Celiac disease (CD) is an autoimmune enteropathy associated with gluten ingestion. In extended families of celiac patients that live in close proximity of one another, shared genetic and environmental factors can predispose them to CD.
AimThe aim of this study was to provide evidence about the genetic and environmental factors involved in the development of CD in the extended family of a pediatric patient.
MethodsThe medical history, environmental conditions, and participant weight, height, and peripheral blood samples were evaluated. The HLA-DQ2/DQ8 haplotypes were genotyped through qPCR testing and the IgA anti-gliadin and anti-transglutaminase antibodies were quantified using the ELISA test.
ResultsTwelve close-living maternal relatives of the index case participated in the study. Eight of them presented with the HLA-DQ2 haplotype, inherited from the grandfather, and 7/12 and 9/12 were positive for IgA anti-gliadin and IgA anti-transglutaminase antibodies, respectively. The main intestinal symptoms stated by the participants were abdominal bloating, excess flatulence, constipation, and gastroesophageal reflux. The most frequent extra-intestinal symptoms were fatigue, stress, and anxiety. In addition, 6/13 participants had bronchial asthma.
ConclusionThe extended family living in close proximity of one another shared a genetic predisposition, environmental conditions, and asthma, which could have predisposed them to celiac disease.
La enfermedad celiaca (EC) es una enteropatía autoinmune asociada a la ingestión de gluten. En las familias extendidas de pacientes celiacos en estrecha convivencia, los factores genéticos y ambientales compartidos pueden predisponer a EC.
ObjetivoProporcionar evidencia sobre factores genéticos y ambientales implicados en el desarrollo de la EC en una familia extendida de un paciente pediátrico.
MétodosSe evaluaron historial clínico, condiciones ambientales, peso y talla de los participantes y se les tomó una muestra de sangre periférica. Se genotipificaron los haplotipos HLA-DQ2/DQ8 mediante qPCR y se cuantificaron por ELISA los anticuerpos IgA antigliadinas y antitransglutaminasa.
ResultadosParticiparon 12 familiares maternos del caso índice, que tenían estrecha convivencia vecinal. Ocho presentaron HLA-DQ2 heredado del abuelo, 7/12 y 9/12 fueron positivos para los anticuerpos IgA antigliadinas e IgA antitransglutaminasa, respectivamente. Los principales síntomas intestinales referidos por los participantes fueron distensión abdominal, exceso de gases, estreñimiento y reflujo gastroesofágico. Los síntomas extraintestinales más frecuentes fueron fatiga, estrés y ansiedad. Además, 6/13 participantes tuvieron asma bronquial.
ConclusiónLa familia extendida convive estrechamente, comparte la predisposición genética, condiciones ambientales y el asma, que podrían haberlos predispuesto a la EC.
Celiac disease (CD) is an autoimmune enteropathy triggered by the ingestion of gluten in genetically predisposed persons. Its estimated prevalence in Mexico is 1:168,1 similar to that found in Native American (1:167)2 and Caucasian (1:100-1:200)3 populations. CD symptoms can be intestinal, such as diarrhea and malabsorption syndrome that cause weight loss and growth failure, or they can be extra-intestinal, such as headache, anemia, dermatitis herpetiformis, and osteoporosis.4 The variability in the clinical manifestations makes diagnosis difficult.
Thirty to 50% of CD is explained by the genetic risk from the HLA-DQ2/DQ8 haplotypes,5 as well as by environmental factors. Relatives of persons with CD are at greater risk for developing the disease, due to genetics and their common history. The risk for presenting with CD increases by 5.6-10% in first-degree relatives and by 2.3-5% in second-degree relatives.6,7 Thus, some authors have proposed the implementation of CD detection strategies in that high-risk group, in an effort to reduce related morbidity and mortality.8,9
The traditional lifestyle of many Mexican families is to live in the same physical area, and so not only do they share genetics, but they also share environmental conditions that can increase the risk for CD. In this context, the aim of the present study was to provide evidence about the genetic and environmental factors, as well as diseases and treatments, involved in the development of CD in the extended family of a boy with said disease.
Materials and methodsParticipantsThe study was conducted in Hermosillo, Sonora, Mexico. Twelve first and second-degree relatives of a boy with CD (index case) were included. The boy, currently 10 years old, was diagnosed with CD at the age of 2, after presenting with acute symptoms and undergoing biopsy and antibody testing. Our study protocol was approved by the institutional Bioethics Committee (CE/016/2014), and written statements of informed consent were obtained from all the participants. The parents signed the statements corresponding to the participants under 18 years of age.
All participants were interviewed to obtain their medical history. In the case of children, the mothers provided the information. The weight and height of the participants were measured, and their body mass index was calculated. The nutritional status of the adults was classified according to the cutoff points of the World Health Organization,10 and the body mass index-for-age Z-score was calculated for the nutritional status of the children.11
A venous blood sample was collected from each participant. A fraction was used to analyze the risk haplotypes and the rest was centrifuged to obtain serum and analyze antibodies.
DNA extraction and genotypingGenomic DNA was extracted from the samples of dried blood drops, following the protocol described by Aguayo-Patrón et al.12 Primers previously designed for the DQA1*0501 and DQB1*0201 (DQ2) alleles and the DQA1*0301 and DQB1*0302/3 (DQ8) alleles were used for the risk haplotype analysis.13 Duplex real-time PCR reactions were carried out and the alleles were identified through dissociation curve analysis.12
Antibody analysisThe IgA anti-gliadin (IgA-Gd) and anti-transglutaminase (IgA-TG) antibodies were evaluated in the serum samples through the enzyme-linked immunosorbent assay (ELISA) test, following a previously described technique.14Antibody indices were calculated using the absorbance values obtained, with respect to the mean absorbance of the healthy population. Values above 1.0 were considered positive.
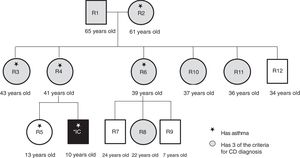
ResultsThirteen subjects, including the index case, participated in the study: the maternal grandparents, the mother, the sister, 4 aunts, one uncle, and 3 cousins. Eight of the participants were females, with a median age of 36 years (fig. 1). The 13 relatives lived in close proximity in a traditional Mexican extended family environment, in houses adjoining, or close to, that of the grandparents. They shared the environment and customs related to upbringing, alimentation, life-style, and treatment of illnesses.
The maternal grandparents moved from a rural zone to the city of Hermosillo in Sonora, Mexico. The second generation (mother and aunts and uncle of the index case) spent their childhood in the rural zone in a farm environment. They all stated they had been breastfed, but also received cow's milk or goat's milk. In addition, 2 participants had complicated births, due to the lack of adequate health services in the rural zone. The father of the index case was tested for specific antibodies and haplotypes, when his son was diagnosed with CD. The results were negative, not associated with the disease, and therefore he was not included in the present study.
The third generation (index case, sister, and cousins) were born in a partially urbanized section of the city of Hermosillo. Not all the houses had a sewage system or concrete floors, and cats, dogs, chickens, and horses were kept in the homes and patios. At the time of the study, there was improved access to health services and medications, but self-medication practices were common. The parents shared medications with their children to treat similar illnesses, rarely completing the adequate doses and/or cycles. All the members of the youngest generation were delivered by cesarean section, and none of them were exclusively breastfed during their first 6 months.
Even though the CD diagnosis of the index case had been known for 8 years, the majority of the family members were not aware of their risk for developing the disease. Most of the adults attributed their symptoms to age and/or menopause. A relevant datum resulting from the medical histories was that six of the participants had been medically diagnosed with bronchial asthma (fig. 1).
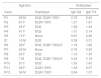
In the genetic risk analysis, 9 of the 13 participants presented with the HLA-DQ2 haplotype or some of its alleles (DQA1*0501). Two cases had a combination with the DQB1*0302 allele of the HLA-DQ8 haplotype. In two other cases, there were no alleles predisposing to CD (Table 1). The antibody analysis resulted in positive titers for IgA-Gd in 7 participants and positive titers for IgA-TG in 9 participants. In both tests, the index case had titers below 1.0, which was due to 8 years of a gluten-free diet as treatment for CD (Table 1).
Haplotypes and antibody titers of the extended family relatives (R) of the index case (IC).
| Age/Sex | Antibodies* | |||
|---|---|---|---|---|
| Case | Haplotype | IgA-Gd | IgA-TG | |
| R1 | 65 M | DQ2, DQA1*0301 | 0.72 | 2.40 |
| R2 | 61 F | DQA1*0501 | 1.31 | 1.67 |
| R3 | 43 F | DQ2 | 1.58 | 1.54 |
| R4 | 41 F | DQ2 | 1.51 | 2.14 |
| R5 | 13 F | None | 0.87 | 0.90 |
| IC | 10 M | DQ2 | 0.38 | 0.31 |
| R6 | 39 F | DQ2, DQB1*0302/3 | 1.19 | 1.62 |
| R7 | 24 M | None | 0.78 | 0.90 |
| R8 | 22 F | DQ2 | 1.12 | 1.33 |
| R9 | 7 M | DQ2, DQB1*0302/3 | 0.24 | 0.19 |
| R10 | 37 F | DQ2 | 1.52 | 1.42 |
| R11 | 36 F | DQA1*0301 | 1.34 | 2.07 |
| R12 | 34 M | DQA1*0301 | 0.84 | 1.07 |
F: female; IgA-Gd: IgA against gliadins; IgA-TG: IgA against transglutaminase; M: male.
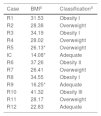
In relation to body mass index, the participants had some grade of overweight or obesity, except for the index case, relative (R) 9, and R12 (Table 2). The intestinal symptoms associated with CD were more recurrent than the extra-intestinal ones, in the relatives studied. Table 3 shows that the most frequent symptom was abdominal bloating (11/13 participants), followed by food intolerance (9/13 participants), and excess flatulence (8/13 participants). Participants R3 and R10 reported having bloating and abdominal pain after eating foods containing corn.
Nutritional status of the extended family relatives of the index case.
| Case | BMIa | Classificationa |
|---|---|---|
| R1 | 31.53 | Obesity I |
| R2 | 28.38 | Overweight |
| R3 | 34.19 | Obesity I |
| R4 | 28.02 | Overweight |
| R5 | 26.13* | Overweight |
| IC | 14.08* | Adequate |
| R6 | 37.26 | Obesity II |
| R7 | 26.41 | Overweight |
| R8 | 34.55 | Obesity I |
| R9 | 16.25* | Adequate |
| R10 | 41.32 | Obesity III |
| R11 | 28.17 | Overweight |
| R12 | 22.83 | Adequate |
BMI: body mass index; IC: index case; R: relative.
Intestinal symptoms of the extended family relatives of the index case.
| Symptoms | n/No. | Cases |
|---|---|---|
| Abdominal bloating | 11/13 | IC, R1, R2, R4, R5, R6, R7, R8, R9, R10, R11 |
| Food intolerance | 9/13 | IC, R2, R3, R4, R6, R7, R8, R9, R10 |
| Excess flatulence | 8/13 | IC, R1, R2, R5, R7, R9, R10, R11 |
| Constipation | 6/13 | IC, R2, R6, R7, R9, R10 |
| Gastroesophageal reflux | 6/13 | IC, R2, R5, R6, R7, R9 |
| Abdominal pain | 5/13 | IC, R2, R7, R9, R10 |
| Diarrhea | 4/13 | IC, R7, R9, R10 |
| Nausea | 4/13 | IC, R2, R7, R9 |
| Vomiting | 2/13 | R9, R10 |
IC: index case; R: relative.
With respect to extra-intestinal symptoms, a greater number of participants presented with fatigue (10/13 participants), stress, anxiety, sleep disturbances, and muscle cramps (8/13 participants). Other clinical signs, such as urticaria, aphthous mouth ulcers, damaged tooth enamel, dermatitis, and weight loss were reported less frequently (3 a 4/13 participants) (Table 4).
Extra-intestinal signs and symptoms of the extended family relatives of the index case.
| Symptoms | n | Cases |
|---|---|---|
| Fatigue | 10/13 | R1, R2, R3, R4, R5, R6, R7, R8. R9, R10 |
| Stress and anxiety | 8/13 | IC, R2, R3, R4, R5, R7, R9, R10 |
| Sleep disturbances | 8/13 | IC, R3, R4, R5, R7, R8, R9, 10 |
| Muscle cramps | 8/13 | R1, R2, R3, R4, R6, R7, R9, R11 |
| Weakness | 7/13 | R2, R3, R4, R5, R6, R7, R9 |
| Headache | 7/13 | R2, R3, R4, R5, R7, R9, R10 |
| Depression | 5/13 | R2, R3, R4, R5, R10 |
| Urticaria | 4/13 | R2, R5, R6, R7 |
| Aphthous mouth ulcers | 3/13 | IC, R2, R9 |
| Damaged tooth enamel | 3/13 | R5, R8, R9 |
| Anorexia | 3/13 | IC, R7, R9 |
| Dermatitis | 3/13 | IC, R5, R9 |
| Weight loss | 3/13 | R1, R4, R11 |
IC: index case; R: relative.
As is still a custom in some places of Mexico, the extended family in our study lives in houses adjoining, or close to, that of the grandparents. Thus, not only do they share genetics, but they also share the environment, child-raising customs, and lifestyle. Feeding practices during infancy and microbial infections and their treatment can influence the composition of the microbiota, affect the intestinal barrier, and possibly trigger CD.15 This can have repercussions in both childhood and adulthood,16 signifying that the members of the family under study shared the environmental risk for CD.
Most of the study participants had the HLA-DQ2 haplotype, inherited from the grandfather (Table 1), whereas in a Mexican population, the most common haplotype in cases of CD was found to be HLA-DQ8. The haplotype of greatest risk was HLA-DQ8, alone (1:9), or combined with the DQB1*0201 allele of the HLA-DQ2 haplotype (1:6), whereas the HLA-DQ2 haplotype had a risk of 1:27.17 In contrast, 88% of the CD cases in a European population presented with the HLA-DQ2 haplotype or its variants,18 with a mean risk of 1:10.5 The phenotype of our study participants was mestizo, but that of the grandfather was Caucasian.
Seventy-five percent of the study relatives had positive titers for IgA antibodies against tissue transglutaminase (9/13 participants), which was much higher than the 47% with positive serology reported by Rodrigo et al.19 in a family case. If the fact that 0.6% of the Mexican population presents with positive serology for CD1 is considered, then high risk for CD of relatives of celiac patients can be inferred. Likewise, a combined prevalence of 7.5% in first-degree relatives and 2.3% of second-degree relatives has been described.7
Regarding anthropometric indicators, in general, the relatives had short stature, as did the index case. The height of two of the adult males was below the regional mean (162.7cm and 165cm vs 166.5cm),20 and the mean height of the females was far below the regional mean (131.04cm vs 155.7cm).21 It has been reported that short children with CD do not reach their maximum height as adults,22 and this is inversely related to the time of diagnosis.23 In other words, even though the participants in our study were apparently asymptomatic, they possibly presented with a less acute form of CD since childhood that could have affected their development. Even though the index case was diagnosed with classic CD at the age of 2, he was not given adequate dietary follow-up until he was 6 years old.
In relation to symptoms, most of the participants stated the most frequent were gastrointestinal, such as abdominal bloating, food intolerance, excess flatulence, and constipation, coinciding with that found in Mexican adults with CD.24 Extra-intestinal symptoms were less frequent and the most reported included fatigue, headache, dermatitis, and anorexia.
Duodenal biopsies were not taken to confirm the diagnosis of CD in the relatives, because even though it is the criterion standard, it is an invasive and costly procedure. Therefore, serologic analysis was contemplated as a viable option, given its high sensitivity and specificity (98-100%).4 Catassi and Fasano25 proposed that CD diagnosis can be considered, if the patient has at least four of the five following criteria: typical signs and symptoms of CD, positive serology, risk genetics, intestinal villous atrophy (determined through biopsy), and response to a gluten-free diet. Thus, the majority (8/12) of the relatives of the index case (with previous CD diagnosis) presented with the clinical and serologic indicators that corresponded to the first three criteria mentioned. Only their response to a gluten-free diet would be lacking to consider them celiac patients.
An interesting datum in the present study was that 6/13 relatives, including the index case, had been diagnosed with bronchial asthma, possibly associated with the shared genetic and environmental conditions. Although the relation between CD and asthma has not been widely studied, there is evidence of their association. According to Tang et al.,26 intraepithelial lymphocytes from biopsies of celiac patients express more cytosolic phospholipase A2 than their healthy counterparts. In addition, the NKG2D receptor expressed in lymphocytes can activate the intracellular cytosolic pathway of the p-cPLA2s, in the presence or not of IL-15, and mediating the release of arachidonic acid. That acid is a substrate of the 5-lipoxygenases and C4 leukotriene synthase that leads to the production of cysteinyl leukotrienes, responsible for enterocyte destruction. Said leukotriene overproduction induces mucosal inflammation and causes the majority of inflammatory conditions that have previously been associated with allergies and asthma.27 Thus, presenting with asthma could be a risk factor for triggering CD.
Some of the study participants (index case, R3, R5) had been treated with montelukast for bronchial asthma, but they suspended it due to medical recommendation and currently continue treatment with loratadine or chlorpheniramine. Montelukast is a drug that inhibits cysteinyl leukotrienes for the treatment of asthma symptoms. Its activity has been confirmed in vitro in intraepithelial lymphocytes, suppressing NKG2D cytotoxicity and inhibiting cysteinyl leukotriene production.26 Even though this drug has not been approved for CD treatment, it is capable of blocking the T CD8+ cells that are responsible for causing intestinal damage. Therefore, its efficiency and safety for the treatment of CD is being evaluated in a phase II clinical trial.28
Some epidemiologic studies have also examined the relation between asthma and CD. In a cohort of Italian children, an increased risk for CD development was found in those subjects that had a previous asthma diagnosis (IRR 1.37, 95% CI: 1.09-1.71). Antibiotic use, which has been related to both diseases, was not a confounding factor in the association.29 To the contrary, in a Danish population cohort, it was concluded that there was no increased risk for presenting with asthma, if the parents had CD.30 Thus, asthma can be considered a predisposing factor to CD, but not vice versa. However, more studies are required to sustain the relation between CD and the inflammatory mechanisms triggered by asthma.
The study family naturally shared genetics and a hereditary background that predisposed to CD. Moreover, they lived in close proximity of each other, sharing environmental factors that could increase the risk for developing CD. The HLA-DQ2 haplotype and customs in upbringing, alimentation, and the treatment of illnesses could be determining factors in an increased risk for CD that the study family had. Another factor involved in the development of CD in several of the participants was asthma, which could trigger CD through inflammatory mechanisms. This has not yet been widely studied.
It is our conclusion that the majority of the relatives of the boy with celiac disease presented with CD indicators through the influence of the following factors: risk genetics, living in close proximity, sharing environmental conditions and cultural patterns, and the fact that some of the family members presented with asthma.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureSigala-Robles R. and Aguayo-Patrón S.V. received support from the Consejo Nacional de Ciencia y Tecnología (CONACYT), grants: 394851 and 377998, respectively.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Sigala-Robles R, Aguayo-Patrón SV, Calderón de la Barca AM. Genética, ambiente y asma asociados a enfermedad celiaca en la familia extendida de un niño afectado. Revista de Gastroenterología de México. 2018;83:79–85.
See related content at DOI: http://dx.doi.org/10.1016/j.rgmxen.2018.05.003, Remes-Troche JM. Doctor, why am I a celiac if I'm Mexican? Breaking another paradigm of celiac disease in Mexico. Rev de Gastroenterol Méx. 2018;83:77–78.