Proton pump inhibitors (PPIs) are widely known drugs that are used quite frequently and indicated in both the short and long terms, in numerous acid-related diseases. Our aim was to produce an expert review that establishes recommendations for the adequate prescription and deprescription of PPIs.
MethodsA group of experts in PPI use that are members of the Asociación Mexicana de Gastroenterología (AMG), after extensively reviewing the published literature and discussing each recommendation at a face-to-face meeting, prepared the present document of good clinical practice recommendations. This document is not intended to be a clinical practice guideline or utilize the methodology said format requires.
ResultsEighteen experts on PPI use developed 22 good clinical practice recommendations for prescribing short-term, long-term, and on-demand PPIs, recognizing adverse events, and lastly, deprescribing PPIs, in acid-related diseases.
ConclusionsAt present, there is scientific evidence on PPI use in numerous diseases, some in the short term (4-8 weeks), others on-demand (for short periods until symptoms improve), or in the long term (without suspending). Numerous adverse effects have been attributed to PPIs, but the majority have no well-established causal association. Nevertheless, PPIs should be suspended when there is no clear indication for their use. These recommendations aim to aid general physicians and specialists, with respect to PPI prescription and deprescription.
Los inhibidores de la bomba de protones (IBP) son fármacos ampliamente conocidos, son utilizados con bastante frecuencia e indicados en múltiples patologías relacionadas al ácido, tanto a corto como a largo plazo.
ObjetivoEsta es una revisión de expertos que establece recomendaciones de buena práctica clínica para la adecuada prescripción y deprescripción de IBP.
MétodosLas recomendaciones de buena práctica clínica se generaron por un grupo de expertos en uso de IBP, miembros de la Asociación Mexicana de Gastroenterología (AMG), después de hacer una extensa revisión de la literatura publicada y de discutir cada recomendación en una reunión presencial. Este documento no pretende ser una guía de práctica clínica con la metodología que este formato requiere.
ResultadosUn total de 18 expertos en el uso de IBP elaboraron 22 recomendaciones de buena práctica clínica para la prescripción a corto, largo plazo y a demanda, reconocimiento de efectos adversos y finalmente la deprescripción de IBP en las enfermedades relacionadas al ácido.
ConclusionesActualmente, existe evidencia científica para el uso de IBP en múltiples enfermedades, en algunas de ellas a corto plazo (4-8 semanas), en otras a demanda (por cortos periodos de tiempos hasta mejorar los síntomas) o a largo plazo (sin suspender). Se les han atribuido múltiples efectos adversos, sin embargo, la mayoría no tienen una asociación causal bien establecida. No obstante, los IBP debe ser suspendidos cuando no exista una indicación clara. Estas recomendaciones pretenden ayudar a médicos generales y especialistas en la prescripción y deprescripción de IBP.
Proton pump inhibitors (PPIs) are among the most widely prescribed medications worldwide. Approximately one-fourth of patients on PPIs continue taking them for at least one year,1 and in observational studies, two-thirds of patients take them without an appropriate indication.2 There is recent concern about the growing prevalence of patients that receive long-term treatment with PPIs, regarding adverse effects and their inadequate use. Thus, the aim of the present review was to make recommendations, based on recent scientific evidence and discussed by a group of experts, for the adequate prescribing and deprescribing of PPIs.
MethodsThis review, conducted by 18 experts, was commissioned by the Asociación Mexicana de Gastroenterología (AMG). The specialists were selected based on their academic careers in teaching, research, and practice, and their specialized knowledge of PPI use and deprescription. An extensive review of the literature, spanning the last 20 years, was carried out on PPIs, their indications, short and long-term doses, on-demand use, adverse effects, and their adequate deprescribing. The experts were divided into 6 working groups to review the publications and formulate recommendations on: 1) the definition of the PPIs available in Mexico, and their pharmacokinetic and pharmacodynamic differences; 2) indications for the short-term use of PPIs and doses; 3) indications for on-demand PPI use and doses; 4) indications for the long-term use of PPIs and doses, 5) long-term adverse effects with PPI use; and 6) deprescribing PPIs. Version 1.0 of the recommendations made by each working group was discussed and voted on by all the experts at a face-to-face meeting. Version 2.0 of the statements, created at the face-to-face meeting, was reviewed and corrected by each of the working groups, resulting in version 3.0. This last version underwent a final reading by all the participants for their approval, producing the document presented below.
Proton pump inhibitorsInhibitors of the H+, K+ -ATPase proton pumps in the parietal cells of the glands of the gastric mucosa are imidazole derivatives formed by pyridine and benzimidazole rings bound by a methylsulfinyl group. The first of these medications approved for clinical use in 1989 was omeprazole (OME); later, substitutions were made in the pyridine and/or benzimidazole rings to create pharmacologic alternatives, in an attempt to improve the speed and efficacy of these drugs, as well as prevent possible side effects.
PPIs are acid-labile weak bases that need to be protected by an enteric coating or as coated granules, sometimes combined with bicarbonate, to achieve a temporal neutralization of the intragastric pH that guarantees their integrity and passage into the duodenum for their absorption and better effect; hence the importance of not opening the capsules or breaking the tablets when they are ingested. Once absorbed, they arrive at the parietal cells of the gastric glands through the systemic circulation. The parietal cells require the active canalicular expression of the H+, K+ -ATPase pumps for binding to occur in response to a meal.3 Not all the pumps are active at the time of a meal, so only two-thirds of the pumps can be inhibited; between 3 to 5 days are needed for PPIs to achieve their maximum effect.
PPIs are metabolized in the P450 cytochrome system (CYP450) in the liver; their main pathways are the 2C19 cytochrome (CYP2C19) and 3A4 cytochrome (CYP3A4) routes.4 These pathways are shared with other medications, and so there can potentially be a lesser or greater degree of interactions with PPIs.5
Despite being safe medications for the patient, the use of PPIs for prolonged periods is not exempt from adverse events. The majority of adverse effects have been described in association studies and causality has only been demonstrated in some of them.
The advent of PPIs established a paradigm in the treatment of all diseases related to hydrochloric acid. Their superiority to other medications in the prevention and control of symptoms and the healing of erosions and ulcers, their role in the management of complications, and their benefit in eradicating Helicobacter pylori (H. pylori) are well-established.6
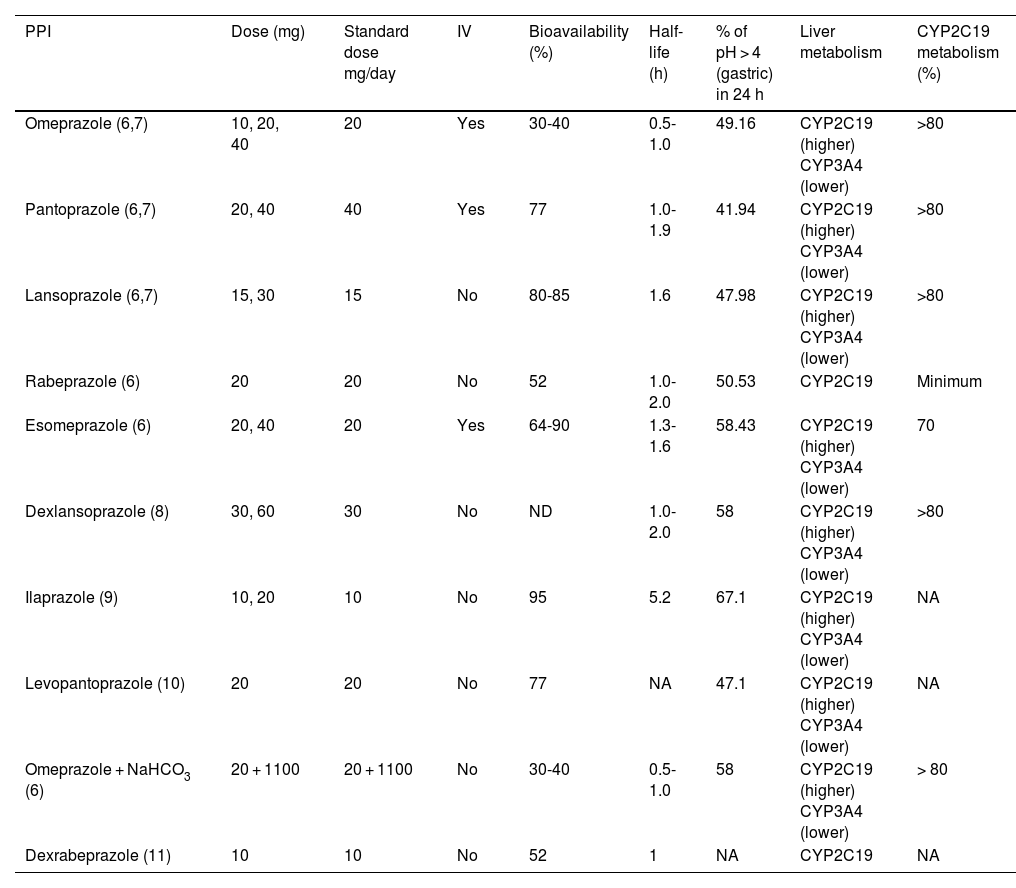
Table 1 lists the PPIs that are available in Mexico, including their doses, administration route, half-life, and the main pathway of liver metabolism.
Commercial PPIs available in Mexico and their pharmacokinetic and pharmacodynamic characteristics.
| PPI | Dose (mg) | Standard dose mg/day | IV | Bioavailability (%) | Half-life (h) | % of pH > 4 (gastric) in 24 h | Liver metabolism | CYP2C19 metabolism (%) |
|---|---|---|---|---|---|---|---|---|
| Omeprazole (6,7) | 10, 20, 40 | 20 | Yes | 30-40 | 0.5-1.0 | 49.16 | CYP2C19 (higher) CYP3A4 (lower) | >80 |
| Pantoprazole (6,7) | 20, 40 | 40 | Yes | 77 | 1.0-1.9 | 41.94 | CYP2C19 (higher) CYP3A4 (lower) | >80 |
| Lansoprazole (6,7) | 15, 30 | 15 | No | 80-85 | 1.6 | 47.98 | CYP2C19 (higher) CYP3A4 (lower) | >80 |
| Rabeprazole (6) | 20 | 20 | No | 52 | 1.0-2.0 | 50.53 | CYP2C19 | Minimum |
| Esomeprazole (6) | 20, 40 | 20 | Yes | 64-90 | 1.3-1.6 | 58.43 | CYP2C19 (higher) CYP3A4 (lower) | 70 |
| Dexlansoprazole (8) | 30, 60 | 30 | No | ND | 1.0-2.0 | 58 | CYP2C19 (higher) CYP3A4 (lower) | >80 |
| Ilaprazole (9) | 10, 20 | 10 | No | 95 | 5.2 | 67.1 | CYP2C19 (higher) CYP3A4 (lower) | NA |
| Levopantoprazole (10) | 20 | 20 | No | 77 | NA | 47.1 | CYP2C19 (higher) CYP3A4 (lower) | NA |
| Omeprazole + NaHCO3 (6) | 20 + 1100 | 20 + 1100 | No | 30-40 | 0.5-1.0 | 58 | CYP2C19 (higher) CYP3A4 (lower) | > 80 |
| Dexrabeprazole (11) | 10 | 10 | No | 52 | 1 | NA | CYP2C19 | NA |
NA: not available; PPI: proton pump inhibitor.
Deprescribing is a carefully planned and supervised clinical process that involves reducing or suspending medications that are not useful, necessary, can cause damage, or do not provide additional benefits in the long term. The primary aim of deprescribing is to reduce the quantity of medications, minimize possible damage, and improve patient quality of life. However, this process can be difficult, especially if the medication is causing no apparent damage and the patient is accustomed to its use. In the specific case of PPIs, there is little solid evidence on how to effectively deprescribe them.7,8
All patients that take PPIs should undergo a periodic check at 4 or 8 weeks of the indications for the use of these medications, and if there is no indication, they should be considered for a deprescribing strategy.8,9
Some operational definitions follow below:8–11
- –
Deprescribing: the process of reducing and/or suspending a medication after considering its therapeutic indication, risks, and benefits.
- –
Abrupt/rapid deprescribing: immediate complete suspension of the customary dose of a medication.
- –
On-demand treatment: administration of a medication decided by the patient based on the presence of symptoms. The patient takes the medication when symptoms are present and suspends it when they disappear.
- –
Intermittent treatment: daily administration of the antisecretory agent for predetermined periods, usually 2-8 weeks, followed by the same time of suspension.
- –
Long-term or continuous treatment: any form of uninterrupted treatment, whether a double dose or a standard dose.
- –
Gradual deprescribing: a gradual reduction of the total daily dose of the antisecretory agent, within a determined period of time, until reaching a minimum maintenance dose that controls symptoms. It can be divided into an intermittent or lower dose, which can be a double dose reduced to a standard dose, a standard dose every other day, or a standard dose divided into 2-3 times a week.
The deprescribing process of a medication consists of 5 steps:11
- 1
Review indications and effectiveness.
- 2
Evaluate the balance between the risk and benefit of chronic use.
- 3
Evaluate patient preferences.
- 4
Decide upon whether to continue, taper, or suspend the medication.
- 5
Deprescribe and monitor the patient.
Recommendation 1: In nonerosive reflux disease (NERD), we recommend standard dose PPI use for 4 weeks.
The short-term treatment goal with PPIs in NERD is to achieve adequate symptom control. This is achieved in 50-60% of patients with a standard dose, and the symptom response rates are similar between all PPIs. The low response rate can be explained due to the great heterogeneity in the studies, given that the majority of clinical trials only consider patients with typical symptoms of gastroesophageal reflux disease (GERD) and endoscopy with no erosions as having NERD, without carrying out the objective measuring of reflux through pH monitoring.12,13 In a meta-analysis, the short-term response with a standard dose of PPI for 4 weeks in patients with erosive reflux disease (ERD) was 72%. It was 50% in patients with NERD and no pH monitoring, and 73% in patients with NERD and positive pH monitoring. It was concluded that in patients with well-defined NERD, the response rate at 4 weeks with a PPI was comparable to the response rate in patients with ERD.14
Recommendation 2: Cicatrization treatment for GERD with a standard dose for mild esophagitis (Los Angeles A and B) for 8 weeks and a double dose in severe esophagitis (Los Angeles C and D) for 8 weeks is recommended. Patients with grade B, C, or D esophagitis should later be given a maintenance dose.
PPIs are the first-choice drugs in erosive GERD.12 The treatment goal is symptom control and mucosal healing, which is achieved with standard dose PPI use in almost 80% of cases of erosive esophagitis.15 A double dose is recommended in patients that do not respond to treatment with the standard dose, as well as in cases of severe esophagitis (Los Angeles C and D), to achieve greater acid suppression.16
In the cases of Los Angeles grade B, C, and D erosive esophagitis, the recommendation is to continue with a maintenance dose, once the first 8 weeks of treatment have passed, to maintain mucosal healing. In those cases, there can be recurrence in just one to 2 weeks after PPI suspension,17 and recurrence in almost 100% of the cases of grade C esophagitis, after 6 months.
Grade A esophagitis is not considered a complication of erosive GERD and less than 60% of those patients have reflux symptoms,18 and so maintenance therapy is not recommended.
Recommendation 3: In functional dyspepsia (FD) for both subtypes (postprandial discomfort syndrome and epigastric pain syndrome), PPIs at the standard dose for 4 weeks are recommended.
Different guidelines recommend standard PPI therapy once a day for 4 weeks as first-line treatment for patients with FD.19,20 A Cochrane meta-analysis that included 6,172 patients from 18 randomized clinical trials (RCTs) confirmed that PPIs are more effective than placebo in reducing overall symptoms in FD (RR 0.88, 95% CI 0.82-0.94, NNT 11). There was no difference between low and high PPI doses, the type of PPI, or the presence of H. pylori.21 Recently, a network meta-analysis of 16 RCTs on 6,017 patients with FD, analyzed symptom improvement with a PPI at a low dose < 20 mg, a standard dose ≥ 20 to ≤ 30 mg, and a high dose > 30 mg. The conclusion was that PPIs were more effective than placebo, in the standard-dose PPI group (RR 0.86, 95% CI 0.78-0.95), as well as at low doses (RR 0.89, 95% CI 0.81-0.97), with no benefit at high PPI doses (RR 0.86, 95% CI 0.74-1.01).22
Even though PPIs are antisecretory drugs, some studies have shown that patients with FD have an increase in duodenal permeability and inflammation, with eosinophil and mast cell infiltration near the submucosal plexus neurons.23 In a recent prospective study, pantoprazole at a dose of 40 mg/day for 4 weeks, not only produced symptom improvement in patients with FD, but also a decrease in duodenal permeability and eosinophilia, as well as in the mast cell count, which could explain the effect of PPIs in FD.24
Recommendation 4: In Helicobacter pylori eradication, we recommend a double-dose PPI for 14 days.
Inadequate acid suppression can reduce the efficacy of H. pylori infection eradication treatment through several mechanisms. Optimum PPI dosing is often overlooked, and their adequate prescription can improve H. pylori eradication rates. In cases of H. pylori infection, utilizing a double-dose PPI and adequate antibiotic regimens for 14 days can increase the probability of success in H. pylori eradication.25
Recommendation 5: In acute nonvariceal upper gastrointestinal bleeding, we recommend an initial PPI 80 mg intravenous (IV) bolus, followed by IV infusion or high-dose PPI every 12 h.
The medical treatment goal is to maintain intragastric pH > 6 for promoting platelet aggregation, stabilizing the clot, and healing.26 Clinical trials have shown that the IV infusion of high-dose PPIs was superior to placebo for reducing the risk of peptic ulcer rebleed.
In patients with active bleeding or nonvariceal upper gastrointestinal bleeding, a recent meta-analysis by Laine et al. found significant benefit in IV high-dose PPI use after endoscopic therapy, given that it reduced the risk of recurrent bleeding.27 However, in a more recent meta-analysis of 6 RCTs, only a decrease in the need for endoscopic treatment in the index endoscopy was shown and it had no impact on mortality or the need for surgery.28 Two consensuses suggest a regimen with high-dose PPI (80 mg bolus at a single dose, followed by 8 mg/h continuous infusion for 72 h),29,30 but intermittent dosing, compared with the infused bolus regimen, has shown similar effectiveness rates.31
Recommendation 6: In the treatment of gastric ulcer, a standard-dose PPI for 4-8 weeks is recommended.
Since the advent of PPIs in the 1990s, numerous clinical trials have shown that these drugs were more efficacious than the histamine-2 receptor antagonists (H2RAs) in treating gastroduodenal ulcers,32 due to their greater potency in and duration of inhibiting acid production. The standard dose is recommended (e.g., OME 20 mg, esomeprazole 20 mg, pantoprazole 40 mg, etc.), once a day. Symptom resolution is fast, and healing is produced at 4 weeks of treatment in most patients. For patients that do not have complete healing after initial treatment, cicatrization generally occurs during the next four weeks of treatment.33,34 If the ulcer was associated with H. pylori, eradication treatment is necessary to prevent recurrence.
The patients with idiopathic ulcers (negative for H. pylori and with no nonsteroidal anti-inflammatory drug [NSAID]/aspirin use) can have a high recurrence rate, and most likely require long-term treatment (>8 weeks)35.
Recommendation 7: In the treatment of duodenal ulcer, a standard-dose PPI for 4 weeks is recommended.
Standard dose PPIs for 4 weeks provide healing rates above 90% for duodenal ulcers.32 The recommended dose in patients with active duodenal ulcer is the standard dose, once a day. Symptom resolution is rapid, and healing is produced in 2 weeks in the majority of patients.26,32 For patients that do not heal after initial treatment, cicatrization generally occurs during the following 2 weeks of treatment. In a meta-analysis of 24 controlled clinical trials that included 6,188 patients, pantoprazole 40 mg/day and lansoprazole 30 mg/day for 4 weeks significantly increased the healing rate (RR 2.96, 95% CI 1.78-5.14 and RR 2.04, 95% CI 1.13-3.53, respectively), compared with H2RAs.36 In addition, a network meta-analysis showed no significant differences in the 4-week healing rate of duodenal ulcer treated with different PPIs.
As in cases of gastric ulcer, if there is H. pylori infection, it should be treated with the appropriate eradication regimen.
Recommendation 8: PPI use as a prophylactic measure is recommended in patients admitted to the intensive care unit with risk factors for stress ulcers.
Stress ulcers (part of stress-related mucosal disease) are often present in patients admitted to the intensive care unit (ICU), and are one of the significant causes of prolonged stay, as well as of increased mortality.37 Approximately 75 to 100% of patients that undergo endoscopy within the first 72 h following the appearance of critical entities are estimated to have lesions in the mucosa and clinically present with manifestations, such as blood in stools, melena, coffee-ground emesis through a nasogastric tube, and hematochezia that conditions hemodynamic instability.38
Calculations in meta-analyses suggest that significant clinical bleeding is around 1%, but despite its low frequency, it is a cause of significant mortality in that group of patients.37 The main risk factors include mechanical ventilation for more than 48 h (RR 15.6) and the presence of coagulopathy (RR 4.3).39 In different meta-analyses, the use of prophylaxis in that group of patients reduces risk by 60%. There is no difference between the frequency of PPI use (one or two times a day), dose, or administration route (enteral vs IV), for reducing the bleeding risk. Therefore, the standard dose is recommended, and the treatment should be given until the patient no longer presents with risk factors.37,40,41
On-demand PPI indications and dosesRecommendation 9: We recommend on-demand PPI use in the maintenance treatment of NERD and mild esophagitis.
On-demand treatment with PPIs consists of the patient deciding on the PPI dose to take when they experience GERD symptoms and suspending treatment when the discomfort disappears. This regimen is different from intermittent treatment, in which the physician prescribes a daily PPI dose for a short period (1-2 weeks), regardless of the presence of symptoms. The standard dose of PPIs is utilized, and the minimum dose necessary for controlling symptoms is recommended.12,42,43
There are two indications for treatment with on-demand PPIs: NERD and Los Angeles grade A mild esophagitis.12,42,43 The efficacy of this treatment in NERD and mild esophagitis has been evaluated in 2 recent meta-analyses.44,45 In the first, 10 RCTs with 4,574 patients were evaluated, comparing on-demand treatment with daily treatment or placebo. The results showed that on-demand treatment with PPIs was superior to daily treatment (RM 0.50, 95% CI 0.35-0.72) in the two patient groups. Compared with placebo, on-demand treatment was effective in patients with NERD or mild esophagitis (OR 0.22, 95% CI 0.13-0.36 and OR 0.18, 95% CI 0.11-0.31, respectively). The second meta-analysis included 11 RCTs on patients with NERD and mild esophagitis, and the results showed that on-demand treatment with PPIs had similar outcomes to continuous treatment, in terms of therapeutic failure (OR 1.26, 95% CI 0.76-2.07). Both meta-analyses concluded that on-demand therapy is effective in the management of NERD and mild esophagitis. It should be clarified that the definition of mild esophagitis in those two studies was based on Savary Miller classification grade I, which is equivalent to Los Angeles classification grade A. Even though expert opinion is that on-demand maintenance treatment can be recommended in patients with Los Angeles grade B esophagitis, controlled clinical trials that support said recommendation are needed.12,42,43
In addition, on-demand PPI use has been shown to be the most cost-effective strategy in the maintenance treatment of NERD.46
Long-term PPI indications and dosesRecommendation 10: Standard dose maintenance treatment is recommended in patients that have the B, C, and D erosive variants of GERD.
The chronic and recurrent nature of reflux disease affects quality of life and causes complications; thus, it requires optimum control for preventing said situations. This is especially true in the case of the erosive phenotype of reflux disease because it has the potential to induce complications (bleeding, stricture). There is greater scientific evidence on the use of PPIs in reflux disease, especially in the erosive phenotype.
The erosive phenotype spectrum of reflux disease is currently based on the Los Angeles classification, which has been extensively validated.47 Subsequent research has determined that interobserver variability is high for the Los Angeles grade A classification and that a conclusive diagnosis of GERD cannot be made in that subgroup of patients.48 On the other hand, patients with Los Angeles grade B classification have a conclusive reflux diagnosis by demonstrating the presence of elevated acid exposure and a similar treatment response to the Los Angeles C variant.49
Given the above, the treatment for inducing healing is required in these erosive variants (B, C, and D), as well as maintenance treatment. The evidence suggests that the erosive variant relapse rate, 6 months after having suspended the treatment of single-dose PPIs, is from 6 to 42%. Studies suggest that 6 months after suspending treatment with PPIs, only 10% of the patients with erosive esophagitis are still in remission.50 Therefore, PPIs are indicated as maintenance therapy for preventing the recurrence of both symptoms and mucosal lesions, and the long-term use of PPIs has been approved by the US Food and Drug Administration (FDA).51–56
A RCT showed that OME 20 mg, once a day (QID), was capable of maintaining 80% of patients with erosive esophagitis free from mucosal lesions after 12 months of continuous treatment. It also reported that OME was significantly superior to both ranitidine and cisapride.50 Similar results were later obtained with lansoprazole (15 and 30 mg, QID), which was shown to be capable of maintaining around 80% of patients with erosive esophagitis in remission at one year.57 Thus, PPIs are useful as maintenance therapy for preventing recurrences of symptoms, as well as mucosal lesions, in patients with the erosive variant, especially those with more severe lesions (Los Angeles B to D). With few exceptions, such as severe erosion grades, half the healing dose of a PPI tends to be adequate for maintenance.58 As a result, the maintenance of the lowest PPI dose necessary for controlling symptoms and esophageal mucosa healing, as a long-term treatment goal, is recommended.
Recommendation 11: Maintenance PPI use at the dose that achieves symptom control is recommended in patients with Barrett’s esophagus, as well as the standard dose for chemoprotection.
Barrett’s esophagus is the replacement of normal squamous epithelium of the distal part of the esophagus with specialized metaplasia (with goblet cells). It is the most important complication of GERD because it is considered a premalignant lesion. It is associated with maximum acid exposure, compared with other forms of the disease, strongly justifying the use of potent antisecretory therapy with PPIs.59 Some studies have shown that PPIs can reduce its progression to adenocarcinoma.60
A meta-analysis based on observational studies concluded that PPI administration led to a reduced risk for progression to low and high-grade dysplasia and to adenocarcinoma (OR 0.29, 95% CI 0.12- 0.79). The same meta-analysis also found that risk reduction was greater with PPI use above 2-3 years (OR 0.45, 95% CI 0.19-1.06 vs OR 1.09, 95% CI 0.47-2.56, respectively).61
Therefore, a standard PPI dose once a day is recommended for preventing the transformation of metaplasia into dysplasia and neoplasia, and this treatment should be continued for life.34,62,63 Based on these results, the recommendation is for asymptomatic patients with Barrett’s esophagus to maintain long-term treatment with PPIs, even if there is no solid evidence in this regard.
Recommendation 12: In peptic stricture, long-term, continuous treatment with a standard-dose PPI is recommended.
The incidence of esophageal stricture has drastically decreased over the past 2 decades, in parallel with a marked increase in PPI use. Consequently, the number of studies and their quality have decreased, and evidence is scarce. One study compared lansoprazole 30 mg, QID, versus ranitidine 300 mg twice a day (BID). The lansoprazole study arm required fewer dilatations and had a higher number of patients with no dysphagia than the ranitidine arm.64
Another study compared OME 20 mg, QID, and ranitidine 150 mg, BID, in patients with the erosive variant and concomitant peptic stricture. OME administration improved dysphagia and lowered the frequency of stricture dilatation.65
In patients with peptic stricture, the intervention by experts in therapeutic endoscopy is required for performing dilatation and rehabilitating the esophagus. Uninterrupted concomitant PPI use is necessary in this population for preventing progression and recurrence.
Recommendation 13: In patients with acid hypersecretion syndromes, continuous treatment with PPIs at a dose that is sufficient for long-term symptom control is recommended.
Acid hypersecretion syndromes, e.g., Zollinger-Ellison syndrome (ZES), are characterized by extreme acid secretion due to a neuroendocrine tumor that ectopically secretes gastrin, resulting in diseases, such as peptic ulcer and severe, continuous, recurrent GERD that is dependent on antacid treatments.66 The exact prevalence and incidence of ZES are unknown but it is a rare disease. In the US, its frequency is estimated at one per every million inhabitants and presentation age is 7 to 90 years.67
Diagnosis is made with a plasma gastrin level > 1,000 pg/mL and a baseline acid secretion of >15 mEq/h or even >5 mEq/h in patients with gastrectomy or hypergastrinemia associated with a pH < 2. If it is not possible to obtain baseline acid secretion, hypersecretion syndrome can be ruled out with a gastric pH > 2, in the absence of antisecretory medications. If the pH is >2 and the plasma gastrin level is between 100-1,000 ng/mL, a secretin stimulation test should be carried out.67
The most common symptoms are abdominal pain, diarrhea, and heartburn, followed by nausea and vomiting in some patients. The endoscopic presence of prominent gastric folds is a sign of acid hypersecretion and 25% can present with upper gastrointestinal bleeding.67
In the 1980s, with the generalized use of gastric H+, K+ -ATPase inhibitors, the medical control of gastric acid hypersecretion was made possible in almost all patients. Due to the greater potency and action duration of PPIs, enabling their doses to be given once or twice a day, they have become the drugs of choice for the treatment of this disease. Numerous acid secretion control studies on patients with hypersecretory states support the use of PPIs at varying doses, and they should be titrated individually, utilizing the established criteria for acid suppression in those patients. In patients with intact stomachs, with no GERD or moderate-to-severe multiple endocrine neoplasia type 1 (MEN1), the generally accepted criterion is the use of antisecretory drugs that induce acid suppression at <10 mEq/h for the hour before the next dose of the drug. In complicated patients (moderate-severe GERD, previous Billroth 2 surgery, or MEN1), greater acid inhibition at < 1 mEq/h may be necessary, depending on the upper gastrointestinal endoscopy findings.66 Lansoprazole showed safety and efficacy in secretion control for 10 years, in treating hypersecretors.68 The largest cohort series, with 303 patients, reported a median 14 years of treatment with PPIs and/or H2ARs and treatment of up to 48 years.66
Patients with acid hypersecretion syndrome have 2 problems: the control of acid hypersecretion and the treatment of gastrinomas, which are malignant in 60 to 90% of patients. Surgery continues to be the only possibility for the cure and treatment of the two problems.66
Recommendation 14: In eosinophilic esophagitis, we recommend long-term, continuous, standard-dose PPI use.
Eosinophilic esophagitis (EoE) is a disease that causes symptoms of esophageal dysfunction. At least 15 eosinophils per high power field (60 eos/mm2) are seen in biopsies, and other causes of esophageal eosinophilia must be ruled out in this group of patients.69
The first evidence on the potential usefulness of PPIs for achieving histologic and clinical remission of EoE was published in the pediatric literature.70 Treatment with high doses of PPIs used to be a diagnostic strategy for differentiating EoE from peptic esophagitis; at present it is used as treatment for EoE.71 The recommended PPI doses in adults are 20 to 40 mg of OME, BID, or its equivalent, and in children, 1-2 mg/kg of OME daily or its equivalent. The long-term strategy is to use the minimum efficacious dose for maintaining remission. PPIs at half the initial dose maintain histologic and clinical remission in a minimum of 75% of patients after at least one year of follow-up. The majority of patients that relapse recover remission after increasing the dose. There are no published data on safety problems of PPIs in patients with EoE.70
The benefits of treatment with PPIs in EoE are probably multifactorial, and include esophageal epithelial barrier repair and possible direct anti-inflammatory effects on certain cytokines.72–74 in vitro, PPIs inhibit Th2-cytokine-induced eotaxin-3 expression, by interfering with the binding of the signal transducer promoter and the transcription activator.75 A recent meta-analysis demonstrated that treatment with PPIs induced clinical response and histologic remission in 60.8 and 50.5% of patients, respectively.71,74
Recommendation 15: In patients with chronic NSAID/ASA use and high risk for upper gastrointestinal bleeding, we recommend continuous PPI use at the standard dose.
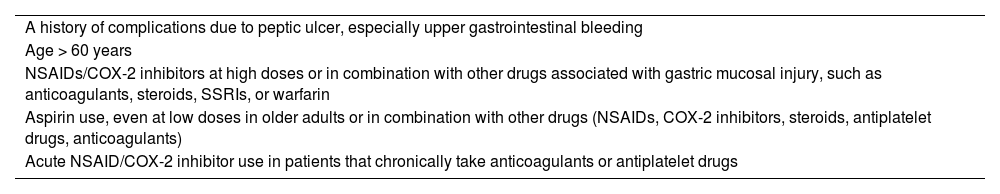
Both NSAIDs and ASA are often chronically indicated, especially in older adults. This is particularly true with ASA, for the prevention of cerebrovascular events and ischemic heart disease. However, there is a risk for developing complications in the upper gastrointestinal tract, such as peptic ulcer disease and gastrointestinal bleeding. In said context, numerous studies have been conducted, with results favoring the use of PPIs in combination with NSAIDs or ASA for reducing that risk. Table 2 identifies the risk factors for gastrointestinal bleeding and prophylaxis. In a systematic review of 41 RCTs, PPIs reduced the endoscopic risk for duodenal (RR 0.44, 95% CI 0.26-0.74) and gastric (RR 0.40, 95% CI; 0.32-0.51) ulcers associated with chronic NSAID use.76
Risk factors for upper gastrointestinal bleeding.
| A history of complications due to peptic ulcer, especially upper gastrointestinal bleeding |
| Age > 60 years |
| NSAIDs/COX-2 inhibitors at high doses or in combination with other drugs associated with gastric mucosal injury, such as anticoagulants, steroids, SSRIs, or warfarin |
| Aspirin use, even at low doses in older adults or in combination with other drugs (NSAIDs, COX-2 inhibitors, steroids, antiplatelet drugs, anticoagulants) |
| Acute NSAID/COX-2 inhibitor use in patients that chronically take anticoagulants or antiplatelet drugs |
COX-2: cyclooxygenase-2; NSAID: nonsteroidal anti-inflammatory drug; SSRIs: selective serotonin reuptake inhibitors.
In a meta-analysis, the effect of co-administering PPIs in patients with no history of peptic ulcer disease, chronically taking low-dose ASA (30-325 mg) was evaluated. Concomitant PPI use was associated with a 73% reduction in the risk for developing peptic ulcer, compared with not using a PPI (RR 0.27, 95% CI 0.17-0.42, p < 0.001), and 50% reduction in the risk for gastrointestinal bleeding (RR 0.50, 95% CI 0.32-0.80).77
On the other hand, in a case-control study conducted on 1,382 NSAID/COX-2 inhibitor users with gastrointestinal complications, the combination of a COX-2 inhibitor with a PPI resulted in a greater reduction in the risk for upper gastrointestinal complications associated with NSAIDs (OR 0.36; 95% CI 0.28-0.47); celecoxib was superior to the combination of a nonselective NSAID with a PPI.78
Recommendation 16: In patients treated with steroids, we do not recommend chronic PPI use, unless the steroids are prescribed in combination with an NSAID/ASA.
The routine use of PPIs is not indicated in patients taking steroids alone for a medical condition, unless the steroids are associated with NSAIDs. In a study conducted on 138 patients with autoimmune disease treated with corticosteroids, 20% presented with peptic ulcer disease. In the multivariate analysis, risk factors were identified: age ≥ 60 years (OR 6.80, p = 0.001), smoking (OR 7.94, p = 0.004), and NSAID use different from COX-2 inhibitors (OR 4.71, p = 0.030), whereas the protective factor was H. pylori infection (OR 0.20, p = 0.022).79 Likewise, in a meta-analysis that included 6,602 patients, the frequency of gastrointestinal complications was compared with a placebo group (n = 3,267) and a steroid group (n = 3,335). In the placebo group, 0.3% developed peptic ulcer versus 0.4% in the steroid group (p > 0.05), concluding that peptic ulcer disease is a rare complication of steroid therapy.80 In a more recent meta-analysis, there was also a low prevalence of peptic ulcer disease in patients with systemic corticosteroids, occurring in 0.4-1.8% of patients. Therefore, routine prophylaxis with PPIs is not recommended.75
Recommendation 17: In patients with upper gastrointestinal bleeding risk factors that are taking antiplatelet drugs or anticoagulants, we recommend prophylaxis with PPIs.
Gastrointestinal bleeding is the most frequent bleeding complication in patients with chronic antiplatelet drug use. In a systematic review, 71,277 participants were included. The results showed that PPI use in patients with dual antiplatelet therapy (ASA and clopidogrel) was associated with a significant reduction in adverse gastrointestinal events (OR 0.38, 95% CI 0.21-0.68, p = 0.001); specifically, upper gastrointestinal bleeds with clopidogrel plus PPI exposure (OR 0.31, 95% CI 0.19-0.51, p < 0.001).81
In the randomized, double blind, placebo-controlled phase 3 Clopidogrel and the Optimization of Gastrointestinal Events Trial (COGENT), the efficacy and safety of the combination of clopidogrel (75 mg) plus OME (20 mg) versus clopidogrel alone, was evaluated. For financial reasons, the study was stopped at 3,761 patients. Upper gastrointestinal events were reported at 1.1% in the OME group and 2.9% in the placebo group, 180 days after the randomization (OR 0.34, 95% CI 0.18-0.63, p < 0.001). More importantly, there was no significant increase in the risk of cardiovascular events with the concomitant use of OME and clopidogrel (p = 0.98).82 The risk for gastrointestinal bleeding was found to be higher in patients with dual antiplatelet therapy in the form of ticagrelor or prasugrel, compared with clopidogrel. In their 2017 guidelines on dual antiplatelet therapy in coronary artery disease, the European Society of Cardiology recommends PPI use in that context (class I, level of evidence B).83
Recommendation 18: In patients taking two or more antiplatelet drugs or an antiplatelet drug plus oral anticoagulation, we recommend prophylaxis with a PPI.
In their 2020 expert review on antiplatelet therapy and anticoagulation in patients with atrial fibrillation or atherosclerotic cardiovascular disease, the American College of Cardiology (ACC) recommends starting or continuing a PPI in patients with ≥ 2 antithrombotic agents, as well as avoiding concomitant NSAID use, to reduce the risk for gastrointestinal bleeding. Once the patient is only taking oral anticoagulation, deprescribing the PPI is recommended, unless there is some other indication for its continuation.84
Recommendation 19: In chronic pancreatitis with exocrine pancreatic insufficiency, we do not recommend the addition of a PPI, given that only enteric-coated pancreatic enzymes are available in Mexico.
The aim of pancreatic enzyme supplementation, in the context of chronic pancreatitis, is suppression of the steatorrhea caused by the condition. All the factors involved in the adequate response to therapy must be kept in mind, even if there has not been therapeutic failure. The most important of these is corroborating that the dose of the supplemented pancreatic enzymes is the correct one. Physiologic studies conducted on the correct absorption of pancreatic enzymes focused on the effects of the gastrointestinal tract on the formulation. Postprandial gastric pH decreased to < 4 after 40 minutes and postprandial duodenal pH after 100 minutes.85 Said pH value is the point at which lipase is irreversibly inactivated and considerably reduces its activity at more advanced sites of the gastrointestinal tract. This is the rationale behind the theory that inhibiting acid, thus maintaining the gastric and duodenal pH above 4, is a factor involved in pancreatic enzyme supplementation to consider. That evidence was established in publications in the 1970s and led to the evolution of enteric coating of the formulations. This advance meant the drug would not have a pH-dependent degradation, preserving its lipolytic activity. The recommendation to inhibit acid through a PPI remains absolute for the non-enteric-coated enzyme formulations.86 However, there is a subgroup of patients that have normal-to-high acid secretion, together with insufficient pancreatic bicarbonate secretion for neutralizing the pH of chyme. PPI administration is suggested in those patients, even when using enteric-coated enzyme supplementation formulations.87 Thus, the recommendation to inhibit acid with a PPI is still valid, simply as a factor that can aid in the correct absorption, together with the factors of adequate dose and enteric coating.88
Adverse effects of long-term PPI useRecommendation 20: In all patients undergoing long-term treatment with PPIs, we recommend that the physician monitor the possible presentation of adverse effects and indicate deprescribing the PPI when the patient no longer needs it or there is no indication for its use.
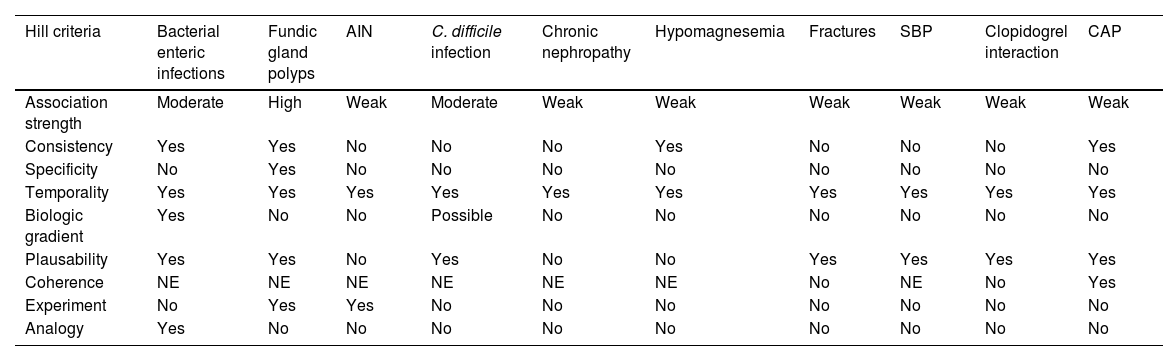
The present expert panel analyzed the evidence, and these are the adverse effects in which there is cause and effect (causality) (Table 3).
Bradford Hill criteria for PPI adverse effect causality.
| Hill criteria | Bacterial enteric infections | Fundic gland polyps | AIN | C. difficile infection | Chronic nephropathy | Hypomagnesemia | Fractures | SBP | Clopidogrel interaction | CAP |
|---|---|---|---|---|---|---|---|---|---|---|
| Association strength | Moderate | High | Weak | Moderate | Weak | Weak | Weak | Weak | Weak | Weak |
| Consistency | Yes | Yes | No | No | No | Yes | No | No | No | Yes |
| Specificity | No | Yes | No | No | No | No | No | No | No | No |
| Temporality | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Biologic gradient | Yes | No | No | Possible | No | No | No | No | No | No |
| Plausability | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes |
| Coherence | NE | NE | NE | NE | NE | NE | No | NE | No | Yes |
| Experiment | No | Yes | Yes | No | No | No | No | No | No | No |
| Analogy | Yes | No | No | No | No | No | No | No | No | No |
AIN: acute interstitial nephritis; CAP: community-acquired pneumonia; NE: not evaluated.
The main mechanism of action of PPIs is the inhibition of gastric acid secretion. One of the main functions of hydrochloric acid is gastric acidification, which prevents the passage of bacteria from the gastric chamber to the small bowel. Gastric acid inhibition has inconsistently been associated with small intestinal bacterial overgrowth (SIBO).89 In a cohort study of 17,598 patients with anticoagulant and ASA use that were randomized to receive pantoprazole 40 mg versus placebo, prolonged PPI use (23 months) conferred a risk for acute diarrhea (HR 1.33, 95% CI 1.01-1.75, p = 0.04, and a number needed to harm of 900).90
Evidence shows that PPI use increases the risk of acute interstitial nephritisAcute interstitial nephritis (AIN) is one of the main causes of acute kidney injury (AKI). The common etiologies of AIN are medication-induced, infectious, and idiopathic. The cause associated with medications is responsible for more than two-thirds of cases. AIN etiology changes with aging, given that it is more frequent in the elderly, especially due to PPIs.91
In 1992, Ruffenach et al. reported the first case of AIN, after which there were several anecdotal reports and numerous cross-sectional and cohort studies that described a consistent association between PPI use and the risk of AIN.92 In this context, current PPI use has been documented to be associated with a significantly higher risk for AIN. In the first reports related to PPI use, the appearance of AIN was considered rare, idiosyncratic, and difficult to predict. Suarez et al. reported the analysis of 64 cases (59 histologically confirmed) and the majority were associated with OME use, and to a lesser degree, with pantoprazole, esomeprazole, lansoprazole, and rabeprazole.93
In a systematic review conducted by Nochaiwong et al. that reported the results of 3 studies (n = 585,296), PPI use was associated with a significantly higher risk for AIN, compared with no PPI use, with a combined RR of 3.61, 95% CI 2.37-5.51, and p < 0.001.94
Given the above, due to the publication of several nonrandomized epidemiologic studies on the possible association of PPIs with AIN and the fact that those studies have critical limitations inherent to data sources, there is the possibility of a risk of surveillance bias. To prevent those confounding factors, Rajan et al. conducted a study that systematically reviewed the literature and evaluated the risk for bias, utilizing the Risk Of Bias In Nonrandomized Studies – of Interventions (ROBINS-I) tool. Of the 620 registers initially identified, 26 studies met the a priori eligibility criteria and underwent a risk of bias evaluation. Only 3 of those studies evaluated AIN, reporting the association between PPIs and AIN, with an adjusted HR of 3.00 and 95% CI of 1.47- 6.14 reported in a single study on 581,184 patients in Canada, whereas the adjusted OR varied from 2.05, 95% CI 1.52-2.72, in 4,143 patients in the United States to 3.20, 95% CI 0.80-12.79, in 3,415 patients in the United Kingdom.95
Another setting that has been studied is the development of renal complications as a cause of hospitalization in PPI users. Several reports have shown there is a higher risk for hospital admission due to AKI and AIN, within 120 days after PPI exposure. The reported AIN rate was higher in patients that received a PPI, compared with controls: 0.32 versus 0.11 per 1,000 person-years, respectively (HR 3.00, 95% CI 1.47-6.14).96
In conclusion, the association between PPI use and the risk of AIN has been documented. Nevertheless, more research is justified for clarifying the underlying mechanisms and establishing the precise causal relation between PPIs and AIN.
The evidence shows that PPI use increases the risk of fundic gland polypsThe first report on fundic gland polyps (FGPs) during treatment with OME was conducted in 1992 by Graham.97 FGPs are the most prevalent type of gastric polyps in recent studies in Western populations and they have been found in up to 5% of patients undergoing upper gastrointestinal endoscopy. The exact mechanism by which PPIs induce FGPs is not yet fully understood but may be related to the stagnation of fluid in the oxyntic glands, conditioning cystic dilations; this occurs without direct association of the secondary hypergastrinemia the PPI induces.98 Huang et al. reported a prevalence of 2% in a study on 10,094 patients that underwent gastroduodenal endoscopy, albeit there are reports describing a higher prevalence of 28%. In addition, those authors found that 66.8% of the patients with FGPs had H. pylori infection, and that age and prolonged PPI use were risk factors for the presence of FGPs; the long-term use of PPIs was a particularly strong risk factor for the appearance of FGPs.99 In 2016, Martin et al. conducted one of the first meta-analyses to attempt to clarify the relation of PPIs with FGPs, analyzing 339 peer-reviewed articles and abstracts; 20 of those articles met all the criteria and included a total of 40,218 subjects. The meta-analysis of 12 studies revealed an increase in FGPs in PPI users, compared with controls (OR 2.46, 95% CI 1.42-4.27, p = 0.001), particularly in persons taking PPIs for at least 6 months (OR 4.71, 95% CI 2.22-9.99, p < 0.001) or 12 months (OR 5.32, 95% CI 2.58-10.99, p < 0.001). Even though that meta-analysis was limited due to the quality of the grouped studies, it provided solid evidence on an association between PPI use and FGP development that was most likely causal.100 The results of another meta-analysis that analyzed 12 studies with 87,324 patients coincided with the findings that long-term use of PPIs (≥ 12 months) was associated with a higher risk for FGPs.101
With no established causalityRecommendation 21: We do not recommend suspending PPIs, given that no cause-effect relation has been established in the following conditions: Clostridioides difficile or COVID-19 infection, vitamin B-12 deficiency and hypomagnesemia, cardiocerebrovascular events, clopidogrel use, bacterial overgrowth, osteoporosis/fractures, gastrointestinal tumors, dementia, pneumonia, chronic nephropathy, and spontaneous bacterial peritonitis in the context of cirrhosis.
Even though PPI use has been associated with a higher risk of Clostridioides difficile (C. difficile) infection, the pathophysiologic mechanism involved in the increased risk is not clear.102 In a meta-analysis of observational studies, PPI users were found to have an increased risk for C. difficile infection (RR 1.3, 95% CI 1.1-1.4) and infection recurrence (OR 1.5, 95% CI 1.2-1.9).103,104 However, by limiting the analysis to cohort studies and randomized trials, great heterogeneity and the quality of the evidence restrict the risk to only older adults and critically ill hospitalized patients.105
Long-term treatment with PPIs has been associated with vitamin B deficiency due to the role of gastric acid and pepsin in the release of the vitamin from ingested nutrients.106,107 Even though a meta-analysis of 25 observational studies found that PPI users had a slightly higher risk (OR 1.42, 95% CI 1.16-1.73, I2 = 54%), the great heterogeneity and inconsistency in the levels for defining deficiency, as well as the low OR, reduced the accuracy for establishing causality.107
PPI use could cause hypomagnesemia due to mutations that decrease the affinity of the transient receptor potential melastatin 6 and 7 channels (TRPM6 and TRPM7) in the enterocytes due to changes in the intraluminal pH, even though most magnesium absorption at the intestinal level occurs through the paracellular route.108 A meta-analysis of 14 observational studies found an increased risk (RR 1.44, 95% CI 1.13-1.76, I2 = 85.2%) in PPI users but definitive associations cannot be made due to their great heterogeneity.109
Clopidogrel is a prodrug that requires CYP2C19 activation to exert its antiplatelet effect, but OME is also metabolized by said enzyme. OME has been theorized to interfere with clopidogrel activation, reducing its therapeutic efficacy and increasing the risk of cardiovascular events in patients with dual antiplatelet therapy. Initially, there was concern about that interaction and ex vivo and observational studies were conducted that produced mixed results.110 However, later clinical trials, such as the PRINCIPLE-TIMI 44 and TRITON-TIMI 38, found no significant differences in the adverse clinical results between PPI users and non-users.82 Finally, in 2009, the FDA warned against the combination of OME with clopidogrel, and in 2016, despite the evidence on the combination’s safety, the FDA reiterated avoiding said combination. It is worth mentioning that there is no such restriction with other PPIs.
The risk of developing SIBO has been associated with PPI use. A meta-analysis conducted in 2017 reported that the combined OR for developing SIBO was 1.71 (95% CI 1.20-2.43).111,112 A study carried out in Mexico showed that a short course (7 days) of a PPI produced SIBO in 7.8% of healthy subjects.113 Additional research is possibly needed to better understand the mechanisms underlying said association and to develop strategies that aid in reducing the risk for SIBO in patients treated with PPIs.114
PPI-induced hypochlorhydria can increase osteoclastic activity and reduce calcium absorption, thus decreasing bone density.115 Even though an association between PPI use and bone fractures has been postulated (OR 1.41, 95% CI 1.16-1.71; I2 = 73%),116 no greater risk in women with rheumatoid arthritis, above 80 years of age, or healthy postmenopausal women has been demonstrated.117 A prospective study on 17,598 stable older adults with cardiovascular or peripheral arterial disease treated with rivaroxaban and/or ASA, with follow-up at 3 years, found there was no higher risk of fractures in pantoprazole users (OR 0.96, 95% CI 0.79-1.17, p = 0.71).118
Observational studies and meta-analyses have shown a link between PPIs and a greater risk for gastric cancer.119 Nevertheless, if the underlying disease for administering PPIs is associated with gastric cancer, this could result in an apparent association between PPIs and gastric cancer. A recent systematic review and meta-analysis found no association between PPI use and gastric cancer.120
PPIs have been associated with an increased risk for dementia.121 Gomm et al. analyzed data from more than 73,000 older adults without dementia at the start of the study and evaluated the risk for developing dementia in relation to PPI use. They found that regular PPI use was associated with a higher risk for developing dementia, compared with the subjects that did not use them. In addition, they observed that this association was stronger in those with a greater number of PPI prescriptions. However, the fact that said study was observational and cannot show a causal relation between PPI use and dementia should be kept in mind.122
In the cohort study conducted by Laheij et al., an association between PPI use and greater risk for community-acquired pneumonia was found.123 The mechanisms that could explain said effect include the increase in gastric pH that favors bacterial and viral colonization, as well as the inhibition of certain immune cell function, which could increase the susceptibility to respiratory infections. It is striking that patients on assisted mechanical ventilation have a greater risk for PPI-associated pneumonia due to the colonization of intestinal pathogens in the respiratory tract and the risk for gastric aspiration.124
A study carried out by Klatte et al. that involves more than 100,000 PPI users in Sweden revealed that both the start and the cumulative use of PPIs was associated with a higher risk for progression of chronic kidney disease (CKD), compared with other antacids.124 Nevertheless, that association is recognized as not demonstrating causality, and the exact mechanisms by which PPIs could contribute to CKD progression are still unclear. Even though alterations of the gut microbiota and hypomagnesemia are mentioned, the need for more research is emphasized.125
The association between PPI use and the risk for COVID-19 infection or the development of complications is not well-defined.105,126,127 Among the meta-analyses of retrospective and cohort studies, the one conducted by Alhumaid et al. reported an OR of 1.80 (95% CI 1.41-2.31, I2 = 72%). If only the cohorts are taken into account (OR 1.55, 95% CI 1.16-2.06, p < 0.00001, I2 = 74%), there is an important heterogeneity of the results, resulting in a low association strength in the meta-analysis.128
With respect to spontaneous bacterial peritonitis, a meta-analysis by Wong et al. was designed to search for infectious complications and mortality in patients with cirrhosis. Despite the low heterogeneity of their results (HR 1.75, 95% CI 1.64-1.85, p < 0.001, I2 = 0%), the association strength was also low. However, we must consider this possible effect of PPIs in patients with cirrhosis and suspend them when they have no clear indication.129
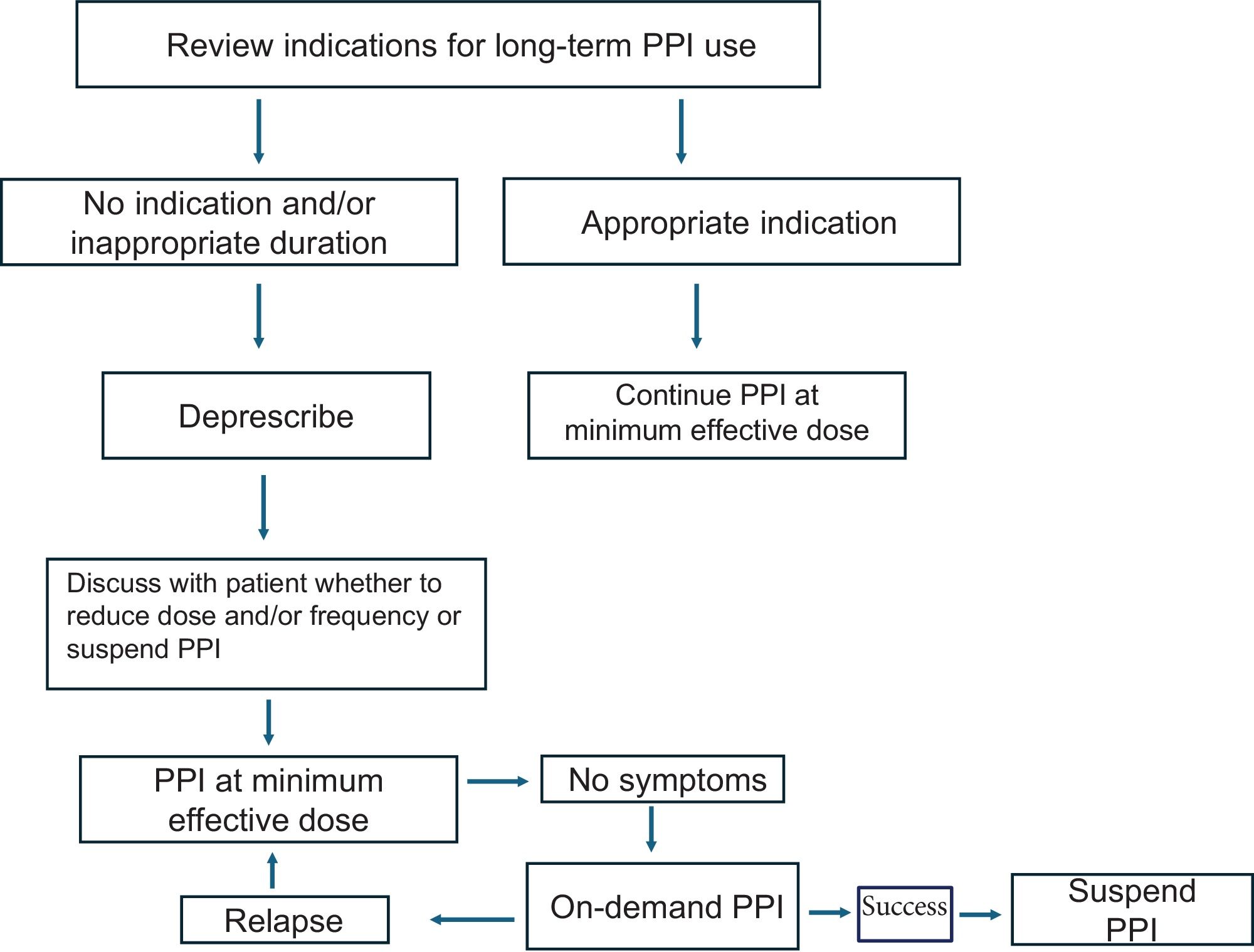
How can a PPI be deprescribed?Recommendation 22: We recommend the sudden, gradual, or intermittent deprescribing of PPIs in patients for whom PPI use is not indicated.
Deprescribing PPIs is justified under conditions in which there is no indication for their continuous use, or once the symptoms for which the PPI therapy was started are now resolved (Table 4). Deprescribing PPIs can be recommended after 4 to 8 treatment weeks. At present there are no studies that identify the best manner for deprescribing: abrupt, gradual, or intermittent (on-demand). Abrupt deprescribing can lead to acid secretion rebound due to a state of hyperacidity, which has been reported with therapies of 4 weeks and longer, even at low PPI doses.131
Indications for deprescribing PPIs.130
| Mild esophagitis, Los Angeles A, with symptom control after 8 weeks |
| NERD controlled after 4 weeks |
| Dyspepsia controlled after 4 weeks |
| Treatment of gastric ulcer after 4-8 weeks |
| Treatment of duodenal ulcer after 4 weeks |
| After Helicobacter pylori eradication therapy for 14 days |
| Inappropriate indication for gastroprotection in patients with comorbidities |
| Patients admitted to the intensive care unit that no longer present with risk factors for stress ulcers |
NERD: nonerosive reflux disease; PPI: proton pump inhibitor.
Gradual deprescribing is carried out in a reduction period of 2 to 4 weeks, decreasing the double-dose to standard dose for 2 weeks and then suspending the PPI. Another approach is reducing the standard dose to a dose every 48 h and then suspending the PPI. Other authors have used the standard dose, switched to an H2RA, and then suspended the PPI.132
In their study, Inadomi et al. studied 73 patients with GERD that had adequate symptom relief in treatment with PPIs. Deprescribing was started as follows: The double-dose was reduced to the standard dose and then the drug was suspended. If the symptoms of heartburn and regurgitation recurred 2 weeks before the follow-up appointment, the lowest PPI dose was restarted. If the symptoms recurred after the 2 weeks, treatment with an H2RA and/or a prokinetic was started. In the case of symptom persistence, those 2 drugs were used and if the symptoms still persisted the PPI therapy was restarted. The results showed that of the 73 subjects, 58% were asymptomatic at one year, with no treatment. Of those patients, 34% required an H2RA, 7% a prokinetic, 1% required the two drugs, and 15% required no medication after one year of follow-up. In the multivariate analysis, heartburn and younger age were found to possibly predict the need for PPIs.133
At present, gradual reduction is the most widely recommended deprescribing method, given that it reduces the risk of recurrence and deprescribing-associated symptoms, but evidence is still lacking on this modality (Fig. 1).
Algorithm for deprescribing a PPI.
In patients taking PPIs, their indication should be reviewed and if there are none, they should be suspended. If there is an indication, the appropriate dose and duration should be prescribed; if the dose or duration is not appropriate, they should be suspended. The most adequate deprescribing strategy should be personalized for each patient, as well as the maneuvers to be employed, in case of symptom relapse.
Source: Adapted from Helgadottir H, Bjornsson ES.11
On the other hand, deprescribing through intermittent treatment is useful in patients with adequate control, but with symptom recurrence at 2-4 weeks.
The clinical evidence on these strategies is summarized below (Table 5):
Clinical evidence of deprescribing strategies.
| Strategy for deprescribing PPIs | Study description | Results |
|---|---|---|
| Reduction of double dose to standard dose129 |
| 79.5% with no recurrence |
| Reduction of double dose to a delayed dual release PPI130 |
| 88% remained controlled, with significant improvement in quality of life (p = 0.016), PAGI-QOL scores |
| Abrupt suspension versus gradual reduction131 |
| There was no significant difference between groups at 6 months (31% vs 22%, respectively) |
| Gradual versus intermittent reduction133 |
| Remission rates: |
| ||
| On-demand versus continuous treatment136 |
| The percentage of patients with no heartburn at the end of the study was 72.2% (esomeprazole 20 mg QID continuously); 45.1% (esomeprazole on-demand); and 32.5% (ranitidine continuously), with similar treatment satisfaction percentages (82.2%, 75.4%, and 33.5%, respectively) |
BID; twice daily; GERD: gastroesophageal reflux disease; OME: omeprazole; PPI: proton pump inhibitor; QID: once a day.
Fifteen percent of PPI users are reported to take a double dose or a dose higher than that recommended by the FDA in different acid-related diseases. Even though there is evidence supporting the use of high doses in gastrointestinal bleeding associated with peptic acid disease or for the prevention of bleeding in high-risk patients, there is no evidence supporting the long-term use of high doses, nor is a double-dose PPI superior to a standard dose in Barrett’s esophagus or in laryngopharyngeal reflux. In a study on 117 patients with heartburn or regurgitation successfully treated with double-dose PPI, the dose was progressively reduced, with 80% remaining recurrence-free.134
Switching the double-dose PPI to a delayed dual release PPIIn a study by Cote et al., lansoprazole 30 mg, BID, was switched to rabeprazole 20 mg, QID, with 60% of the patients maintaining symptom remission. Fass et al. evaluated patients with heartburn that was well-controlled with PPIs, BID. They were switched to dexlansoprazole 30 mg, QID; 88% maintained symptom control, with significant improvement in quality of life (p = 0.016), PAGI-QOL scores, diet, and habits (p < 0.001).135
Abrupt suspension versus gradual reductionIn a controlled study by Bjömsson et al., the probability of remaining asymptomatic without PPIs, after abrupt PPI suspension, was compared with gradual reduction for 3 months (single daily dose every other day), with later suspension. There was no significant difference between groups at 6 months (31 vs 22%, respectively).136
Gradual reduction versus intermittent reductionDifferent variants of intermittent treatment have been tested. The traditional one is the administration of medications from one to 4 weeks, with the same rest period. A study evaluated recurrence rates with 2 doses of OME and ranitidine, with courses given every 2-4 weeks at the dose that completely controlled symptoms. Those authors found that remission was maintained at 12 months, with intermittent treatment every 2-4 weeks, in 72% of the patients and 93% had fewer than 3 recurrences at 12 months.137 Variants of this type of deprescribing include administering the medication only on weekends (Friday, Saturday, and Sunday), every other day, and 2 or 3 times a week. Erosive esophagitis recurrence rates with 4 different reduction regimens were: 75% (ranitidine 150 mg, BID); 68% (OME 20 mg every weekend), and 11% (OME 20, QID).138
On-demand treatment versus continuous treatmentIn patients with erosive GERD, a higher number of cases of permanent endoscopic remission has been described, when the patients had continuous treatment at a low dose, compared with on-demand therapy. Nagahara et al. reported higher remission rates (85.3% vs 44.4%, p < 0.01) with continuous OME 20 mg, QID, vs on-demand OME, for 8 weeks.139 Another study reported that 5% of a group with NERD using on-demand PPIs developed esophageal erosions versus no patient with continuous treatment.140
A study evaluated symptom control in patients with NERD and previous treatment with esomeprazole 40 mg, QID, through 3 strategies: reduction to 20 mg, QID, taken continuously, on-demand, or switching to continuous ranitidine 150 mg, BID, for 6 months. The percentage of patients with no heartburn at the end of the study was 72.2% (continuous esomeprazole 20 mg, QID); 45.1% (on-demand esomeprazole); and 32.5% (continuous ranitidine), with similar treatment satisfaction percentages (82.2%, 75.4%, and 33.5%, respectively).141
Recommendation 23: In patients programmed for PPI deprescribing, we recommend informing them of possible upper gastrointestinal symptom recurrence, such as heartburn, regurgitation and/or epigastralgia after treatment withdrawal.
Upon indicating the deprescribing of PPIs, it is important to inform patients that they may experience gastrointestinal symptoms, such as heartburn, regurgitation, and dyspeptic symptoms within 2 to 4 weeks after treatment is withdrawn, and that this does not necessarily mean they have to immediately return to continuous PPI use. That situation can be explained by rebound acid hypersecretion, defined as an increase in gastric acid secretion above pre-treatment levels after therapy with PPIs.9
In a double-blind RCT on 120 persons with no history of upper gastrointestinal symptoms, the deprescribing of PPIs after 8 weeks of treatment resulted in a higher incidence of gastrointestinal symptoms (heartburn, regurgitation and/or epigastralgia), compared with subjects that continued PPI use.137 Another study was carried out on 48 healthy volunteers (24 women) that were negative for H. pylori. They were randomly assigned to receive treatment with 40 mg of pantoprazole or placebo, QID, for 28 days. Dyspeptic symptoms were recorded daily, utilizing the Glasgow dyspepsia score (GDS) for 2 weeks before, during, and 6 weeks after treatment. After the first and second week, the pantoprazole group had higher dyspepsia scores than the placebo group.142
In a systematic review that included 5 studies, 2 of which involved healthy volunteers, 44% of subjects experienced acid-related symptoms up to 4 weeks after treatment withdrawal; said symptoms were mild-to-moderate and the most common were heartburn and regurgitation. In 3 studies, in which patients with reflux disease participated, no symptoms caused by rebound acid hypersecretion were found. Even though the studies on healthy volunteers showed that upper gastrointestinal symptoms presented upon deprescribing PPIs, its clinical importance is still unknown.143
Ethical considerationsNeither informed consent from any patient nor authorization from any bioethics committee were required for the development this document, given that it is based solely on the bibliography published in indexed journals to serve as a good clinical practice guideline. This document was authorized by the scientific committee of the AMG and the committee of the Revista de Gastroenterología de México of the AMG and contains no information through which any patient could be identified or recognized.
Financial disclosureThe present document was prepared with the support of the Asociación Mexicana de Gastroenterología (AMG), which paid for the authors’ transportation from their cities and their accommodations, so that the document could be produced. No honoraria were received.