A 72-year-old woman with no medication allergies had a past history of diabetes mellitus, hypercholesteremia, high blood pressure, a ventriculoperitoneal shunt due to subarachnoid hemorrhage after a ruptured aneurysm, and atrophied chronic gastritis. She received eradication therapy for positive Helicobacter pylori, has a history of iron-deficiency anemia of at least 4-year known progression that is treated empirically, and continues treatment with omeprazole, insulin, metformin, olmesartan, nebivolol, and oral iron.
She was admitted to our service for the first time due to symptoms of 2-week progression of 5-10 diarrheic, watery stools per day, with no blood, mucus, or pus, and no fever. Her chronic anemia worsened (hemoglobin 9.4mg/dl; usual values were around 11mg/dl). Upper and lower endoscopic study identified atrophied chronic gastritis with no other alterations. Biopsy was not taken. Stool cultures were negative. After metformin was suspended as a possible triggering factor and the corresponding insulin readjustment was made, the diarrhea disappeared and the patient was released.
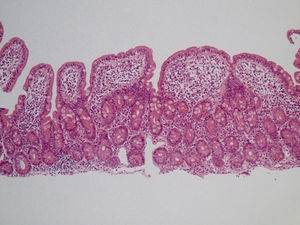
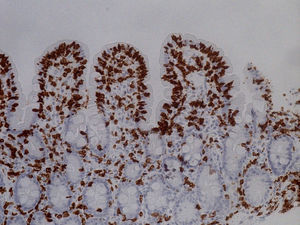
She was readmitted 2 months later because the diarrhea reappeared with similar characteristics. At physical examination, the patient had low blood pressure (89/56mmHg), was pale, and presented with oligoanuria, all in the context of hypovolemic shock secondary to gastrointestinal losses, requiring treatment in the intensive care unit. Laboratory work-up: urea 197mg/dl, creatinine 3.51mg/dl, sodium 150 mmol/l, and potassium 5.24 mmol/l; hemoglobin 9.8mg/dl, MCV 80 fl, and iron 15μg/dl. Once she was clinically stable, thorough testing was undertaken, with the following results: liver function, folic acid, vitamin B12, thyroid hormones, basal cortisol, serum proteinogram, glycosylated hemoglobin, and digestion in feces were all normal. Stool cultures, stool ova and parasite exam, Clostridium difficile toxin, ANA, ANCA, and anti-transglutaminase IgA antibody (0.30 U/ml [reference values < 7 U/ml]) tests were all negative. No significant alterations were observed in the chest x-ray, abdominopelvic CAT scan, or bowel transit. Endoscopic study was repeated with biopsy from the second portion of the duodenum and the bulb, where partial atrophy with a villous pattern (fig. 1) with increased intraepithelial lymphocytes (modified Marsh 3b)1 was observed (fig. 2).
With fluid and electrolyte resuscitation and a gluten-free diet, the laboratory test parameters normalized within 3 weeks and the number of stools decreased significantly and their consistency increased. Once the patient's blood pressure figures stabilized, the antihypertensive medication was reintroduced and the watery diarrhea reappeared. Under those circumstances, we interpreted the symptoms as sprue-like enteropathy, associated with olmesartan (which she had been taking for 3 years at a dose of 40mg daily). The olmesartan was suspended and substituted with verapamil. Six months after that suspension, the patient continues to be asymptomatic.
Olmesartan is an angiotensin receptor II selective blocker (ARB) that is commonly used to treat high blood pressure. Since July 2013, its label states sprue-like intestinal alterations as an adverse reaction to the medication.2 Increasingly more cases associated with its use are being described, but not with other ARBs.3 Olmesartan should be included in the differential diagnosis in cases of chronic diarrhea with repeatedly negative serologic celiac disease tests.4
The causal mechanism of this pathology is unknown,5 but it could be due to an immunologic lesion, with elevated CD8+ and IL15 overexpression on the part of epithelial cells.6 Villous atrophy and intraepithelial lymphocyte infiltration characteristic of celiac disease can be seen in other situations, such as bacterial overgrowth, Crohn's disease, intestinal lymphoma, or drug treatment,7 highlighting mofetil mycophenolate, azathioprine,8 and olmesartan, as was the case of our patient. Not only symptoms, but also histologic alterations, have been observed to remit with the suspension of the drug.9 Given our patient's good progression, intestinal biopsy has not been repeated. According to the Naranjo algorithm, causality was considered probable (7 points), and was reported to the Spanish Drug Surveillance Agency.
We therefore consider it essential to include this entity in the differential diagnosis of chronic diarrheic syndromes of unclear etiology, emphasizing the performance of a thorough anamnesis, including the detailed review of chronic drug treatments of patients, given that the associated complications can be potentially severe.
Financial disclosureNo financial support was received in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Solano-Iturri G, García-Jiménez N, Solano-Iturri JD, Blanco-Sampascual S. Enteropatía sprue-like asociada a olmesartán: causa emergente de diarrea crónica asociada a fármacos. Revista de Gastroenterología de México. 2018;83:71–72.