Neuroendocrine neoplasms are rare tumors accounting for approximately 2% of all neoplasms, most of which (62–70%) originate in the gastrointestinal tract and pancreas. Our primary aim was to describe the clinical presentation and pathologic features of these tumors, to improve our knowledge and early identification of them.
Materials and methodsA retrospective, cross-sectional, observational, and descriptive study was conducted on patients with a confirmed diagnosis of neuroendocrine neoplasm treated at the Hospital Ángeles del Pedregal within the time frame of 2018 and 2024. All cases diagnosed with neuroendocrine neoplasm of the pancreas or gastrointestinal tract in surgical specimens from the pathology service were included. The clinical, laboratory, and imaging data were obtained from the patients’ hospital charts.
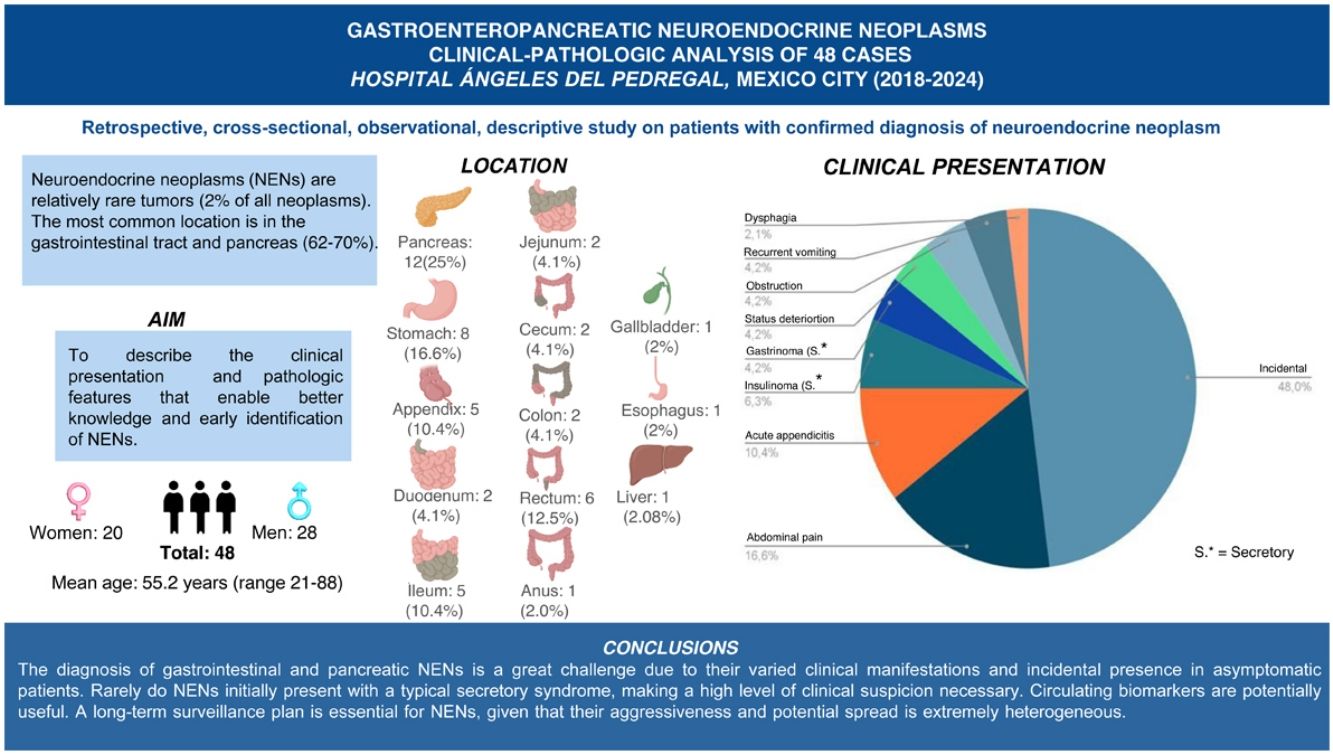
ResultsMean patient age was 55.2 years (range: 21–88 years). Of the 48 cases, 28 were men and 20 were women. The most frequent locations of the neuroendocrine neoplasms were the pancreas (25%), stomach (16.6%), rectum (12.5%), and appendix (10.4%). In 23/48 (47.9%) cases, the diagnosis of neuroendocrine neoplasm was incidental during surgery, endoscopy, or in imaging studies.
ConclusionsThe great variety of gastroenteropancreatic neuroendocrine neoplasms was described. Given that these tumors rarely produce a typical secretory syndrome, a high level of clinical suspicion is needed. Circulating biomarkers may be useful for their early diagnosis.
Las neoplasias neuroendocrinas (NNE) son tumores infrecuentes que representan aproximadamente el 2% de todas las neoplasias. La mayoría (62–70%) se originan en el tubo digestivo y páncreas. Nuestro principal objetivo es describir la presentación clínica, las manifestaciones y características patológicas que permitan un mejor conocimiento e identificación de estos tumores.
Materiales y métodosSe trata de un estudio retrospectivo, transversal, observacional y descriptivo de los pacientes con diagnóstico confirmado de neoplasia neuroendocrina que fueron atendidos en el Hospital Ángeles del Pedregal en el período comprendido entre los años 2018 y 2024. Se incluyeron en esta serie todos los casos con diagnóstico de neoplasia neuroendocrina de páncreas o tubo digestivo en piezas quirúrgicas del servicio de patología. Los datos clínicos, estudios de laboratorio e imagenología se obtuvieron de los expedientes hospitalarios.
ResultadosLa edad media de los pacientes fue de 55.2 años (rango 21 a 88 años). De los 48 casos, 28 son del género masculino y 20 del femenino. Las localizaciones más frecuentes de las neoplasias neuroendocrinas fueron: páncreas (25%), estómago (16.6%), recto (12.5%) y apéndice (10.4%). En 23/48 (47.9%) casos, el diagnóstico de NNE fue incidental en cirugía, endoscopía o estudios de imagenología.
ConclusionesSe describe la gran variedad de presentaciones clínicas de las neoplasias neuroendocrinas de páncreas y tubo digestivo. Dado que estos tumores rara vez presentan un síndrome secretor típico, es necesario un alto índice de sospecha clínica, siendo potencialmente útiles los biomarcadores circulantes.
Neuroendocrine neoplasms (NENs) are relatively rare tumors, given that they account for approximately 2% of all neoplasms.1 Their annual incidence is 5.25 per 100,000 inhabitants, and most cases (62–70%) originate in the gastrointestinal tract and pancreas.2,3 In recent years, the incidence of NENs has been on the rise due to greater knowledge about them, as well as to the advent of new technologies that facilitate their diagnosis.4
NENs originate in cells with a neural/endocrine hybrid phenotype. Their neural component characteristics include the presence of dense-core granules, similar to those of serotonergic cells that store monoamines, but they lack synapses. Because of their endocrine nature, these cells are capable of synthesizing amines, peptides, and hormones and secreting them into the circulation, generating metabolic effects and systemic clinical manifestations.1,5–7
The neuroendocrine cells may agglomerate at specific anatomic sites, such as the pancreatic islets of Langerhans, or scatter throughout the gastrointestinal tract, lung, thyroid, and pituitary gland, among other organs. The neuroendocrine cells make up the so-called diffuse endocrine system or amine precursor uptake and decarboxylation (APUD) system and are in charge of relevant physiologic functions.5
The clinical presentation of NENs depends on their anatomic location and capacity to secrete amines or biologically active peptides. Patients may present with a specific syndrome, such as fasting hypoglycemia due to insulinoma, recurrent peptic ulcer, atypical location of tumors that secrete gastrin (Zollinger-Ellison syndrome), the development of diabetes mellitus, migratory necrolytic erythema associated with glucagon-producing tumors, and “carcinoid syndrome” characterized by chronic diarrhea, abdominal discomfort, fecal urgency, flushing, hypotension, and bronchospasm associated with the episodic release of serotonin.4,6,8 Chronic diarrhea may be diagnosed when stool weight is above 240 g per day, with at least 3 daily bowel movements for more than 4 weeks.9 However, a large percentage of NENs are not secretory, and so are discovered incidentally during surgery, endoscopy, or in imaging studies due to the presence of metastasis, bowel obstruction, or in appendectomy specimens.10
Many NENs are indolent and produce nonspecific symptoms, and as a result, are confused with disorders of gut-brain interaction (DGBIs) (irritable colon, spastic colon, functional gastrointestinal disorders), considerably delaying diagnosis. Between 21 and 69% of cases are detected in advanced stages, presenting with metastatic disease, worsening prognosis and reducing the life expectancy of the affected patients.5,10
Chromogranin A, gastrin, and serotonin blood tests are used as markers for neuroendocrine cells that can identify NENs, enabling early diagnosis. The most widely used immunohistochemical markers for identifying neuroendocrine tumor (NET) differentiation are chromogranin A and synaptophysin.10,11
The present work is a detailed review of 48 cases of NENs diagnosed at the Hospital Ángeles del Pedregal (HAP) within the time frame of 2018 and 2024. Our review is limited to gastroenteropancreatic tumors that correspond to the most frequent locations of said tumors and are a greater diagnostic challenge. All the cases were confirmed by the pathology department of the HAP, and most were complemented with immunohistochemistry studies. The data from patient hospital charts were carefully analyzed and additional information from the attending physicians was collected to clarify the clinical characteristics of the patients and their progression.
The results of our case series were compared with those in national and international publications. We believe it is very important to know the clinical-pathologic characteristics of NENs to improve the diagnostic process, having greater suspicion of them for early diagnosis, and consequently achieving better therapeutic results.
The aims of our study were to know the incidence of gastroenteropancreatic NETs at the HAP over a 6-year period, compare the epidemiologic data of our hospital with those reported in Mexico and internationally, describe the clinical presentation of the gastroenteropancreatic NENs and the probability of their metastatic spread, and review the auxiliary diagnostic methods for diagnosing said tumors at the HAP.
Materials and methodsThe protocol planning began in November 2023, together with the bibliographic review of the most recent articles on NETs. Once we had the approval of the ethics committee (January 2024), we began to collect the clinical and pathologic information, and in April 2024, started the analyses of the data collected.
Our case series is a retrospective, cross-sectional, observational, descriptive study on patients with a confirmed diagnosis of NEN, treated at the HAP within the time frame of 2018 and 2024. The study was structured according to the strengthening the reporting of observational studies in epidemiology (STROBE) checklist. Given the cross-sectional and observational nature of our analysis, defining outcomes, predictors, potential confounders, or effect modifiers was not necessary. The diagnostic criteria were those following the World Health Organization (WHO) norms that establish both the diagnostic and classification criteria of NENs.
The database of the pathology service of the HAP covering the period from 2018 to 2024 was reviewed. Forty-eight cases with a histopathologic diagnosis of a NET of the pancreas, liver, or any segment of the gastrointestinal tract were included in the study. To establish a clinical-pathologic correlation, only cases treated at the HAP were included that had clinical and laboratory information, as well as an available hospital chart with the NET diagnosis confirmed by immunohistochemistry or histopathology report.
Given the low incidence of NETs, the decision was made to exclude those outside of the gastrointestinal tract, liver, or pancreas, from our analysis. The data are presented through simple descriptive statistics, like those utilized in similar studies found in the literature.
Statistical analysisSimple descriptive statistics were exclusively utilized for the data analysis, including absolute and relative frequencies, means, and percentages. Standard deviation estimates tend to be inexact or susceptible to random variation when the sample size is reduced. Given that ours was not a case-control comparative study, tests for evaluating statistical differences between the groups studied were not necessary. We utilized descriptive methods, similar to those used in other published studies on NENs in the national and international literature.
Ethical considerationsThe present work meets the specifications of the bioethical research norms. To conduct the study, documents guaranteeing absolute confidentiality of the clinical and personal data of the patients and the identity of the attending physicians were signed and the study was approved by the research and ethics committees of the HAP (registration number 2711, January 5, 2024). The authors had no contact with the patients; only the pathology specimens were studied and the hospital charts reviewed to evaluate the clinical data in a general and anonymous manner. Thus, the authors declare that this manuscript contains no information that could undermine the privacy of the patients or identify the patients or attending physicians.
A bibliographic search was conducted spanning 12 years on the PubMed®, UpToDate®, and ClinicalKey® search engines, utilizing the following keywords: neuroendocrine neoplasm, gastroenteropancreatic tumor, synaptophysin, chromogranin, epidemiology, and treatment of NENs (Mexican and international data).
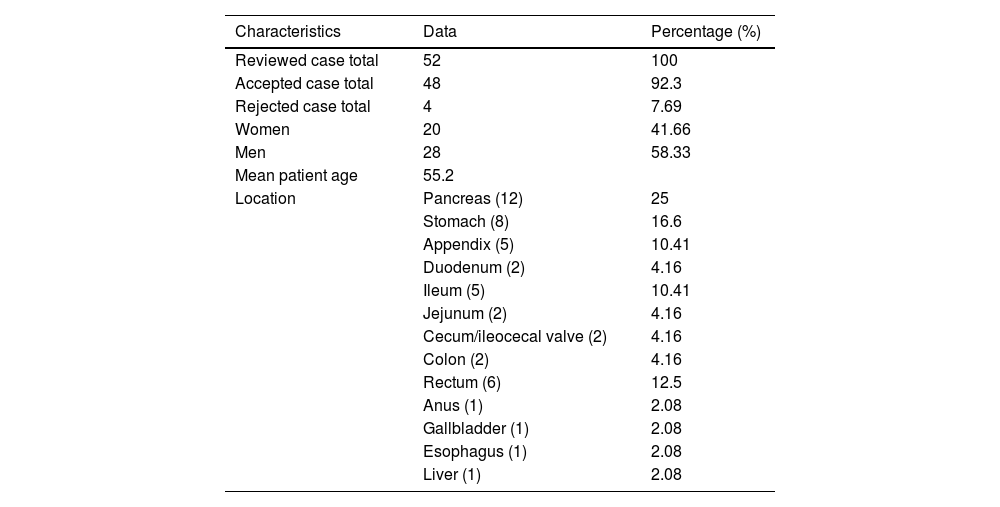
ResultsDemographicsFifty-two cases diagnosed with NENs of the pancreas, liver, and gastrointestinal tract, treated at the HAP within the time frame of 2018 and 2024, were identified. Of those cases, 4 were eliminated due to insufficient clinical-pathologic information. The 48 remaining cases were included in the present case series. Mean patient age was 55.2 years (range: 21−88 years). Twenty-eight of the 48 cases were men and 20 were women (Table 1).
Demographic data and tumor location.
| Characteristics | Data | Percentage (%) |
|---|---|---|
| Reviewed case total | 52 | 100 |
| Accepted case total | 48 | 92.3 |
| Rejected case total | 4 | 7.69 |
| Women | 20 | 41.66 |
| Men | 28 | 58.33 |
| Mean patient age | 55.2 | |
| Location | Pancreas (12) | 25 |
| Stomach (8) | 16.6 | |
| Appendix (5) | 10.41 | |
| Duodenum (2) | 4.16 | |
| Ileum (5) | 10.41 | |
| Jejunum (2) | 4.16 | |
| Cecum/ileocecal valve (2) | 4.16 | |
| Colon (2) | 4.16 | |
| Rectum (6) | 12.5 | |
| Anus (1) | 2.08 | |
| Gallbladder (1) | 2.08 | |
| Esophagus (1) | 2.08 | |
| Liver (1) | 2.08 |
The most frequent locations of the NENs analyzed were the pancreas (25%), stomach (16.6%), rectum (12.5%), and appendix (10.4%). Other less frequent locations were the ileum (6.25%), cecum and ileocecal valve (4.1%), jejunum (4.1%), and colon (4.1%). We found only one case of NEN located in the anus (2.0%), one in the gallbladder (2.0%), and one in the esophagus (2.0%) (Table 1).
All the cases of NENs located in the liver corresponded to metastatic disease, with the primary tumor in the pancreas or gastrointestinal tract.
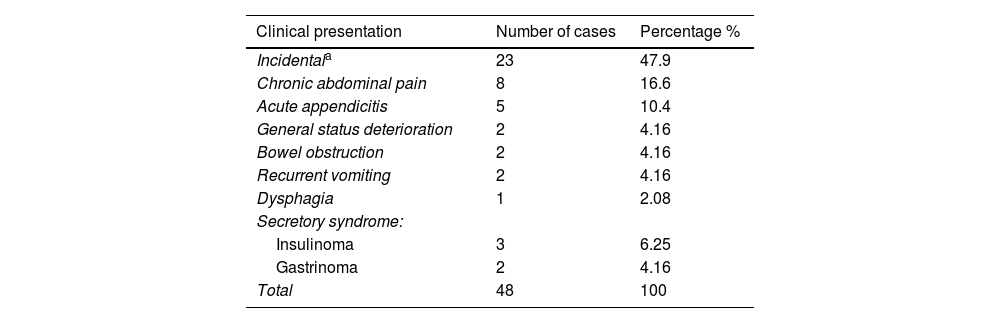
Clinical presentationSecretory syndromeOf the NENs of the pancreas, 2 were associated with an organic hypoglycemic syndrome related to hyperinsulinemia and were diagnosed as insulinomas. In both cases, the clinical presentation was typical of those tumors, with severe hypoglycemia and fasting neuroglycopenia. In one additional case, the immunohistochemical stains were positive for insulin, but there was no association with a clinical syndrome of hypoglycemia and hyperinsulinemia. Two of the cases of NENs were associated with hypergastrinemia, one corresponded to a gastrinoma, with Zollinger-Ellison syndrome and severe ulcer disease that was recurrent and resistant to medical treatment, with gastrointestinal bleeding. Another patient had positive immunohistochemical stains for gastrin in a pancreatic NET with a clinical ulcerous syndrome of short progression (Table 2).
Clinical presentation of the neuroendocrine neoplasms.
| Clinical presentation | Number of cases | Percentage % |
|---|---|---|
| Incidentala | 23 | 47.9 |
| Chronic abdominal pain | 8 | 16.6 |
| Acute appendicitis | 5 | 10.4 |
| General status deterioration | 2 | 4.16 |
| Bowel obstruction | 2 | 4.16 |
| Recurrent vomiting | 2 | 4.16 |
| Dysphagia | 1 | 2.08 |
| Secretory syndrome: | ||
| Insulinoma | 3 | 6.25 |
| Gastrinoma | 2 | 4.16 |
| Total | 48 | 100 |
The rest of the cases (43/48 or 89.5%) did not present with clinical syndromes associated with the release of biologically active substances with systemic effects.
Chronic abdominal painA frequently found clinical presentation (8 cases or 16.6%) of the NENs was chronic abdominal pain of poorly defined characteristics, mainly located in the epi-mesogastrium and upper abdomen. The habitual history was poorly systematized, persistent, colicky abdominal pain of several months of progression, almost always treated by various physicians who had attributed the symptoms to DGBIs, gastrointestinal infections, or other diagnoses.
Acute appendicitisNENs may present as acute abdomen with typical signs of appendicitis. However, the presence of an appendicular neoplasm of neuroendocrine origin is revealed in the surgical specimen, as occurred in 5 of the 48 patients of our case series (12.5%). The 5 cases corresponded to 4 men and one woman, with an age range of 21–57 years.
Bowel obstructionTwo patients (4.16%) with a NEN of the small bowel sought medical attention due to mechanical bowel obstruction that caused pain, nausea, vomiting, and dehydration, requiring emergency surgical intervention. An additional patient with a small bowel NEN presented with pain and clinical signs of bowel subocclusion, who also required surgical treatment.
General status deteriorationOnly 2/48 patients (4.16%) presented with weight loss, fatigue, asthenia, and adynamia. Said symptoms led the attending physicians to order imaging and/or endoscopic studies, with which they found a NEN in the stomach of one of the patients and in the pancreas of the other case.
Incidental findingSymptoms alerting to the possible presence of an occult neoplasm were not identified in nearly half of the cases of NENs (23/48 or 47.9%), and said tumors were discovered incidentally in routine check-ups, imaging studies carried out for other diseases, from a family history of malignant tumors, in gastrointestinal endoscopies, and in surgical procedures unrelated to NENs.
Laboratory and imaging characteristicsCirculating markersCirculating biomarker measurement in the patients with NENs was rarely ordered, except in the cases with secretory syndrome, including the 2 cases of insulinoma and the 2 of gastrinoma. Chromogranin A and serotonin levels were not utilized for diagnostic purposes in the present case series, which is not surprising, given that a high number of cases were discovered incidentally. However, NEN biomarkers (chromogranin A and serotonin) were utilized for therapeutic monitoring in the majority of patients.
Imaging studiesThe most widely used methods for diagnosing NEN were abdominal computed tomography (CT) in 14 cases (29%), endoscopy in 8 cases (16.6%), abdominal ultrasound in 4 cases (8.3%), and positron emission tomography (PET)-CT in 4 cases (8.33%).
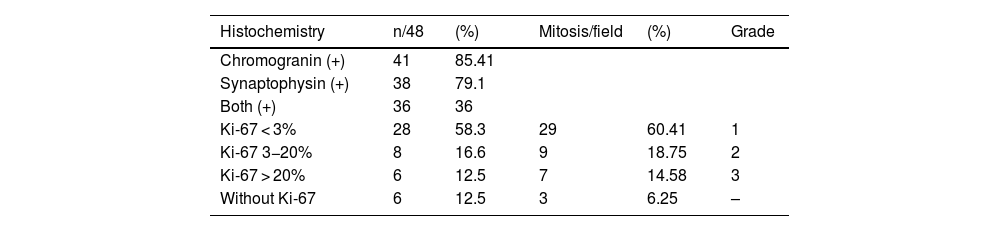
Histopathologic and immunochemical characteristicsTumors were positive for chromogranin in 41/48 (85.4%) of our patients, for synaptophysin in 38/48 (79.1%), and for both biomarkers in 36/48 (75%). A total of 19/48 patients (39.5%) had tumors that were positive for CD56 (Table 3).
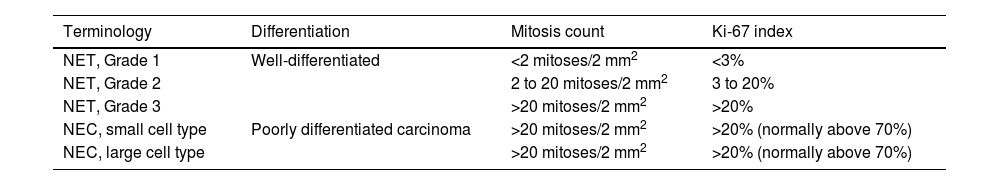
To evaluate the histopathologic grade, we used the 2022 WHO classification (Table 4).12,13 The cell replication immunohistochemical biomarker, Ki-67, was utilized, and in the cases in which it was not used, the tumors were classified based on the number of mitoses per high power field (×40). In 28/48 cases, Ki-67 was lower than 3% and in one additional patient the number of mitoses per field was below 2/mm2; those 29 cases (60.4%) were classified as grade 1 according to the WHO classification, corresponding to well-differentiated NENs, with a low cell replication rate, indicating good prognosis. In 8/48 patients, Ki-67 showed a 3–20% cell replication rate, with an additional patient with a mitosis percentage in the same range; those 9 patients (18.7%) were classified as grade 2, according to the WHO classification. In 6/48 patients, Ki-67 was above 20% and in an additional case the number of mitoses was above 20/mm2; those 7 cases (14.5%) were classified as grade 3. In that subgroup, the replication rate was high, generally indicating poor prognosis and aggressive biologic behavior of the neoplasm.
Classification and staging criteria for gastrointestinal and hepatopancreatic neuroendocrine neoplasms. WHO 2022.
| Terminology | Differentiation | Mitosis count | Ki-67 index |
|---|---|---|---|
| NET, Grade 1 | Well-differentiated | <2 mitoses/2 mm2 | <3% |
| NET, Grade 2 | 2 to 20 mitoses/2 mm2 | 3 to 20% | |
| NET, Grade 3 | >20 mitoses/2 mm2 | >20% | |
| NEC, small cell type | Poorly differentiated carcinoma | >20 mitoses/2 mm2 | >20% (normally above 70%) |
| NEC, large cell type | >20 mitoses/2 mm2 | >20% (normally above 70%) |
NEC: neuroendocrine carcinoma; NET: neuroendocrine tumor.
Previously, grade 3 tumors and poorly differentiated tumors were included in the diagnosis of neuroendocrine carcinoma (NEC). However, since 2019, there was a modification in the WHO classification because it was demonstrated that NETs were not genetically related to NECs. Therefore, it is important to differentiate between grade 3 tumors and NECs, given that they are molecularly different entities.14,15 NEC morphology suggests greater aggressiveness, a high infiltrative capacity, a very large number of mitoses, and a lower grade of cell differentiation. In our review, we decided to group the grade 3 NETs and NECs together because only 2 cases of that type of aggressive tumor were detected after the 2022 WHO classification was published.
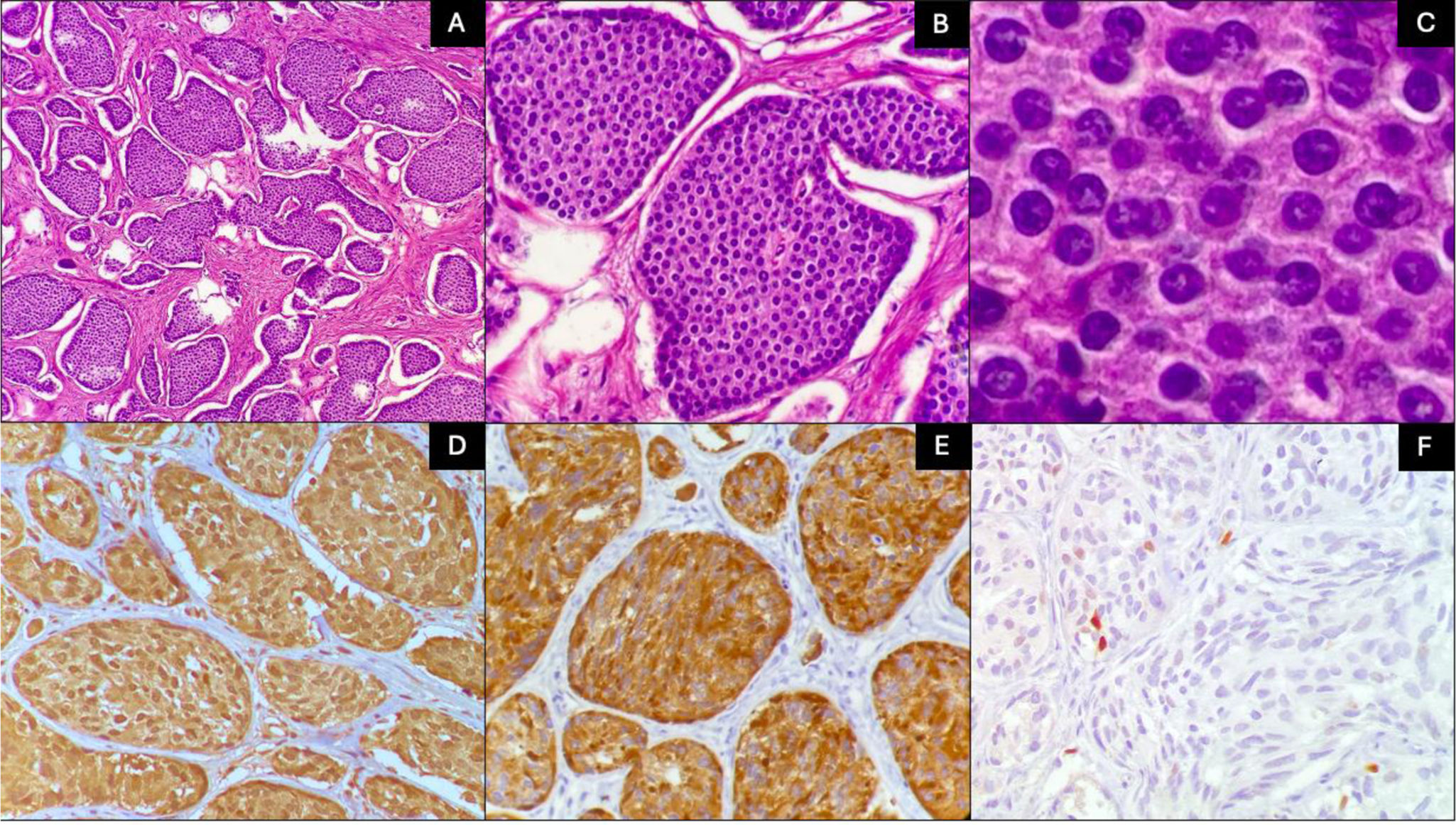
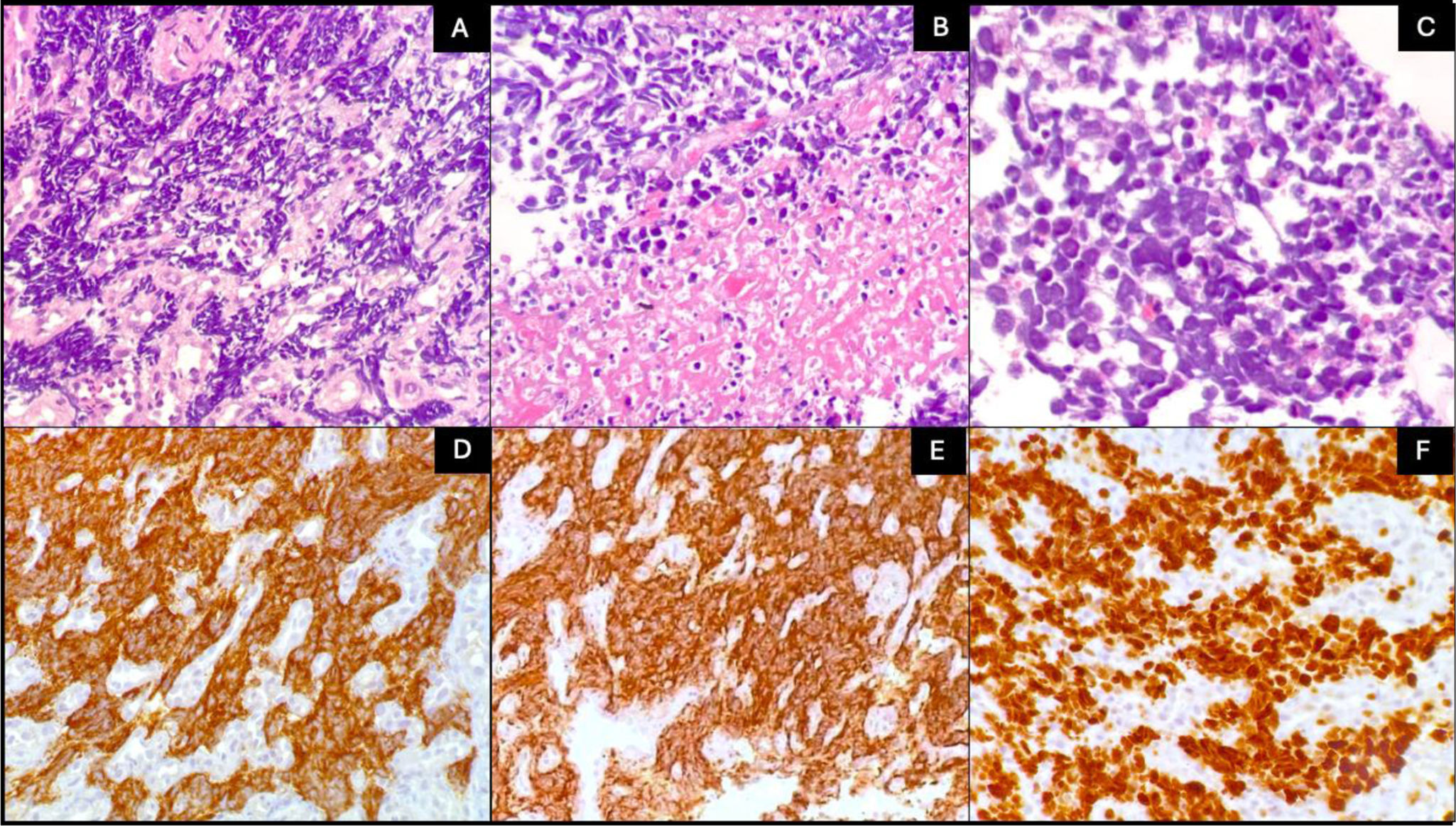
Fig. 1 shows the typical images of 2 NENs of our series corresponding to grade 1 disease, usually associated with good prognosis. The immunohistochemical markers (chromogranin and synaptophysin) confirmed the neuroendocrine lineage of the tumor cells. Fig. 2 shows the histopathologic findings of a NEC, in which the invasive tendency and large number of mitoses confirmed by Ki-67 can be seen. There was positivity for chromogranin and synaptophysin.
Histologic characteristics of 2 grade 1 neuroendocrine tumors. A) The tumor is arranged in a nest pattern (H&E ×10). B) Monotonous cells with no mitosis (H&E ×40). C) Nuclei show coarse granular chromatin and a moderate quantity of eosinophilic cytoplasm (H&E ×100). D) Chromogranin positivity. E) Synaptophysin positivity. F) Ki-67 quantified at 2%.
Histopathologic characteristics of a neuroendocrine carcinoma. A) The tumor presents an infiltrating pattern (H&E ×10). B) The presence of tumor necrosis (H&E ×20). C) Pleomorphic cells with hyperchromatic nuclei, scant cytoplasm (H&E ×40). D) Chromogranin positivity. E) Synaptophysin positivity. F) Ki-67 quantified at 90%.
The clinical-pathologic data of 48 cases diagnosed with gastroenteropancreatic NENs, treated at the HAP in Mexico City within the time frame of 2018 and 2024, are presented. This is the first case series on NENs describing patients from a private hospital in Mexico.
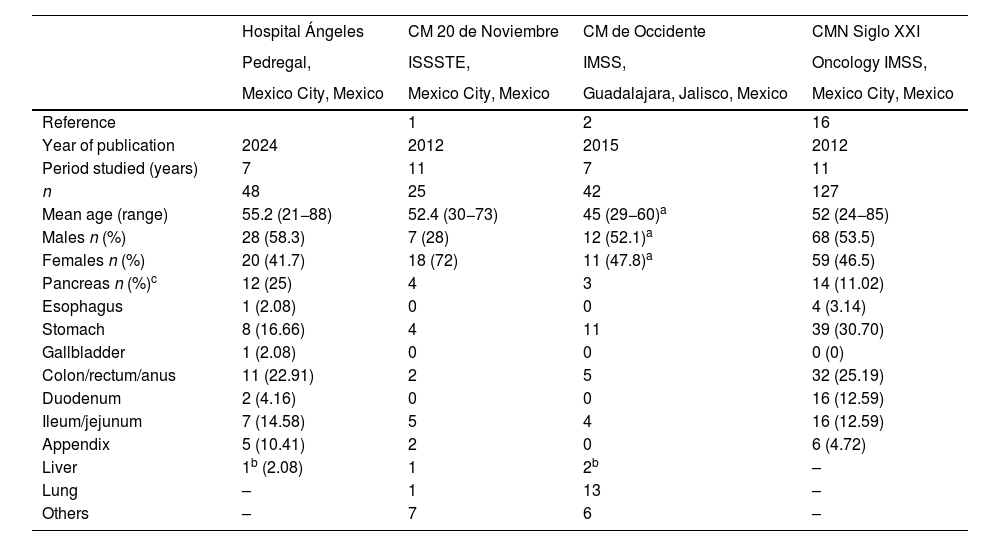
Our retrospective design was similar to that used in 3 other Mexican studies conducted in Mexico City1,16 and Guadalajara, Jalisco.2 The cases were identified through the diagnoses of NENs made from surgical specimens received at the anatomic pathology service. The large majority of cases were classified by histopathologic grade, according to the Ki-67-based cell replication rate (42/48 or 87.5%) or the number of mitoses per field, and were confirmed by immunohistochemical markers (75%).
Table 5 shows a comparison of the data obtained in the present study with those of other Mexican studies,1,2,16 revealing great demographic similarity. The median patient age was between the fifth and sixth decades of life, with a wide range of presentation ages and no clear predominance of sex. The most frequent sites of tumor origin were the stomach and pancreas. The NENs were not frequently associated with hormone or peptide secretory syndromes and most patients had a history of long-term abdominal pain, gastrointestinal bleeding, or recurrent ulcer disease.
Comparison with studies published in Mexico.
| Hospital Ángeles | CM 20 de Noviembre | CM de Occidente | CMN Siglo XXI | |
|---|---|---|---|---|
| Pedregal, | ISSSTE, | IMSS, | Oncology IMSS, | |
| Mexico City, Mexico | Mexico City, Mexico | Guadalajara, Jalisco, Mexico | Mexico City, Mexico | |
| Reference | 1 | 2 | 16 | |
| Year of publication | 2024 | 2012 | 2015 | 2012 |
| Period studied (years) | 7 | 11 | 7 | 11 |
| n | 48 | 25 | 42 | 127 |
| Mean age (range) | 55.2 (21−88) | 52.4 (30−73) | 45 (29−60)a | 52 (24−85) |
| Males n (%) | 28 (58.3) | 7 (28) | 12 (52.1)a | 68 (53.5) |
| Females n (%) | 20 (41.7) | 18 (72) | 11 (47.8)a | 59 (46.5) |
| Pancreas n (%)c | 12 (25) | 4 | 3 | 14 (11.02) |
| Esophagus | 1 (2.08) | 0 | 0 | 4 (3.14) |
| Stomach | 8 (16.66) | 4 | 11 | 39 (30.70) |
| Gallbladder | 1 (2.08) | 0 | 0 | 0 (0) |
| Colon/rectum/anus | 11 (22.91) | 2 | 5 | 32 (25.19) |
| Duodenum | 2 (4.16) | 0 | 0 | 16 (12.59) |
| Ileum/jejunum | 7 (14.58) | 5 | 4 | 16 (12.59) |
| Appendix | 5 (10.41) | 2 | 0 | 6 (4.72) |
| Liver | 1b (2.08) | 1 | 2b | – |
| Lung | – | 1 | 13 | – |
| Others | – | 7 | 6 | – |
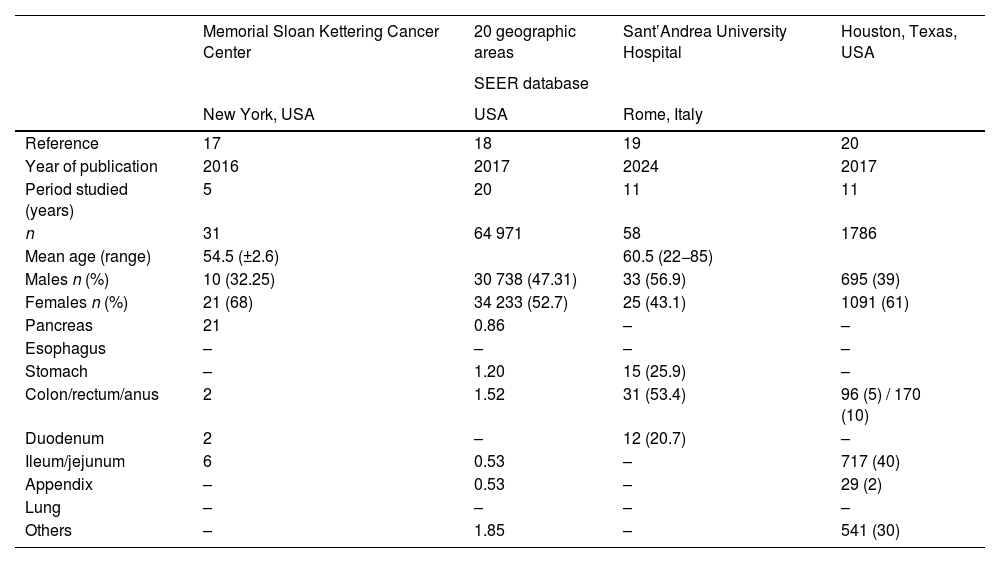
Table 6 shows the main case series of NENs published internationally. Once again, the data were similar to ours. The largest study is a multicenter case series conducted in 20 states of the United States.17–20 Our data coincide in presentation age, the incidence of affected organs, and clinical presentation.
Main international studies on neuroendocrine neoplasms.
| Memorial Sloan Kettering Cancer Center | 20 geographic areas | Sant’Andrea University Hospital | Houston, Texas, USA | |
|---|---|---|---|---|
| SEER database | ||||
| New York, USA | USA | Rome, Italy | ||
| Reference | 17 | 18 | 19 | 20 |
| Year of publication | 2016 | 2017 | 2024 | 2017 |
| Period studied (years) | 5 | 20 | 11 | 11 |
| n | 31 | 64 971 | 58 | 1786 |
| Mean age (range) | 54.5 (±2.6) | 60.5 (22−85) | ||
| Males n (%) | 10 (32.25) | 30 738 (47.31) | 33 (56.9) | 695 (39) |
| Females n (%) | 21 (68) | 34 233 (52.7) | 25 (43.1) | 1091 (61) |
| Pancreas | 21 | 0.86 | – | – |
| Esophagus | – | – | – | – |
| Stomach | – | 1.20 | 15 (25.9) | – |
| Colon/rectum/anus | 2 | 1.52 | 31 (53.4) | 96 (5) / 170 (10) |
| Duodenum | 2 | – | 12 (20.7) | – |
| Ileum/jejunum | 6 | 0.53 | – | 717 (40) |
| Appendix | – | 0.53 | – | 29 (2) |
| Lung | – | – | – | – |
| Others | – | 1.85 | – | 541 (30) |
NENs are relatively rare, compared with other types of malignant neoplasms. Nevertheless, their incidence appears to have risen, perhaps due to improved diagnostic methods through circulating markers, imaging studies, and endoscopy. Endoscopy enables the detection of localized lesions, and CT and magnetic resonance imaging have similar diagnostic yields, albeit tomography is more efficacious in NETs of the small bowel. Endoscopic ultrasound is a very useful technique, given that it can diagnose up to 26% of pancreatic NETs at early stages, whereas CT or other radiologic alternatives are less efficacious.21
There is no doubt that NENs are a diagnostic challenge because only a small percentage of them (10.4% in our series) present with a clinical syndrome associated with the hypersecretion of hormones or vasoactive substances. In our study, we found 3 cases of insulinoma, 2 of which had severe hypoglycemia and neuroglycopenia. One case expressed immunohistochemical markers corresponding to insulinoma, but the patient had not presented with significant episodes of hypoglycemia. Two of our cases had gastrinomas with recurrent ulcers in the gastrointestinal tract. The remaining patients (89.5%) had no specific associated symptoms. Only 5/48 patients (10.4%) presented with specific syndromes that could be clinically related to the presence of a NEN. None of our cases presented with clinical or laboratory data consistent with carcinoid syndrome, such as diarrhea, flushing, or bronchospasm. Carcinoid syndrome is more frequent in NENs of the respiratory tract, which were excluded from our study. In gastrointestinal NENs, carcinoid syndrome is present only in patients with extensive liver metastases capable of releasing serotonin directly into the systemic circulation, preventing its inactivation during its passage through the liver by the monoamine oxidase enzyme.
One of the relatively frequent (16%) clinical presentations in our series was upper abdominal pain, with a progression of several months, misdiagnosed and treated as a DGBI. Importantly, the possibility of NEN should be considered in patients with nonspecific abdominal pain. In that sense, ordering chromogranin A and serotonin (or its urinary metabolite, 5-hydroxyindolacetic acid) tests could be useful, given that elevated levels of those markers in blood could facilitate the early diagnosis of a NEN.
Several of our patients were diagnosed during surgery for acute abdomen; 2 with bowel obstruction due to the mechanical effect of the tumor and 5 were associated with acute appendicitis. Cases of NEN-associated acute appendicitis cover a wide range of ages, and so there are no data for suspecting the neoplasm in those patients. Because they are malignant tumors, once the abdominal emergency is resolved, the patient should receive postoperative surveillance and treatment of possible tumor-related complications. When the tumor in the appendicular area is smaller than 1 cm, resection of colonic segments is not recommended, but in tumors larger than 2 cm, hemicolectomy of the ascending colon may be considered.22
One-third of our cases (16/48) were detected incidentally in endoscopy or imaging studies, signifying that measuring circulating markers for NENs could be of great utility in cases that are difficult to diagnose.23
It is important to define the histopathologic grade of each case of NEN, according to the WHO classification, because tumor grade is correlated with prognosis. NENs should also be classified according to cell differentiation grade, cell proliferation index and/or the number of mitoses per high power field. In cases of grade 3 disease, the well-differentiated tumors are called NETs. Poorly differentiated tumors are called neuroendocrine carcinomas (NECs) and they have a worse prognosis. NECs are divided into small cell or large cell tumors. Not only do the subclassifications have prognostic implications, but they are also useful for selecting specific chemotherapeutic agents.
Even though the majority of NENs are sporadic, a small percentage are associated with type I and II multiple endocrine neoplasms that possibly have a hereditary transmission pattern.
Cases of high-grade NENs (poorly differentiated) have poor prognosis. According to international case series, survival is 16.2 years for grade 1, 8.3 years for grade 2, and 10 months for grade 3.18 However, long-term clinical follow-up is necessary in all cases because even well-differentiated and low-grade NENs occasionally have aggressive biologic behavior and can produce distant metastases. High-grade tumors require systemic management with combined chemotherapeutic agents (etoposide/cis-platin). Sunitinib and everolimus are also being used.23–25 In 2018, therapy with radionuclides targeted at peptide receptors was approved for gastroenteropancreatic tumors that express somatostatin receptors. Receptor expression can be demonstrated through PET before the patient receives therapy, especially in grade 1 and 2 tumors.7,26
Well-differentiated NENs generally respond well to treatment with somatostatin analogues, with favorable effects on symptomatology and possible antiproliferative effects,27 but one of their main adverse effects is uncontrollable diarrhea due to the suppression of the release of hormones and the biologically active substances that inhibit pancreatic enzyme secretion and gastrointestinal motility.9 Resection is indicated for NENs, whenever possible, and radiofrequency ablation and transarterial chemoembolization may also be used for liver metastases. In cases that are surgically unresectable, platin-derived chemotherapy is usually indicated,28 especially in grade 2 or 3 tumors.18,25
Serum chromogranin A measurement is useful for monitoring progression, for prognosis, and for treatment response. Its utility in immunohistochemistry lies in its capacity to evaluate NEN extension and differentiation.
Other markers, such as secreted phosphoprotein 1 (SPP1), have been shown to be efficient for NEN diagnosis, given that they are more sensitive than even chromogranin A, even though chromogranin A has better correlation with tumor aggressiveness.21,29,30 Immunohistochemical markers, such as INSM1, have recently acquired importance; INSM1 may be more specific than conventional markers.31
One of the new genetic tests is the NETest. It is a “liquid biopsy” for NETs that improves molecular diagnostic accuracy by assessing the expression of certain genes, such as Ki-67, SSTR1, and SSTR2, in blood, through reverse transcription polymerase chain reaction.21
ConclusionsThe diagnosis of gastroenteropancreatic NENs was made in 48 patients treated at the HAP in Mexico City over the 6-year study period. Our cases showed very similar demographic data and clinical presentations to those published nationally and internationally. The diagnosis of NENs is a great challenge for the clinician, due to the variety of their clinical manifestations, as well as to their incidental presence in asymptomatic patients. The use of biomarkers may facilitate early diagnosis and should be ordered in patients with a wide range of chronic issues, such as abdominal pain, diarrhea, gastrointestinal bleeding, bowel occlusion or subocclusion, and weight loss. The review of the hospital charts of our patients showed that circulating biomarker measurement was hardly ever ordered before the histopathologic diagnosis; said quantifications could also be very useful in the postoperative surveillance of patients. New circulating biomarkers for improving the diagnostic process in NENs are being developed, based on the genetic expression of the tumor cells (liquid biopsy).
Financial disclosureNo financial support was received in relation to this study/article.
The authors declare that there is no conflict of interest.