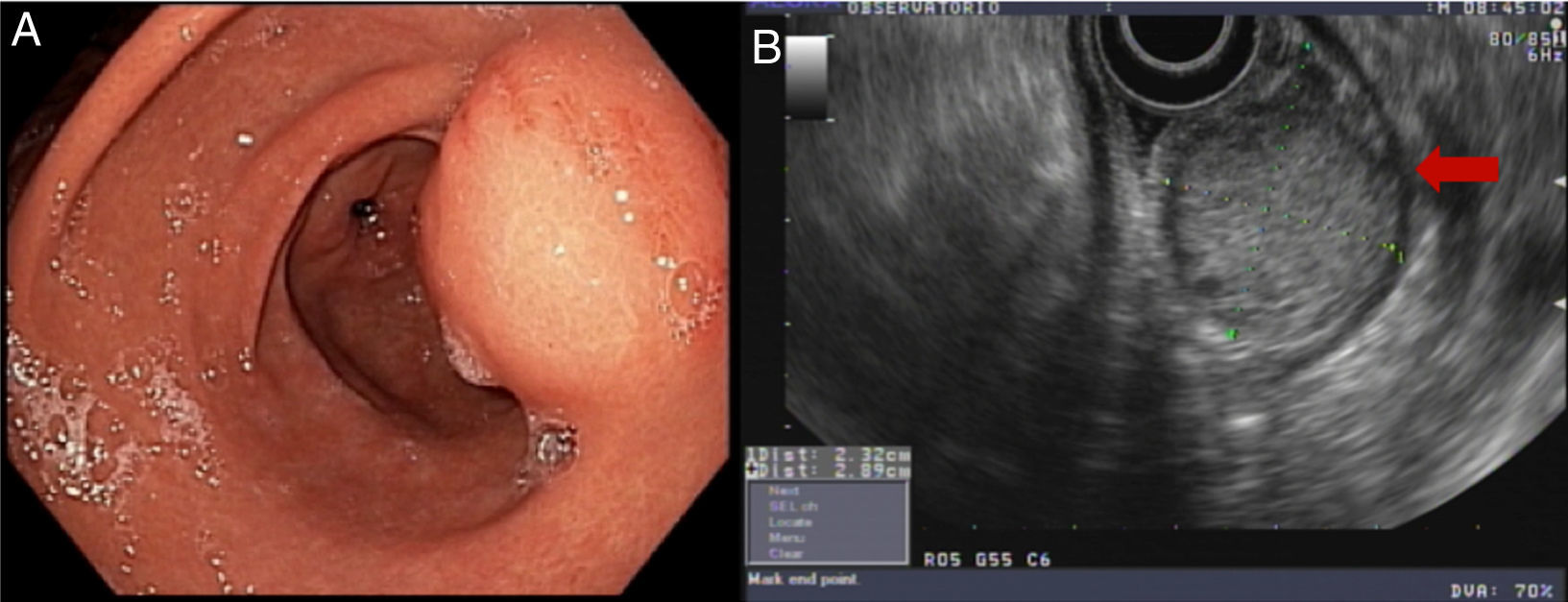
Endoscopy showed an ulcerated subepithelial tumor in the posterior wall of the gastric antrum. No abnormalities in the gastric mucosa were reported in the endoscopic biopsy. Endoscopic ultrasound (EUS) identified a 3 x 2.5cm nodular, hypoechogenic, heterogeneous tumor located in the muscular layer of the gastric wall, through Doppler imaging, with interior vascularity. The EUS data were consistent with a gastrointestinal stromal tumor (GIST) (fig. 1).
Computed axial tomography was performed and distant disease was ruled out. Based on these findings, the tumor was surgically resected.
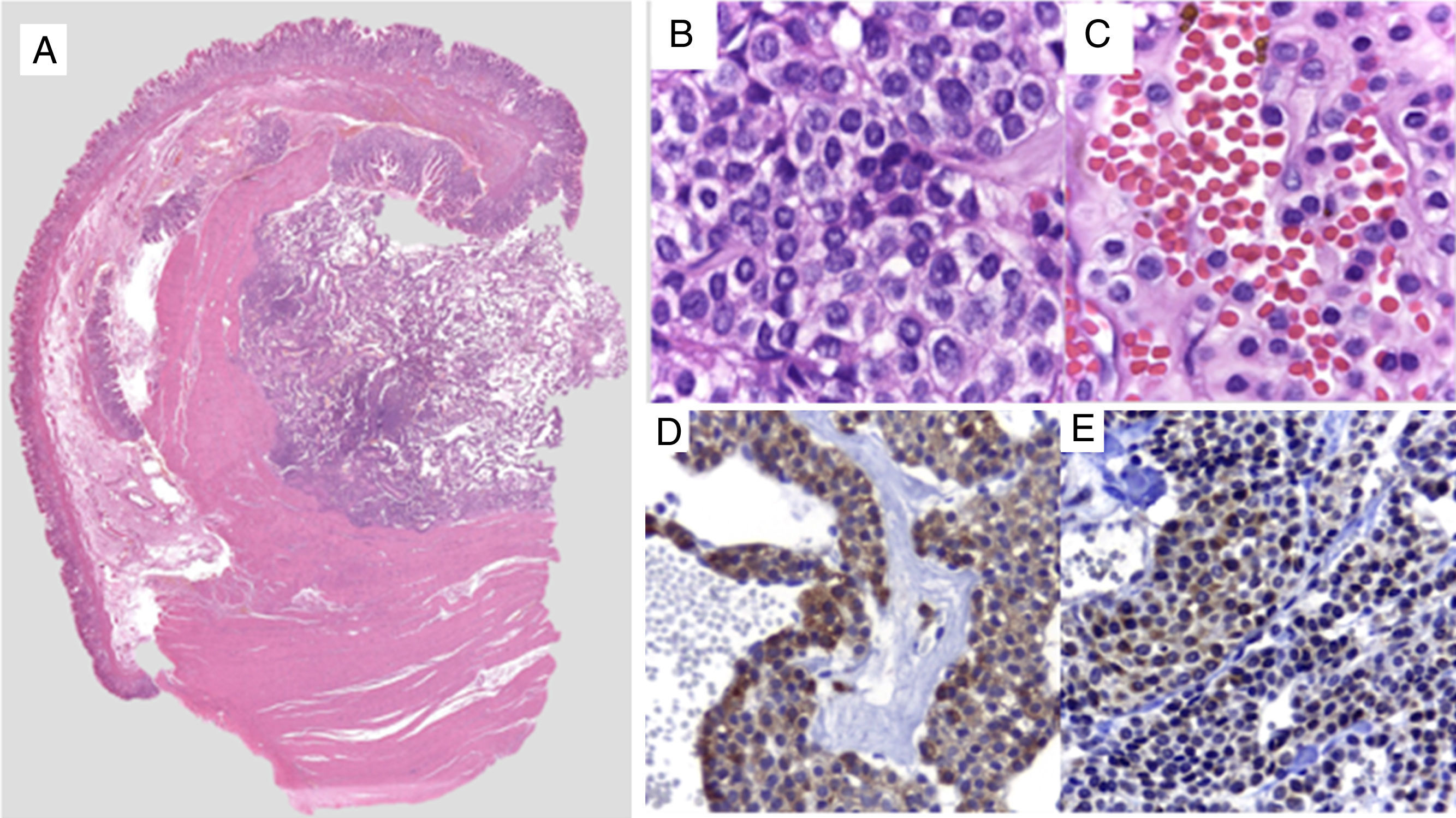
The macroscopic pathology study reported a 3.5 x 2.3cm nodular tumor between the serous and muscular layers, with focal ulceration of the mucosal surface. Microscopically, through hematoxylin/eosin staining, a proliferation of small-to-intermediate-sized round cells was observed, with central nuclei and dense granular chromatin. The immunohistochemical analysis (fig. 2) revealed positive staining for smooth muscle actin (SMA), intense h-Caldesmon, and focal expression of synaptophysin. Staining was negative for CD117, DOG1, CD56, PGP9.5, and chromogranin. Ki-67 was positive in 3% of the tumor cells. Because of the immunophenotype, the diagnosis was glomus tumor, ruling out epithelioid GIST and neuroendocrine tumor, both of which are histologic differential diagnoses of glomus tumor. The patient was released on the third day with no complications. At the follow-up 6 months after discharge, the patient was asymptomatic and only post-resection changes were observed at endoscopy, along with normal mucosa and no tumor recurrence.
A) Comprehensive image of the intramural lesion. B and C) Round, uniform cells with central oval nuclei, homogeneous granular chromatin, clearly delineated and eosinophilic small nucleolus of the cytoplasm. D) Immunohistochemistry positive for smooth muscle actin. E) Immunohistochemistry positive for synaptophysin in perivascular glomus cells.
Described in 1948 by De Busscher, glomus tumors were considered benign lesions,3 but today they are regarded as mesenchymal tumors with malignant potential.1
In 2001, Folpe et al. proposed the following criteria for suspecting malignancy: location of the tumor in the deep layers, size larger than 2cm, and histologically, atypical mitotic figures with a moderate-to-high nuclear grade, with more than 5 mitoses per 50 high power fields.4 If a gastric tumor is larger than 5cm, malignancy can be suspected.5
Preoperative diagnosis of glomus tumors is difficult because of their similarity to GISTs and neuroendocrine tumors in CAT, magnetic resonance (MR), and endoscopic ultrasound imaging studies.6 Radiologically, glomus tumors present as submucosal masses, with or without ulcers, and can contain small calcifications.1,7 Diagnosis based on EUS is presumptive and cannot replace histologic diagnosis.
An alternative that has been shown to be useful in the preoperative diagnosis of submucosal gastric tumors is EUS-guided fine needle aspiration biopsy.8 Given that in 52-93% of the cases it is possible to obtain a sufficient amount of tissue for staining tests, as well as for immunohistochemical analysis, this biopsy is considered essential for the preoperative diagnosis of gastric glomus tumors.6,9
Histopathologically, glomus tumor cells are small, uniform, round or polygonal, with very visible cell membranes.10 Diagnosing and differentiating this tumor from other gastric tumors can most certainly be based on immunohistochemical study. Glomus tumors are positive for smooth muscle actin (SMA), calponin, and h-Caldesmon and negative for desmin, and thus can be differentiated from GISTs.5
Optimum treatment is surgical resection, when there is a single tumor.1 In the case of our patient, the tumor was surgically resected. However, some cases can be managed through endoscopic submucosal resection.11,12
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Rosales-López E, Salceda-Otero JC, Angulo-Molina D, Posada-Torres JA, Canchola-Aguilar MG, Lozoya-Gonzalez D. Glomangioma gástrico, diagnóstico diferencial de tumores del estroma gastrointestinal. Revista de Gastroenterología de México. 2018;83:72–74.