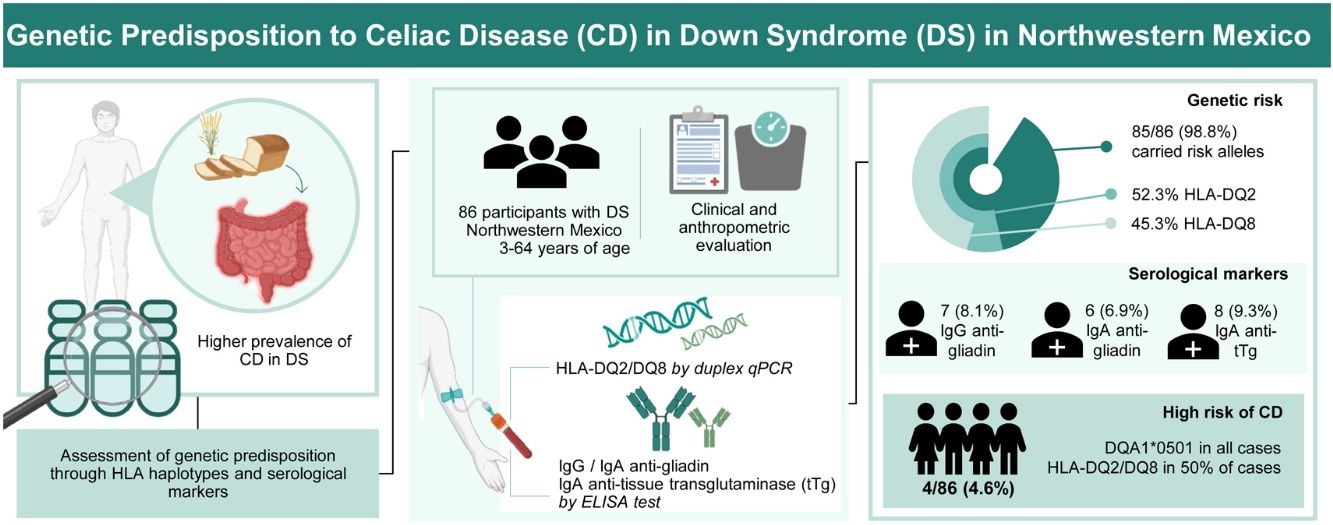
Celiac disease (CD) is an autoimmune enteropathy triggered by ingested gluten, with intra- and extra-gastrointestinal symptoms. Individuals with Down syndrome (DS) have a higher prevalence of autoimmune disorders, including CD. This study aimed to evaluate genetic predisposition (HLA haplotypes) and serological markers of CD in individuals with DS, from Northwestern Mexico.
MethodsEighty-six participants with DS, 3–64 years of age, were included in the study. We assessed HLA-DQ2 and DQ8 haplotypes by duplex PCR and related symptoms, as well as IgG and IgA anti-gliadin antibodies, and IgA anti-tissue transglutaminase antibodies by an enzymatic immunoassay.
ResultsMost participants (98.8%) carried risk alleles, with 52.9% having HLA-DQ2 and 45.9% HLA-DQ8. IgG anti-gliadin antibodies were positive in 8.1% of participants. In comparison, 6.9% were positive for IgA anti-gliadin antibodies, and 9.3% had a positive index for IgA anti-tissue transglutaminase antibodies. Four participants (4.6%) presented full or partial HLA-DQ2/DQ8 haplotypes, positive indexes for IgA anti-gliadin, and anti-transglutaminase antibodies related to CD.
ConclusionsThe study reveals an almost complete genetic predisposition to autoimmunity and a very high risk of CD in individuals with DS. Enhanced diagnostic protocols and ongoing monitoring are recommended to improve the management of CD in this vulnerable population.
La Enfermedad celíaca (EC) es una enteropatía detonada por la ingesta de gluten, con síntomas intra y extra gastrointestinales. Los individuos con síndrome de Down (SD) tienen una mayor prevalencia de trastornos autoinmunes, incluida la EC. El presente estudio tuvo como objetivo evaluar la predisposición genética (haplotipos HLA) y marcadores serológicos de EC en individuos con SD, en el noroeste de México.
MétodosSe incluyó a 86 participantes con SD, de 3 a 64 años. Evaluamos los haplotipos HLA-DQ2 y DQ8 por PCR dúplex y síntomas relacionados, al igual que por anticuerpos antigliadina IgG e IgA y anticuerpos antitransglutaminasa tisular IgA por inmunoensayo enzimático.
ResultadosLa mayoría de los participantes eran portadores de alelos de riesgo, el 52.9% con HLA-DQ2 y 45.9% HLA-DQ8. Los anticuerpos antigliadina IgG fueron positivos en 8.1% de los participantes. Comparativamente, 6.9% dieron positivo para anticuerpos antigliadina IgA y 9.3% presentaron un índice positivo para anticuerpos antitransglutaminasa tisular IgA. Cuatro participantes (4.6%) presentaron haplotipos HLA-DQ2/DQ8 completos o parciales, índices positivos para antigliadina IgA y anticuerpos antitransglutaminasa relacionados con la EC.
ConclusionesEl estudio reveló una predisposición genética casi completa a la autoinmunidad y un riesgo muy alto de EC en individuos con SD. Se recomiendan protocolos de diagnóstico mejorados y monitorización activa para el manejo de EC en esta población vulnerable.
Down Syndrome (DS), also known as Trisomy 21, is a genetic disorder caused by the presence of an extra complete or partial copy of chromosome 21 (Hsa21).1 Globally, it occurs in approximately 1 in 1,000 live births,2,3 and in Mexico, the incidence rate is 23 per 100,000 inhabitants, making it the leading cause of severe aneuploidy in newborns.4 DS exhibits a range of cognitive and physical phenotypic characteristics, with intellectual and motor disabilities being the most prominent. It is also associated with an increased risk of dyslipidemia, primarily due to unfavorable high-density lipoprotein cholesterol (HDL-c) and triglyceride levels.5 Additionally, there is an elevated risk (incidence rate ratios ranging from 1.2 to 94.7) for various clinical conditions such as diabetes, inflammatory bowel disease, obesity, autoimmune and renal diseases, hypothyroidism, and Alzheimer’s disease.6,7
Celiac disease (CD) is an autoimmune systemic disorder triggered by the intake of gluten, the major protein of wheat, rye, and barley, damaging the intestinal mucosa and inducing malabsorption and chronic inflammation. Gastrointestinal symptoms include diarrhea, abdominal pain, nausea, vomiting, abdominal distension, loss of appetite, nutrient malabsorption, and unintended weight loss.8 Extraintestinal manifestations include short stature, fatigue, iron-deficiency anemia, and neurological, musculoskeletal, and cardiac conditions.9,10 The recognition of clinical manifestations of CD in individuals with DS can be challenging, as these symptoms may overlap or be masked by typical DS-related health issues.11
The pathogenesis of CD involves complex interactions between dietary factors (such as gluten consumption), genetic predisposition (HLA-DQ2 and HLA-DQ8), and autoimmune mechanisms (tissue transglutaminase acting as an autoantigen). The genetic component plays a significant role in CD predisposition, as the HLA-DQ2 (DQA1*0501 and DQB1*0201) and HLA-DQ8 (DQA1*0301 and DQB1*0302/3) haplotypes, present on the surface of antigen-presenting cells. They exhibit a strong affinity for deamidated gliadin peptides, which are processed by the enzyme, transglutaminase. These peptides are presented to CD4 + T cells, leading to the production of antibodies against gliadins and autoantibodies against transglutaminase, initiating an inflammatory cascade.12,13
The prevalence of CD is estimated to affect between 0.5 and 1% of the general population. There is a higher prevalence in individuals with DS, ranging from 4 to 13%, with a six to ten-fold increased risk, compared with neurotypical populations.14,15 However, approximately half of the individuals with DS and CD have difficulty expressing symptoms, which can lead to missed or delayed diagnosis.11
Early detection of CD in individuals with DS is crucial, as timely diagnosis and treatment can prevent long-term complications, such as malnutrition, osteoporosis, liver and neurological complications, lymphomas, and increased susceptibility to other autoimmune diseases, thereby improving the quality of life in this vulnerable population. Although the presence of CD in DS is documented in some countries, there is a lack of data regarding these conditions in Mexico. Therefore, the aim of this study was to screen for CD risk in DS patients from Northwestern Mexico by detecting HLA-DQ2/DQ8 haplotypes and serological CD markers.
Materials and methodsStudy design and populationA cross-sectional observational study was conducted on individuals with DS from Northwestern Mexico, recruited through convenience sampling, from August to December 2022. The study adhered to the STROBE guidelines to ensure methodological rigor and transparency. Informed consent was obtained from the parents or guardians of the study participants. The study protocol was approved by the Research Ethics Committee of the Faculty of Medicine, Universidad Autónoma de Sinaloa. All study procedures followed the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Legal guardians were informed about the aim of the study, and written consent was obtained. Participants included individuals of both sexes, above two years of age, with a cytogenetic diagnosis of Down syndrome. Exclusion criteria were incomplete data or insufficient blood samples for the biochemical and genetic analyses. A clinical history was obtained for each participant through interviews with their parents or guardians, inquiring about age, sex, and clinical symptoms.
Anthropometric measurementsAnthropometric evaluation followed the procedures of the International Society for the Advancement of Kinanthropometry (ISAK). Body weight was measured using a Tanita HS-302 scale (Tanita Corporation of America, Inc, Arlington Heights, IL, USA), with participants wearing light clothing. Height was measured with a SECA 213 stadiometer (MFBIA; SECA, Hamburg, Germany). Body mass index (BMI) was calculated (weight in kilograms/height in meters squared) and classified according to Flores Arizmendi et al.16 for participants under 20 years of age, with the 5th percentile or lower considered underweight, and a BMI in the 95th percentile or higher considered obesity. For those over 20 years of age, the classification was based on the World Health Organization (WHO) cut-off points for underweight (≤18.5 kg/m2), normal weight (18.5−24.9 kg/m2), overweight (≥25 kg/m2), and obesity (≥30 kg/m2).
Biochemical profileA peripheral blood sample was obtained via venipuncture after a 12 -h fast, using additive-free SST gel (without an anticoagulant) in EDTA K2 tubes (as an anticoagulant). The samples were centrifuged at 2,500 × g for 10 min at 4 °C to separate the serum and plasma. Hemoglobin levels were measured, using an automated hematology analyzer (Beckman Coulter® LH 780, CA, USA) based on the cyanmethemoglobin method, following the manufacturer’s guidelines. Hematocrit values were determined with the same equipment, using the microcentrifugation method, calculating the percentage of red blood cells in total blood. Plasma glucose was measured using enzymatic kits (HUMAN Diagnostics Worldwide; Wiesbaden, Germany). HbA1c measurement was performed by high-performance liquid chromatography (HPLC), using a certified HbA1c analyzer (Bio-Rad D-10® Hemoglobin Testing System, Bio-Rad Laboratories, Hercules, CA, USA), and the results were reported as percentages.
Genotyping of the HLA-DQ2 and HLA-DQ8 haplotypesDNA extraction was performed in 1.5−2 ml Eppendorf tubes, starting with the mixture of 300 µl of peripheral blood and 600 µl of lysis buffer, incubated at 68 °C for 5 min. Chloroform was added, followed by centrifugation, to separate the phases. The supernatant was treated with 5% CTAB (Sigma-Aldrich, MO, USA) and injectable water, then centrifuged again. Sodium chloride and cold ethanol were added, and the mixture was centrifuged to obtain the pellet, which was washed with 70% ethanol and left to dry. Finally, it was resuspended in a TE buffer at 56 °C and quantified using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific, MA, USA).
After gDNA extraction, the HLA-DQ2 and HLA-DQ8 haplotypes were genotyped by qPCR, employing SYBR Green Supermix (Bio-Rad, California, USA) with the StepOnePlus thermal cycler (Applied Biosystems, California, USA). Duplex reactions, according to Aguayo-Patron et al.,17 were performed for alleles of the HLA-DQ2 haplotype (DQA1*0501, forward 5’-acggtccctctggccagta-3’ and reverse 5’-agttggagcgtttaatcagac-3’; DQB1*0201, forward 5’-gtgcgtcttgtgagcagaag-3’ and reverse 5’-gcaaggtcgtgcggagct-3’) and the HLA-DQ8 haplotype (DQA1*0301, forward 5’-ttcactcgtcagctgaccat-3’ and reverse 5’-caaattgcgggtcaaatcttct-3’; DQB1*0302/3 forward 5’-gacggagcgcgtgcgtta-3’ and reverse 5’-caaattgcgggtcaaatcttct-3’), according to Olearup and Fogdell.18 Beta-actin was used as an internal control (forward 5’-gcaagcaggagtatgacgag-3’ and reverse 5’-gtcaccttcaccgttccagt-3’. For description, HLA-DQ2 was exclusive for the DQA1*0501 and DQB1*0201 alleles, whereas HLA-DQ8 was exclusive for DQA1*0301 and DQB1*0302. Those alleles, forming dimers with a different chain, were named A1*0501, B1*0201, A1*0301, or B1*0302, respectively, as in Mejía-León et al.19
Genetic risk for CD was in accordance with that reported in a Northwestern Mexican population.19 In the Mejía-León study, the risk was expressed as 1:N, where N represents the number of individuals in whom one positive case was found. This value was calculated using the frequency of each HLA-DQ profile in the general population, multiplied by 100, and divided by its frequency in patients.
Serum antibody tests (anti-Gliadin IgG and IgA, anti-Transglutaminase IgA)Serum samples were used to quantify anti-gliadin IgG and IgA antibodies (non-deamidated), as well as anti-transglutaminase IgA, using the enzyme-linked immunosorbent assay (ELISA), following the method described by Cabrera-Chávez et al.20 Briefly, the microplates were coated with gliadins or transglutaminase and then blocked with gelatin. Serum samples were serially diluted and incubated in the plates, followed by the addition of anti-IgG or anti-IgA antibodies conjugated with HRP. Once the reaction developed, it was stopped with H2SO4. The results were the average of 4 duplicate dilutions (8 total absorbance readings), measured at 450 nm, using the iMark Microplate Absorbance Reader (Bio-Rad, California, USA). Positivity was defined as an index > 1.0 of the ratio of the sample mean absorbance divided by the mean plus two standard deviations of the mean absorbance of all the serum samples.20,21
Statistical analysisData normality was verified using histograms and the Kolmogorov-Smirnov test. Descriptive statistics were used to summarize the anthropometric and clinical characteristics of the participants. Continuous variables were reported as means with standard deviations and ranges, including age, BMI, glucose, HbA1c, hemoglobin, and hematocrit. Categorical variables, such as sex, and positive indexes of serological markers (IgG and IgA anti-gliadin antibodies, and IgA anti-tissue transglutaminase antibodies) were expressed as frequencies and indexes. The prevalence of specific HLA haplotypes (HLA-DQ2 and HLA-DQ8) was also determined. All statistical analyses were performed using STATA v.13 (Stata Corp, College Station, TX, USA) software. Data were reported as means ± standard deviations or percentages (%).
Ethical considerationsInformed consent was obtained from all participants before their inclusion in the study. For participants who were minors, written informed consent was provided by their parents or legal guardians, ensuring complete understanding of, as well as agreement with, the research objectives and procedures. The authors declare that this study was conducted following the protocols approved by the Research Ethics Committee of the Faculty of Medicine, Universidad Autónoma de Sinaloa, with registration number CONBIOÉTICA-25-CEI-003-20181012, adhering to the ethical principles established in the Declaration of Helsinki for research involving human participants. All data collected were anonymized to ensure the confidentiality and privacy of the participants, and no identifiable personal information was included. The study strictly complied with applicable ethical and legal standards to safeguard the rights and integrity of the individuals involved.
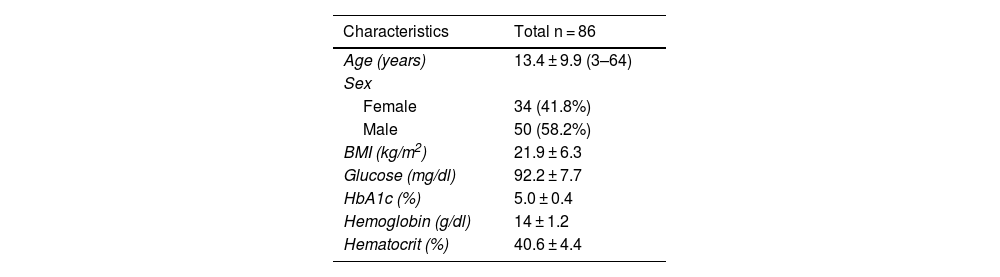
ResultsDemographic, anthropometric, and clinical characteristics of 86 participants with DS are shown in Table 1. According to the BMI, the mean number of DS patients were within normal weight, with only 23.3% of them having overweight or obesity. The participants had normal ranges for fasting glucose and HbA1c, no signs of diabetes, and there were no data to support anemia.
General characteristics of the participants.
| Characteristics | Total n = 86 |
|---|---|
| Age (years) | 13.4 ± 9.9 (3–64) |
| Sex | |
| Female | 34 (41.8%) |
| Male | 50 (58.2%) |
| BMI (kg/m2) | 21.9 ± 6.3 |
| Glucose (mg/dl) | 92.2 ± 7.7 |
| HbA1c (%) | 5.0 ± 0.4 |
| Hemoglobin (g/dl) | 14 ± 1.2 |
| Hematocrit (%) | 40.6 ± 4.4 |
BMI: body mass index, HbA1c: glycated hemoglobin.
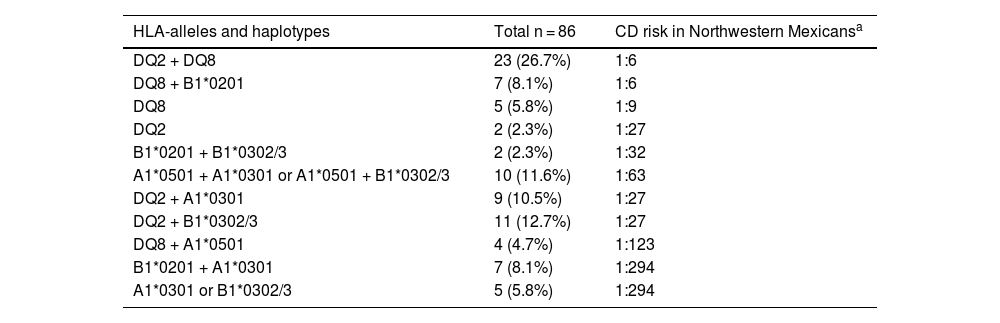
Almost all the participants with DS (85/86) presented with a genetic predisposition to CD, with at least one allele of the HLA-DQ2/DQ8 haplotypes; 69 of them had a risk higher than 1:63 (Table 2). HLA-DQ2 was the most prevalent, presenting in 52.3% of the participants, followed by HLA-DQ8 in 45.3%. Twenty-three (26.7%) participants exhibited the two haplotypes (HLA-DQ2 and HLA-DQ8), 2 (2.3%) presented with only HLA-DQ2, 5 (5.8%) had only HLA-DQ8, and 31 (36%) carried alleles forming dimers with a different chain (A1*0501, B1*0201, A1*0301, or B1*0302). Only one individual carried no risk alleles.
HLA alleles and risk for celiac disease in the Northwestern Mexican population.
| HLA-alleles and haplotypes | Total n = 86 | CD risk in Northwestern Mexicansa |
|---|---|---|
| DQ2 + DQ8 | 23 (26.7%) | 1:6 |
| DQ8 + B1*0201 | 7 (8.1%) | 1:6 |
| DQ8 | 5 (5.8%) | 1:9 |
| DQ2 | 2 (2.3%) | 1:27 |
| B1*0201 + B1*0302/3 | 2 (2.3%) | 1:32 |
| A1*0501 + A1*0301 or A1*0501 + B1*0302/3 | 10 (11.6%) | 1:63 |
| DQ2 + A1*0301 | 9 (10.5%) | 1:27 |
| DQ2 + B1*0302/3 | 11 (12.7%) | 1:27 |
| DQ8 + A1*0501 | 4 (4.7%) | 1:123 |
| B1*0201 + A1*0301 | 7 (8.1%) | 1:294 |
| A1*0301 or B1*0302/3 | 5 (5.8%) | 1:294 |
Regarding serological markers for the diagnosis of CD, 7 (8.1%) participants exhibited positive indexes of IgG anti-gliadin (anti-Gd) antibodies, 6 (6.9%) were positive for IgA anti-Gd antibodies, and 8 (9.3%) had positive indexes for IgA anti-tissue transglutaminase (anti-TG) antibodies.
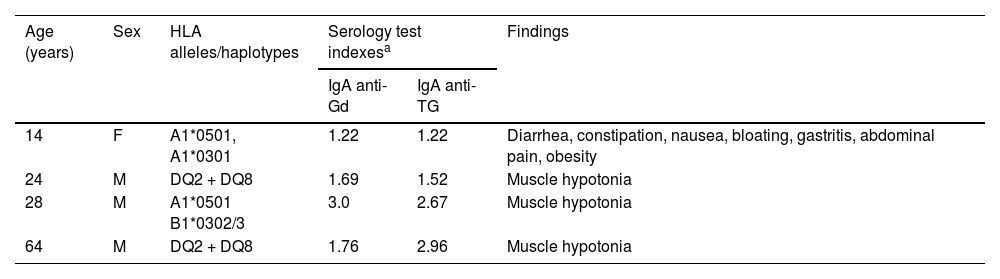
Table 3 shows the participants who presented with the coexistence of risk-haplotypes, antibodies associated with CD, and signs and symptoms. The first participant described in the table is an adolescent who was diagnosed with CD before participating in our study, and according to her parents, she was on a gluten-free diet and experienced gastrointestinal symptoms, including diarrhea, constipation, nausea, gastritis, heartburn, vomiting, abdominal pain, and obesity. In addition, three adult participants exhibited elevated IgA anti-Gd and anti-TG antibodies and reported no apparent gastrointestinal symptoms. Notably, two of the four individuals described in the table carried both HLA-DQ2 and HLA-DQ8, the highest risk for CD in the Northwestern Mexican population.
Characteristics of participants with a high probability of celiac disease.
| Age (years) | Sex | HLA alleles/haplotypes | Serology test indexesa | Findings | |
|---|---|---|---|---|---|
| IgA anti-Gd | IgA anti-TG | ||||
| 14 | F | A1*0501, A1*0301 | 1.22 | 1.22 | Diarrhea, constipation, nausea, bloating, gastritis, abdominal pain, obesity |
| 24 | M | DQ2 + DQ8 | 1.69 | 1.52 | Muscle hypotonia |
| 28 | M | A1*0501 B1*0302/3 | 3.0 | 2.67 | Muscle hypotonia |
| 64 | M | DQ2 + DQ8 | 1.76 | 2.96 | Muscle hypotonia |
F: female; M: male; Anti-Gd: anti-gliadin; Anti-TG: anti-transglutaminase.
As previously stated, DS patients suffer from other diseases, especially autoimmune diseases like CD. Therefore, DS patients have been screened for CD in different geographic regions worldwide, resulting in CD detection in 3.1–13%, compared with 1% or less in the general population.15,22 However, such screening is almost non-existent in Latin American countries, except for 2–3 studies in Brazil and Colombia.15,23,24 To the best of our knowledge, there are no published studies on the Mexican population with DS, and screening is important for evaluating risk and preventing complications in the already fragile health of these patients.
We found that 4.6% of our DS population had a potential risk of CD, based on genetic risk and specific IgA antibodies against gliadins and transglutaminase, compared with 0.6% in the general Mexican population.25 Importantly, all individuals with potential CD in our study carried the DQA1*0501 allele, a component of the HLA-DQ2 haplotype, highlighting the strong association between HLA-DQ2 and CD susceptibility in this group. Furthermore, 2/4 individuals presented the HLA-DQ2/DQ8 haplotypes, suggesting that while both haplotypes contribute to genetic risk, HLA-DQ2 was the most prevalent among individuals with a potential risk of CD. This observation aligns with findings in Colombian children with DS and CD, where HLA-DQ2 also predominated (3/5), compared with HLA-DQ8 (2/5).23
Notably, genetic risk was extremely high, with 85/86 of the DS participants having some grade of genetic risk and 81% of them presenting with a risk >1:100.
Genetic predisposition due to the presence of class II HLA genes plays a significant role in the etiology of CD.26 Clinical guidelines from the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) recommend HLA-DQ2 and HLA-DQ8 genotyping for diagnosing asymptomatic or suspected CD in children, given that the absence of these alleles indicates a very low risk of CD.27,28 In our study, HLA-DQ2 was the most predominant (52.9%), and HLA-DQ8 was expressed in 45.9% of cases. HLA-DQ2 is present in 30–40% of populations, primarily in Europe, and HLA-DQ8 is more common (20–25%) in Central and South America.23,29
Specifically, the DQA1*0501 allele from the HLA-DQ2 haplotype was present in 55/86 DS individuals, concurring with that reported for the Northwestern Mexican population. Our DS study participants had a particular combination of haplotypes, with a DQ2:DQ8 ratio of 1.2:1. The healthy population in the Northwestern region is very different from other mestizo populations in Latin America, including Mexico, mainly because of its lower frequency of the DQB1*0302 allele.19 However, Table 2 shows that DQB1*0302 frequency was twice as high in cases of CD, in most of our participants.
The similar risk haplotypes, HLA-DQ2 and DQ8, predispose to type 1 diabetes (T1D).19 Despite almost 100% of our DS participants having the haplotypes or their alleles, none of them had developed T1D, as shown by the normal levels of fasting glucose and HbA1c in Table 1.
Our study population had positive indexes of IgG anti-Gd, IgA anti-Gd, and anti-TG antibodies, at 8.1, 6.9, and 9.3%, respectively. Similar results have been reported in Latin America, with an 8.2% prevalence of high levels of IgA anti-TG antibodies in children with DS in Colombia,23 and a range of 6.5 to 17.5% in children and adolescents with DS from Brazil.15,24
In European populations with DS, such as in Poland, high levels of IgA anti-TG and IgG anti-Gd antibodies are reported at 6.3 and 9%, respectively.30 The prevalence of IgA anti-TG is 9.4% in Ireland,31 12.2% in Portugal, and 5.2% in the Netherlands.32 Data from India are similar, with a prevalence of 7%.33 Conversely, populations in the Middle East exhibit higher percentages of individuals, with elevated levels of IgG and IgA anti-Gd, as well as IgA anti-TG (57.7–79%, 23–32.1%, and 9.6–15.4%, respectively).34,35
CD is associated with behavioral and emotional difficulties,36 which can be exacerbated in vulnerable populations, such as those with DS. The presence of prominent neurological symptoms and subtle gastrointestinal symptoms complicates the identification of CD. Published studies indicate that up to one-third or more of DS patients with CD present with no characteristic symptoms of CD,11,36 due to the variability in clinical manifestations, including gastro- and extraintestinal symptoms, atypical symptoms, and even asymptomatic cases.37
In our study, one of the four (25%) participants with potential CD presented with various gastrointestinal symptoms (Table 3), highlighting the representational iceberg of CD in DS, in which prevalence is markedly higher, compared with euploid populations.38,39 This relationship might go unnoticed due to the insidious and occasionally silent nature of CD. Given the serological and genetic risk observed, it is essential that these patients undergo intestinal biopsy to confirm the diagnosis and ensure appropriate clinical management.
IgA anti-TG is a reliable predictor of CD, as demonstrated in a study of German children with detected IgA anti-TG antibodies and risk haplotypes, in whom CD was confirmed through duodenal biopsies two years later.40 Elevated IgA anti-TG levels have also been suggested to possibly reflect atrophy in the small intestinal villi.29 Another study involving 14 specialized CD centers across various countries evaluated adults with suspected CD and found that IgA anti-TG was a reliable predictor of duodenal villous atrophy in 98% of cases. However, discrepancies were observed in the histological evaluation of duodenal biopsies, highlighting difficulties, even for histology specialists.41
On the other hand, it is important to consider that IgA anti-TG can be positive in patients with enteric infections, such as giardiasis and other pathogens, as well as other diseases.42 We had eight patients with positive IgA anti-TG, but four were negative for anti-gliadin antibodies. Because the prevalence of enteric infections is common in our populations,43 it is possible our cases were infected.
Diagnosing CD in the DS population presents a significant challenge due to the factors of intellectual disability and behavioral difficulties. Furthermore, the atypical disease presentation, combined with solid recommendations for CD evaluation and monitoring, leads to underdiagnosis or delayed diagnosis. Screening by serological tests and risk haplotype genotyping is needed to ensure the DS population receives appropriate diagnosis and treatment.
In conclusion, the present study shows a high genetic predisposition to CD, with a pattern of HLA-DQ2/DQ8 alleles or haplotypes typical of the Northwestern Mexican population with this disease. Additionally, due to the high risk of CD in our study sample, evidenced by elevated serological IgA against gliadins and tissular transglutaminase, along with the presence of HLA-DQ2 and HLA-DQ8, confirmation through intestinal biopsies is necessary. Furthermore, if CD is confirmed, the timely implementation of a gluten-free diet is crucial for preventing complications. Therefore, the continuous monitoring of signs and symptoms of CD and early diagnostic interventions are essential for individuals with DS, to prevent other comorbidities, such as mineral malabsorption and its consequences, and especially intestinal lymphoma induced by non-treated CD.
Financial disclosureNo financial support was received for this study.
The authors declare that there is no conflict of interest.