Due to its elevated prevalence, complex pathophysiology, and broad spectrum of clinical manifestations, gastroesophageal reflux disease (GERD) requires precision diagnosis and treatment. The aim of this Latin American expert review was to provide good clinical practice recommendations for the rational use of diagnostic tests and the personalized treatment of GERD.
MethodsGood clinical practice recommendations were developed by a group of Latin American experts in GERD. A thorough review of the literature was conducted, and recommendations on the diagnosis and treatment of GERD were issued after three group discussions.
ResultsTwenty-one experts on GERD formulated 30 clinical recommendations for appropriately indicating diagnostic tests for disease phenotypes and their medical treatment, refractory GERD management, endoscopic and surgical treatment indications, the control of GERD in obesity, pregnancy, and older adults, as well as the role of Helicobacter pylori infection and GLP-1 agonists.
ConclusionsDetermining GERD phenotypes in patients through the appropriate use of diagnostic tests enables personalized treatment to be prescribed. The recommendations established in this document may contribute to improving the quality of care of patients with GERD.
La enfermedad por reflujo gastroesofágico (ERGE) por su elevada prevalencia, fisiopatología compleja y su espectro amplio de manifestaciones clínicas requiere de un diagnóstico y tratamiento de precision.
ObjetivoEn esta revisión de expertos latinoamericanos se hacen recomendaciones de buena práctica clínica para el uso racional de las pruebas diagnósticas y la prescripción de un tratamiento personalizado de la ERGE.
MétodosLas recomendaciones de buena práctica clínica se generaron por un grupo de expertos en ERGE de Latinoamérica. Se realizó una revisión detallada de la literatura y se emitieron recomendaciones sobre diagnóstico y tratamiento de la ERGE después de 3 discusiones en grupo.
ResultadosVeintidós expertos en ERGE elaboraron 30 recomendaciones de buena práctica clínica para la indicación apropiada de pruebas diagnósticas y tratamiento médico de los fenotipos de la enfermedad, el manejo de la ERGE refractaria, indicaciones de tratamiento quirúrgico y endoscópico, y el control de la ERGE en obesidad, embarazo, adulto mayor y el papel de la infección por Helicobacter pylori y agonistas de GLP-1.
ConclusionesLa fenotipificación del paciente con ERGE con el uso apropiado de pruebas diagnósticas permite la prescripción de un tratamiento personalizado. Las recomendaciones establecidas en este documento pueden contribuir a mejorar la calidad de atención del paciente con ERGE.
Gastroesophageal reflux disease (GERD) is defined by the presence of symptoms and lesions caused by the return of gastric content into the esophagus that bothers the patient and alters his/her quality of life.1 GERD is very prevalent in the general population and affects one out of every 5 adults.2
Its pathophysiology is multifactorial and the main mechanisms are: 1) loss of the antireflux barrier secondary to transitory relaxations and incompetence of the lower esophageal sphincter (LES) or the presence of a hiatal hernia, 2) abnormal esophageal motility, 3) reduced salivary secretion, 4) poor resistance of the esophageal epithelium, 5) delayed gastric emptying, and 6) duodenogastroesophageal reflux.3 Its clinical manifestations vary and include esophageal symptoms, such as heartburn and regurgitation, and extraesophageal symptoms, such as cough, laryngitis, and asthma. It can also be complicated by stricture, Barrett’s esophagus, and cancer.1 Diagnostic tests are currently available that enable the GERD phenotype to be determined and personalized treatment to be established. The present document aims to provide updated good clinical practice recommendations for the diagnosis and treatment of GERD, based on a review of the literature and the opinions of Latin American experts.
MethodsThe specialists that participated in this Latin American review were selected based on their recognized academic, teaching, research, and healthcare careers, whose area of interest is GERD. The 22 participating gastroenterologists were from different Latin American countries (LATAM): Mexico, Guatemala, Costa Rica, Honduras, Dominican Republic, Colombia, Venezuela, Ecuador, Peru, Brazil, Chile, and Argentina. An extensive review of the literature published over the past 20 years on GERD, diagnostic tests, and treatment was carried out. The experts were divided into 5 working groups to review the publications and formulate recommendations on: 1) clinical manifestations: typical symptoms, extraesophageal symptoms, and complications of GERD, 2) indications for diagnostic tests, 3) personalized treatments based on endoscopic phenotypes and through gastroesophageal reflux measurement, 4) endoscopic and surgical treatments of GERD, and 5) GERD in special populations. Version 1.0 of the recommendations made for each of the groups was discussed and voted on by all the experts at a virtual meeting. Version 2.0 was reviewed and corrected by each of the authors individually and sent to the general coordinator (MAV) who was in charge of editing the recommendations and including them in version 3.0. The same process was carried out for version 3.0. This last version underwent a final review by all participants for their approval, producing the final document presented herein.
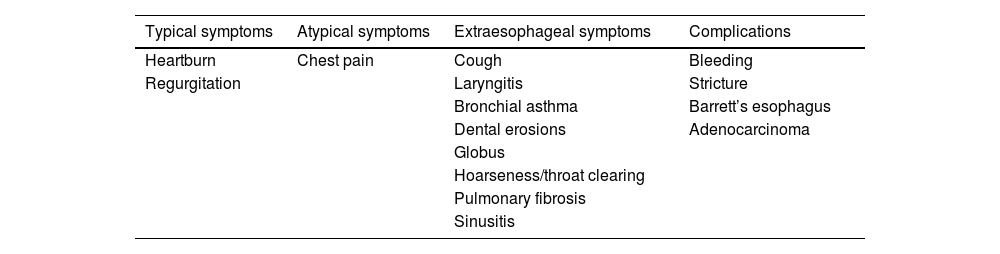
Clinical manifestations: typical symptoms, extraesophageal symptoms, and complications of gastroesophageal reflux diseaseRecommendation 1.Typical symptoms, such as heartburn and regurgitation, or atypical symptoms, such as chest pain, and the extraesophageal symptoms of asthma, laryngitis, cough, and dental erosions, suggest the diagnosis of GERD and require tests that objectively show the presence of the disease.
Typical reflux symptoms include heartburn, defined by the sensation of retrosternal burning that ascends from the epigastrium to the neck, and regurgitation that is the effortless return of gastric content into the mouth accompanied by an acidic or bitter taste. Those two symptoms have 30 to 76% sensitivity and 62 to 92% specificity, enabling the presumptive diagnosis of GERD and requiring an evaluation through objective tests to establish diagnostic certainty.4 The symptoms should be mild and occur at least twice a week, and when moderate to severe, occur at least once a week. They present mainly after meals and their nighttime appearance leads to sleep alterations and quality-of-life decline.5 Chest pain may be a symptom of GERD. Its semiology is indistinguishable from pain due to ischemic heart disease, and therefore a thorough cardiovascular evaluation is first required to rule out a cardiac origin, before considering it a manifestation of GERD.6
Sialorrhea, belching, hiccups, and dysphagia are other symptoms that may be due to GERD, but that are not characteristics of the disease. Dysphagia is an alarm symptom and always requires a precise diagnostic evaluation.
The clinical presentation of GERD may include extraesophageal symptoms. Extraesophageal manifestations that have an established association with GERD are cough, laryngitis, bronchial asthma, and dental erosions. Otitis media, sinusitis, or pulmonary fibrosis are manifestations possibly associated with GERD and always require the performance of objective tests, in order to be attributed to GERD (Table 1).
Symptoms and complications of GERD.
| Typical symptoms | Atypical symptoms | Extraesophageal symptoms | Complications |
|---|---|---|---|
| Heartburn | Chest pain | Cough | Bleeding |
| Regurgitation | Laryngitis | Stricture | |
| Bronchial asthma | Barrett’s esophagus | ||
| Dental erosions | Adenocarcinoma | ||
| Globus | |||
| Hoarseness/throat clearing | |||
| Pulmonary fibrosis | |||
| Sinusitis |
GERD: gastroesophageal reflux disease.
Recommendation 2.Complications of GERD are esophageal stricture, esophageal ulcers, gastrointestinal bleeding, Barrett’s esophagus, and esophageal adenocarcinoma. They should be suspected in subjects with established risk factors, such as male sex, age above 50 years, obesity, tobacco use, and symptoms that have lasted more than 5 years.
Erosive esophagitis (EE) is the most common complication of GERD and occurs in approximately 30% of patients. Barrett’s esophagus (BE), with its carcinogenic potential, is the most serious complication of long-standing GERD and is present in 1-5% of patients with GERD symptoms that undergo endoscopy. The presence of dysphagia, weight loss, or manifestations of gastrointestinal bleeding, such as hematemesis or melena, are alarm symptoms that make suspecting complications in a patient with GERD mandatory.
Central obesity is a risk factor for having GERD and developing some of its complications.7 Obesity has been implicated in a spectrum of esophageal diseases related to reflux, ranging from EE and BE to esophageal adenocarcinoma (EAC).8 Obesity promotes GERD through alterations of the anatomy and physiology of the gastroesophageal junction. In a study conducted on a Japanese population that included 2,608 persons, the area of visceral fat was associated with the presence of reflux esophagitis in both men and women. Smoking and serum triglyceride levels were also associated with the presence of esophagitis in men. However, a significant association between the area of visceral fat and the severity of esophagitis or presence of BE was not shown. In men, alcohol use was associated with the severity of esophagitis, as well as with the presence of BE.9
Rational use of diagnostic testsTherapeutic proton pump inhibitor testRecommendation 3.In patients with typical symptoms and no alarm features, we recommend a standard dose proton pump inhibitor (PPI) test for 2 to 4 weeks, and in cases of noncardiac chest pain, for 4 to 8 weeks.
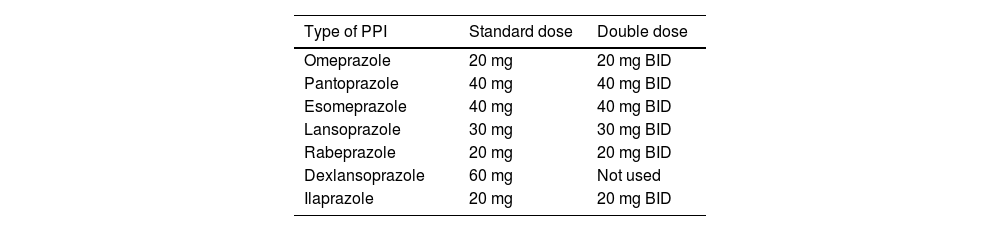
The presence of typical symptoms and their improvement with antisecretory drugs, such as PPIs, led to the use of the so-called PPI test as a diagnostic tool of GERD, instead of performing objective tests on younger patients (<45 years of age) with no alarm symptoms.10 The test consists of administering a PPI for a set time and is considered positive when symptom improvement is greater than 50%. The test was originally described with omeprazole, but upon being validated with other PPIs, became the “PPI test”.11–14 This test can be conducted with a standard or double-dose PPI (Table 2). The duration of the test is not standardized and has been utilized from one to 8 weeks.
PPI doses utilized in GERD.
| Type of PPI | Standard dose | Double dose |
|---|---|---|
| Omeprazole | 20 mg | 20 mg BID |
| Pantoprazole | 40 mg | 40 mg BID |
| Esomeprazole | 40 mg | 40 mg BID |
| Lansoprazole | 30 mg | 30 mg BID |
| Rabeprazole | 20 mg | 20 mg BID |
| Dexlansoprazole | 60 mg | Not used |
| Ilaprazole | 20 mg | 20 mg BID |
BID: twice a day; GERD: gastroesophageal reflux disease; PPI: proton pump inhibitor.
The diagnostic yield varies, depending on the dose, duration, and predominant symptom, but generally has a sensitivity of 71-79% (95% CI 72-84%) and a specificity of 45% (95% CI 4-49%), utilizing endoscopy and pH monitoring as the reference standard.4 Most guidelines suggest indicating the PPI 30 to 60 min before breakfast for 2 to 4 weeks for typical GERD symptoms and no alarm features, and for 4-8 weeks for functional chest pain (FCP). A test is considered positive when there is a symptom reduction of 50% or higher.15 There is still no evidence on the efficacy of potassium-competitive acid blockers (P-CABs), such as vonoprazan, tegoprazan, or fexuprazan, used in a therapeutic test.
Recommendation 4.We recommend conducting diagnostic tests on patients with failed treatment with PPIs or P-CABs, patients with extraesophageal manifestations, and candidates for antireflux surgery.
We recommend performing confirmatory tests when a definitive diagnosis of GERD is needed, i.e., in patients that are nonresponders or have an incomplete response to PPI or P-CAB therapy, patients that are candidates for antireflux surgery, and patients with symptoms, such as cough, excessive belching, and suspected rumination.14
When patients present with symptoms of GERD and are nonresponders to antisecretory therapy with no objective signs of reflux,4 diagnostic tests are required to confirm or rule out the presence of reflux and determine the GERD phenotype.16 In such patients, there is the possibility that treatment refractoriness is due to a disorder of gut-brain interaction (DGBI), given that GERD is not shown in objective tests in over 60% of nonresponders to a therapeutic test with antisecretory agents.17
Before starting antisecretory therapy, we recommend first performing diagnostic tests for extraesophageal manifestations of cough, asthma, or laryngitis, especially when there are no coexisting typical reflux symptoms.18 The symptoms of cough and pharyngeal discomfort have a low probability of being caused by GERD and often occur due to multifactorial mechanisms.14 Performing diagnostic tests at the outset appears to be a more cost-effective strategy than empiric treatment with antisecretory drugs, given the high number needed to treat.17
We recommend performing diagnostic tests on all patients that are candidates for antireflux surgery. Recent consensuses recommend that the preoperative evaluation include upper gastrointestinal endoscopy, esophagogram, high-resolution esophageal manometry, and pH monitoring without the use of antisecretory therapy, regardless of whether there is objective evidence of reflux at endoscopy.19,20
EndoscopyRecommendation 5.We recommend performing endoscopy in patients with symptoms of GERD and alarm features or at risk for Barrett’s esophagus, patients that are nonresponders to PPI or P-CAB therapy, or patients that present with symptom recurrence after suspending antisecretory therapy.
The new 2.0 version of the Lyon Consensus specifies that the conclusive diagnosis of GERD is made when endoscopy finds: a) Los Angeles classification grade B, C, or D esophagitis, b) biopsy-confirmed Barrett’s esophagus, or c) peptic stricture.21 Due to interobserver variability, different expert consensuses establish that Los Angeles grade A esophagitis is not definitive evidence of GERD.14
Given that mucosal healing occurs with the use of antisecretory drugs in approximately 80% of patients,22 the probability of finding significant EE is largely reduced if the endoscopy is performed after 8 weeks of treatment with said medications. To maximize diagnostic yield, endoscopy should be performed 2 to 4 weeks after interrupting antisecretory treatment of unconfirmed GERD. If endoscopy is carried out very soon, possibly no esophagitis or very mild esophagitis will be seen, which may not accurately represent the GERD phenotype. During the time the patient stops taking antisecretory drugs (2 to 4 weeks before the endoscopy), antiacids can be recommended for relieving reflux symptoms.6
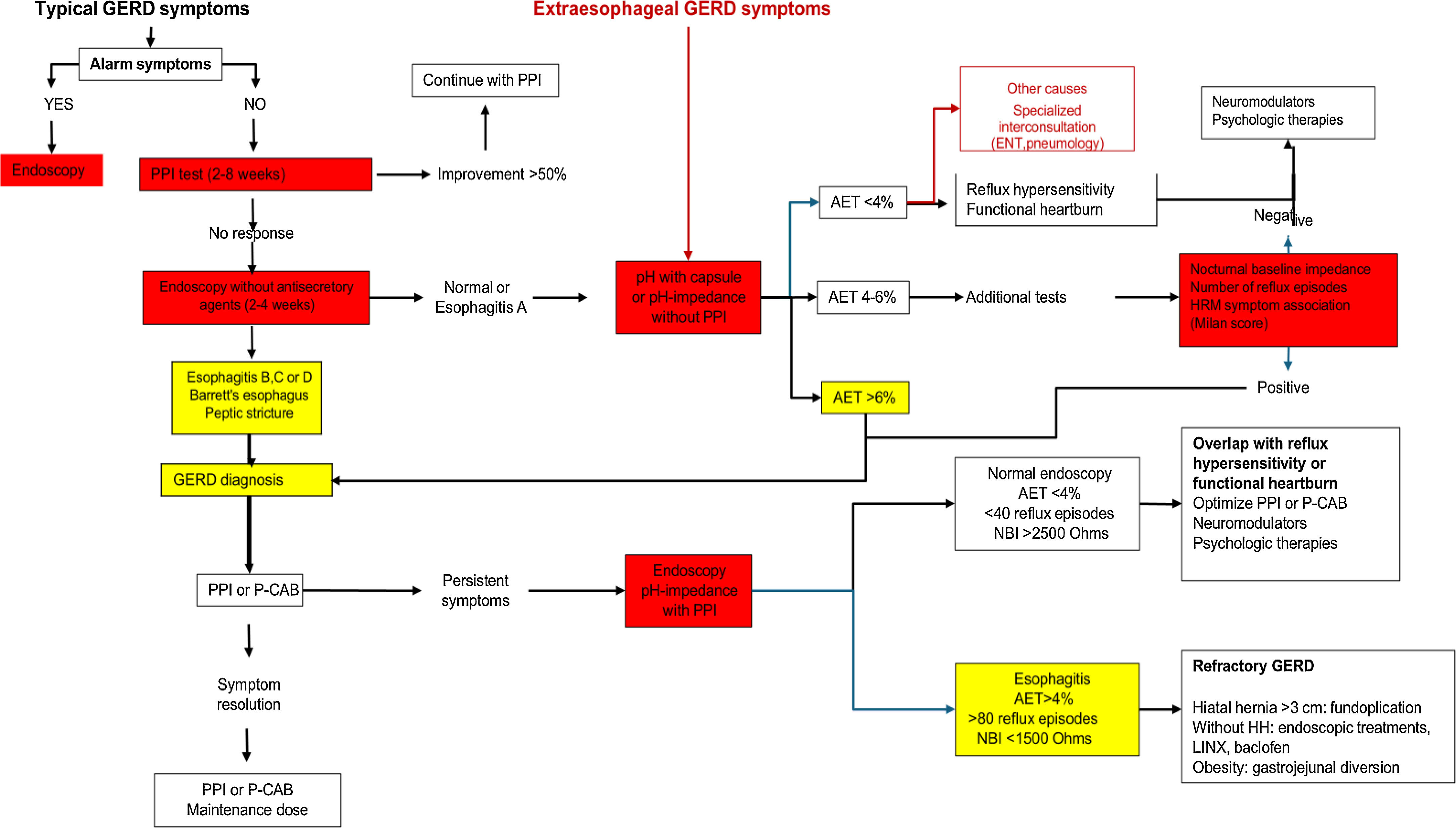
When there is severe EE (Los Angeles grade C/D), repeating endoscopy after treatment with a double-dose PPI for 8 weeks is recommended, to evaluate mucosal healing and rule out BE, which may be difficult to detect when there is severe inflammation.4 The persistence of inflammatory lesions (Los Angeles grade B, C, and D) and/or fibrotic lesions (peptic stricture) at endoscopy, while receiving optimized antisecretory treatment, is indicative of refractory GERD23 (Fig. 1).
Diagnostic and therapeutic algorithm for GERD.
AET: acid exposure time; ENT: ear, nose, and throat; GERD: gastroesophageal reflux disease; HH: hiatal hernia; HRM: high-resolution manometry; NBI: nocturnal baseline impedance; P-CAB: potassium-competitive acid blocker; PPI: proton pump inhibitor.
During the endoscopic procedure, a complete evaluation should be conducted that includes: 1) evaluating the presence of EE according to the Los Angeles classification, 2) describing the diaphragmatic hiatus utilizing the Hill classification, 3) measuring the axial length of the hiatal hernia, and 4) inspecting the BE. In the Hill classification, the diaphragmatic hiatus should be described, observing the gastroesophageal valve in retrovision and with gastric insufflation. Grades I and II are normal and grades III and IV have been independently associated with poor therapeutic response to PPIs and with the presence of EE.5,24
It is important to point out that in countries with a high incidence of gastric cancer, endoscopy should be performed when there are alarm symptoms, such as weight loss, anemia, age above 45 years, melena, and a family history of gastric cancer.
Gastroesophageal reflux measurementRecommendation 6.In patients with typical reflux symptoms that do not respond to PPIs or P-CABs, we recommend measuring reflux through wireless capsule or 24 h pH-impedance monitoring without antisecretory treatment.
In patients with typical reflux symptoms that do not appropriately respond to antisecretory drug administration, upper endoscopy is normal in 70% of cases.25 In such patients, ambulatory reflux measurement is obligatory for diagnosing GERD. Currently, we rely on 24 h measuring using a pH or impedance-pH monitoring catheter, which is placed 5 cm above the LES, identified through manometry. On the other hand, acid exposure can be evaluated for 96 h through wireless capsule pH monitoring, thus reducing daily variability.26,27 The capsule is placed 6 cm above the gastroesophageal junction during endoscopy, enabling better evaluation time, better tolerance on the part of the patient, and less interference with daily activities. The definition of GERD with this technique is more conservative and comes from controlled and randomized clinical trials. GERD is considered when acid exposure is greater than or equal to 6% for at least 2 days and ruled out when acid exposure is less than 4% over 4 days. This is a very appropriate definition because it is a predictor for suspending PPIs.14,28
Unfortunately, the wireless capsule technique is not widely available in Latin America.29 Currently, it is only available in Argentina, Brazil, and Venezuela, and so clinicians frequently use 24 h pH-impedance testing. The most widely utilized metric in impedance-pH monitoring for diagnosing GERD is an acid exposure time (AET) above 6% for making a positive diagnosis and below 4% for ruling out the disease.14 In 12% of patients, diagnosis cannot be made based on AET because it is between 4-6% (indeterminate area).
It is important to consider the presence of a functional esophageal disorder in patients with heartburn that have a negative endoscopy and normal AET.30 Those disorders are classified as reflux hypersensitivity and functional heartburn, according to symptom association indexes, such as the symptom index or symptom association probability index. Adequately categorizing those patients enables the appropriate treatment to be administered, improving quality of life and significantly reducing healthcare costs (Fig. 1).
Recommendation 7.We recommend performing 24 h pH-impedance testing without antisecretory treatment when unconfirmed GERD symptoms are associated with frequent belching, suspected rumination, or extraesophageal manifestations.
In patients that present with regurgitation as a dominant symptom of GERD, confounders, such as supragastric belching and rumination, should be taken into account.31
Belching should be considered part of the pathophysiology of GERD, but when frequent, it is important to distinguish whether the burping is gastric or supragastric.32 The latter is a behavioral disorder that consists of the inhalation of air secondary to a diaphragmatic contraction and its rapid expulsion, which can simultaneously induce reflux.29 Twenty-four- hour impedance-pH testing is the diagnostic method of choice because it can evaluate the movement of air, unlike the studies that only determine pH.33 The diagnosis of supragastric belching is essential for prescribing a specific treatment with diaphragmatic breathing, cognitive behavioral therapy, and phono-audiologic rehabilitation.34
In cases of rumination, clinically defined as the ascent of food content into the mouth in the postprandial period, impedance-pH monitoring can be a useful method for suspecting the condition, although esophageal impedance manometry utilizing a postprandial protocol is the method of choice for its diagnosis.35 The detection of rumination symptoms leads to specific treatment through cognitive behavioral therapy and breathing exercises.36
The evaluation of extraesophageal manifestations, such as cough, dysphonia, and throat clearing, is challenging, especially when they appear in an isolated manner, because their causes are multifactorial. When the evaluation of GERD as a causal mechanism is needed, 24 h pH-impedance monitoring is more sensitive than pH monitoring alone, given that the simultaneous measuring of impedance makes it possible to detect the number of reflux episodes, regardless of acid exposure and symptom association.37 In addition, it enables the evaluation of the mean nocturnal baseline impedance, which is a parameter correlated with mucosal damage secondary to GERD. On the other hand, symptom association is what allows the causal association with events like cough to be evaluated. The use of an acoustic or a combined acoustic and manometric device is recommended for evaluating coughing episodes during ambulatory reflux measurement.
Recommendation 8.We recommend performing 24 h pH-impedance monitoring with antisecretory drugs in cases of confirmed GERD with symptom persistence, despite optimum treatment.
In cases in which GERD was diagnosed through wireless or catheter pH monitoring or 24 h pH-impedance monitoring and the patients had symptom persistence despite optimal antisecretory treatment, 24 h pH-impedance is the only useful diagnostic tool. In such cases, the test should be carried out on antisecretory treatment29 (Fig. 1). In measuring reflux with antisecretory treatment, acid reflux episodes become weakly acid or non-acid, which is why detection is not possible through pH monitoring but through pH-impedance monitoring.38 In such cases, pH-impedance monitoring allows us to determine whether the patient is unable to appropriately control reflux or if antisecretory drugs control reflux but the patient nevertheless presents with a functional disorder.23
Recommendation 9.In patients with inconclusive acid exposure time for GERD, we recommend evaluating the following metrics:
–Nocturnal baseline impedance
–Number of reflux episodes
–Presence of hiatal hernia through manometry
In patients with inconclusive gastroesophageal reflux measurement for GERD (AET between 4-6%), other metrics can be employed that aid us in sustaining the diagnosis. One of them is mean nocturnal baseline impedance, which establishes mucosal integrity. It is determined through 24 h pH-impedance, measured in three 10-min periods during the night. Different researchers have established different nocturnal baseline impedance values. The 2.0 Lyon Consensus utilizes a cutoff point < 1,500 Ohms for supporting the diagnosis of GERD and > 2,500 Ohms for ruling it out.14,39
Another parameter for supporting GERD is the number of reflux episodes. GERD is supported by more than 80 episodes in 24 h and ruled out by fewer than 40 episodes in 24 h. This metric is also a predictor of treatment response when the patient is taking a PPI40 (Fig. 1).
The post-reflux swallow-induced peristaltic wave (PSPW) has been ruled out as an adequate metric for supporting the diagnosis of GERD and is only used in research studies. Lastly, the presence of hiatal hernia at endoscopy and manometry, hypotensive LES, altered esophageal peristalsis, and symptom association are also findings that support the diagnosis of GERD.14
Esophagogram, gastric emptying evaluationRecommendation 10.We do not recommend carrying out an esophagogram or evaluating gastric emptying for diagnosing GERD.
In the age of endoscopy, pH monitoring, and high-resolution manometry (HRM), the esophagogram has been displaced in the diagnosis and management of GERD. In a study by Saleh et al., they evaluated the use of esophagogram for diagnosing GERD, compared with impedance-pH, in 20 patients. They found 46% sensitivity, 44% specificity, 50% positive predictive value (PPV), and 40% negative predictive value (NPV), concluding that esophagogram currently has no role in the diagnosis of GERD.41 The presence of reflux in the esophagogram poorly correlates with esophageal pH monitoring, and therefore the GERD guidelines of the American College of Gastroenterology and the Lyon Consensus do not recommend it as a diagnostic tool. In addition, the expert panel of the Latin American consensus on GERD do not recommend esophagography for diagnosing GERD, with a 100% level of agreement.6,29,42 Esophagogram can be useful in evaluating the anatomy of the esophagus and esophageal strictures and is a mandatory study when short esophagus is suspected.
Previous studies have shown that patients with GERD have slower gastric emptying rates, compared with healthy controls, but prevalence differs due to methodological variations. Buckles et al.43 recently evaluated gastric emptying, utilizing scintigraphy in patients with GERD, and concluded that the delay in gastric emptying was frequent at both 120 and 240 min after solid food ingestion and that symptoms alone were not a useful predictor of its pathophysiology. Having knowledge of that subgroup of patients may be important, with respect to treatment strategies and long-term therapy.
Esophageal manometryRecommendation 11.High-resolution esophageal manometry is a complementary test for supporting the diagnosis of GERD. It is indicated in the preoperative evaluation of antireflux surgery and in patients with failure after fundoplication.
The usefulness of HRM in GERD was previously limited to localizing the LES for the correct placement of the pH catheters and for ruling out major disorders, such as achalasia, in patients that were candidates for antireflux surgery.44 HRM has recently been considered a complementary test. The presence of abnormal morphology of the esophagogastric junction (type III or hiatal hernia), an incompetent esophagogastric barrier (a decrease in baseline pressure or in the esophagogastric junction contractile integral), or the presence of esophageal hypomotility according to the Chicago classification v4.0 (ineffective esophageal motility or absent contractibility) are factors associated with abnormal AET or the presence of EE and can be useful parameters in cases of inconclusive GERD diagnosis. The Milan score was recently developed to evaluate the probability of having GERD utilizing HRM. Different studies have shown that the presence of hiatal hernia, decreased esophagogastric junction contractile integral, and the presence of ineffective esophageal motility are associated with GERD. In addition, the dynamic maneuver during the HRM, known as the straight leg raise (45º), facilitates the evaluation of changes in intra-abdominal and intraesophageal pressure, with respect to the baseline, and is predictive of abnormal AETs.3 An increase in intraesophageal pressure > 11 mmHg predicts an AET > 6%, with 79% sensitivity and 85% specificity.19 The Milan score, which includes all the variables previously contained in a nomogram, indicates that scores < 60 have a 10% risk of GERD and values > 210 have a 90% risk of reflux.45
And lastly, HRM is a diagnostic test that should be conducted on all patients that are candidates for antireflux surgery or that present with symptoms after fundoplication. A recent consensus developed by surgeons and gastroenterologists (the Padua Consensus) places an important value on this test. The evaluation of esophageal motility is essential for ruling out motor disorders that can contraindicate surgery, such as achalasia or outflow obstruction, resulting in a change of treatment strategy. A postoperative classification has also been developed for interpreting HRM findings following laparoscopic fundoplication.4
Diagnostic tests in extraesophageal manifestationsRecommendation 12.In patients with extraesophageal manifestations without typical symptoms, we recommend studying the causes of laryngopharyngeal symptoms different from reflux. The study of GERD through pH-impedance without antisecretory drugs is recommended. We do not recommend esophagogastroduodenoscopy, laryngoscopy, or esophagogram for diagnosing GERD in those patients.
There is a broad differential diagnosis in the patient with isolated chronic laryngopharyngeal symptoms, such as cough and hypersensitive larynx syndromes, phonotrauma, allergic disorders, different lung diseases including bronchopulmonary eosinophilic disorders, postnasal drip syndrome, psychogenic cough, globus, medication side effects (angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, fentanyl, sitagliptin, calcium antagonists, latanoprost), neuropathy associated with previous SARS-CoV-2 infection, and even functional laryngopharyngeal disorders.46 In such patients, comparing the diagnostic yield of the different gastroesophageal reflux measuring techniques, pH-impedance has big advantages: it detects acid reflux that is slightly acid or nonacid, as well as the number of acid reflux episodes, it enables the measuring of nocturnal baseline impedance, and it identifies functional disorder overlap.47–49 Measuring through wireless pH can be useful when transnasal catheters are not tolerated or in cases highly suspected of having a negative result, but it is not superior to pH-impedance in evaluating extraesophageal symptoms. The use of other tests, such as the Restech pharyngeal pH study or salivary pepsin test, are not recommended due to their low sensitivity (<40%) and poor correlation with symptoms and pH-impedance findings.49 Endoscopy is not recommended in those patients because it has 20-35% sensitivity for diagnosing GERD in patients with extraesophageal manifestations.46 Laryngoscopy has 86% sensitivity, 9% specificity, and 44% diagnostic accuracy, compared with measuring AET through impedance-pH monitoring. Laryngeal findings of erythema, edema, lymphoid hyperplasia of the posterior larynx, ulcerations, subglottic stricture, vocal cord polyps, granuloma, and leukoplakia are not specific, do not correlate with the AET, do not predict a response to PPIs or fundoplication, and can be seen in allergic disorders and infections or in the healthy population.50
Personalized treatment based on endoscopic phenotypes and through gastroesophageal reflux measurementLifestyle modificationsRecommendation 13.We recommend that all overweight or obese patients with GERD lose weight, avoid smoking, and not eat foods they have identified as symptom inducers. Patients with nocturnal reflux should eat their evening meal early and elevate the head of the bed or sleep on their left side.
Traditionally, patients with GERD have been advised to adopt elimination diets. However, evidence supporting that practice is limited, given that many studies on the impact diet has on symptoms do not have controls, have small samples, their methodological quality is deficient, and they are often associated with other therapeutic interventions, complicating their interpretation.6 Certain food have been reported to reduce LES tone, such as mint, chocolate, coffee, alcohol, and fats, whereas others can have a direct irritating effect on the esophageal mucosa, such as spicy foods and citrus fruits.51 However, those findings do not always correlate with pH monitoring results or with symptom improvement after their elimination.52 The current recommendation is that diet be individualized based on the symptoms of each patient.5,51
In addition, prolonging the interval between the evening meal and bedtime, avoiding food intake 2-3 h before going to sleep, may contribute to reducing nocturnal symptoms.51,52
Several clinical trials have shown that sleeping with the head of the bed elevated 15-20 cm significantly reduces AET, the number of prolonged reflux episodes, and improves quality of sleep.52,53 A randomized clinical trial reported that sleeping left-side down and utilizing a wedge that elevated the torso and head of the patient approximately 23 cm improved nocturnal acid exposure by 87%.54 Thus, it is reasonable to advise patients with nocturnal symptoms to utilize devices, such as wedges, and special pillows or elevate the head of the bed about 20 cm with books or bricks, in addition to waiting 2-3 h after the evening meal, before going to sleep.
There is greater esophageal acid exposure in obese patients that correlates with greater symptom intensity. Weight loss in persons with overweight has been shown to be effective in reducing both GERD symptoms and esophageal acid exposure, whereas weight gain is associated with a higher risk of complications.55
Several studies have shown that stopping smoking improves symptoms of GERD. Subjects that stopped smoking for a year experienced symptom improvement of 44%, compared with the 18% of subjects that continued smoking.56 Given that the benefits of stopping smoking and losing weight go beyond GERD, they are recommendations that are generally applicable to all patients, not only those with GERD.
Treatment for non-erosive reflux diseaseRecommendation 14.PPIs and P-CABs are drugs of choice in the treatment of patients with nonerosive reflux disease. Treatment should be started with the standard dose for 4 weeks. For maintenance therapy, the minimum dose necessary for symptom control is recommended, considering the intermittent regimen or on-demand treatment.
PPIs are the alternative with the best quality of evidence on the treatment of patients with nonerosive reflux disease (NERD). In a meta-analysis that included 17 randomized controlled trials, with a total of 6,072 patients, PPIs were significantly superior to histamine type 2 receptor antagonists (H2RAs) and placebo, in the control of NERD symptoms.57 Even though it has been reported that response to PPIs might be superior in patients with EE, that is possibly related to the fact that NERD is diagnosed in patients with no objective evidence of GERD. In a meta-analysis that compared the response to PPIs in patients with NERD, defined as a negative endoscopy and positive pH monitoring study, versus patients with EE, symptom remission was similar in the two groups.58
P-CABs are a more recent option for managing acid-related diseases and should be considered according to availability and approval in each country. In a placebo-controlled study that evaluated tegoprazan in subjects with NERD, there were significant differences in typical symptom control, in favor of the treatment at daily doses of 50 mg and 100 mg.59 Another study that evaluated P-CABs in patients with NERD found that 10 mg daily of vonoprazan for 4 weeks was superior to placebo for the remission of heartburn.60 P-CABs have also been evaluated in NERD with no response to PPIs. In a small study on 26 patients, vonoprazan was administered for 12 weeks after ineffective treatment with a PPI. The medication switch was associated with significant symptom improvement.61
Starting dose and maintenance dose are themes that have been analyzed in different publications. The recommended starting dose is the standard dose for one month, but the maintenance dose is a subject of debate due to the high symptom recurrence rate after treatment and for the tendency to utilize antisecretory therapy for short periods due to the risk of adverse effects.62–64
In patients with NERD, maintenance treatment with an intermittent or on-demand regimen with PPIs has been proposed.62 In patients with periods of symptom relapse upon suspending treatment, intermittent therapy is recommended in 2 to 4-week periods. In cases of on-demand treatment, the PPI is used until symptoms remit.62 For both strategies, the standard dose can be started and then decreased until finding the minimum dose needed for symptom control. In patients with NERD, it appears that on-demand treatment with the standard dose is not inferior to continuous treatment.63
P-CABs have also been evaluated as on-demand treatment. In a placebo-controlled trial, vonoprazan was prescribed in patients with NERD. The response was significantly superior in the treatment group, even at half the vonoprazan dose.64
Adjuvant therapyRecommendation 15.We recommend the use of alginates, antacids, and mucosal protectors in the management of occasional symptoms. H2ARs are used for short periods to relieve nocturnal symptoms that are not controlled with PPIs.
Alginates form a protective barrier against reflux at the gastroesophageal junction just above the acid pocket. They are prescribed in patients presenting with symptoms after meals and at night, as well as in patients with hiatal hernia. Antacids are recommended for temporary symptom relief and should not be used as long-term treatment.4,6,65 Esoxx-One®, a bioadhesive that contains hyaluronic acid and chondroitin sulfate, is a mucosal protector that improves symptoms in patients with nonerosive GERD, when used in combination with a PPI.66 The nighttime dose of H2ARs prevents nocturnal acid leakage mediated by histamine in patients with GERD treated with double-dose PPIs. Due to their tolerance or tachyphylaxis, they are only recommended for short periods of 2 to 4 weeks, in patients with nocturnal symptoms that are not controlled with double-dose PPIs.
Recommendation 16.Prokinetics are only indicated in patients with symptoms of delayed gastric emptying. Baclofen is recommended when regurgitation is the predominant symptom.
The use of prokinetics in combination with PPIs has not been shown to be more effective than treatment with PPIs alone in GERD. They are only indicated in patients with GERD and symptoms of gastroparesis, such as nausea, vomiting, fullness, or early satiety. Agonists of the gamma-aminobutyric acid type B (GABA-B) neurotransmitter reduce the number of transient lower esophageal sphincter relaxations (TLESRs). Baclofen is indicated in patients with predominant symptoms of belching and regurgitation that do not respond to PPI monotherapy. It can produce dizziness, confusion, and other adverse events on the central nervous system.4,6,65
Treatment of erosive gastroesophageal reflux diseaseRecommendation 17.As starting treatment of erosive GERD, we recommend standard-dose PPIs or P-CABs for 8 weeks. In patients with severe EE, double-dose PPIs should be used for 8 weeks. The continuous use of standard-dose PPIs or the half-dose of P-CABs should be employed for maintenance treatment.
PPIs and P-CABs are the antisecretory drugs of choice in EE.6 PPIs are used at the standard dose once a day for 8 weeks in cases of esophagitis A and B. In cases of severe esophagitis (C and D), double-dose delayed release PPIs should be prescribed. The healing of esophagitis is achieved in more than 80% of cases with said regimens. P-CABs have been shown to have a faster, stronger, and sustained acid-inhibiting effect.67–69 No significant differences between PPIs and P-CABs have been shown in reflux symptom control, but P-CABs are more effective than PPIs in healing esophagitis, particularly in severe cases.67–71 Standard doses are utilized for 8 weeks.68–71 Consensuses and expert recommendations describe P-CABs as equivalent options to PPIs in mild esophagitis and as a better pharmacologic alternative in severe esophagitis.5 For maintenance treatment of EE, the standard dose of the PPIs that produced the esophagitis healing are recommended. Half of the standard dose of P-CABs has been shown to be effective as maintenance treatment of EE.5,72 On-demand treatment with PPIs or P-CABs in grade A esophagitis is a cost-effective regimen.73
Medical treatment of Barrett’s esophagusRecommendation 18.We recommend continuous treatment with PPIs in patients with Barrett’s esophagus, for controlling symptoms and preventing progression to dysplasia or esophageal adenocarcinoma.
There is evidence on the effect of prolonged acid suppression, particularly with PPI use, on reducing the risk of adenocarcinoma and high-grade dysplasia in patients with BE. For example, a meta-analysis and systematic review that included 7 studies, with 2,813 patients, reported that PPI use was associated with a 71% decrease in the risk of adenocarcinoma and/or high-grade dysplasia (adjusted OR 0.29, 95% CI 0.12-0.79). H2ARs were also studied, finding no significant effect.74 With the advent of P-CABs, it is likely they could be recommended in the future for managing BE due to their stronger and longer-lasting effect.
Statins,75 COX2 inhibitors,76 and aspirin77 have also been studied as probable chemopreventive medications for BE, with more heterogeneous results and with an insufficient level of evidence for considering them in the BE management recommendations.
Treatment of refractory gastroesophageal reflux disease and overlap with functional esophageal disordersKey conceptsThe persistence of GERD symptoms despite medical treatment does not necessarily indicate a diagnosis of refractory GERD. Up to 30% of patients with GERD symptoms can persist with symptoms despite receiving treatment with PPIs.23 The causes of this apparent therapeutic failure may be due to multiple factors that include lack of treatment adherence, incorrect PPI administration, pharmacogenetic differences (such as CYP2c19 polymorphisms), and incorrect diagnoses (eosinophilic esophagitis [EoE]). According to current definitions, it is essential to identify and classify patients with “therapeutic failure” into 2 main groups:23,78,79
- 1
Patients with persistent symptoms of previously undiagnosed GERD: These patients assume they have GERD and do not respond to treatment, but they have no objective evidence of GERD (through endoscopic or physiologic studies). In such cases, conducting the necessary studies for confirming the diagnosis, before restarting treatment, is recommended.
- 2
Patients with persistent symptoms of previously diagnosed GERD: These patients have objective evidence of GERD (EE, abnormal esophageal acid exposure, etc.) but continue with symptoms despite treatment. Reviewing PPI use adherence and ruling out other causes of therapeutic failure is essential. If other causes are ruled out, patients should be re-evaluated while being treated with PPIs.
Refractory GERD is defined as the presence of objective evidence of GERD (EE, abnormal AET, or numerous reflux episodes in pH monitoring), despite double-dose PPI therapy for 8 weeks. This definition is based on expert consensuses and its cost-effectiveness is not well established.14,79,80
Recent studies have shown that there are physiologic parameters that have been obtained during pH-impedance in patients taking double-dose PPIs that predict the response to treatment of refractory GERD. The study by Gyawali et al. is the most important analysis and reported that an AET > 0.5% and > 40 reflux episodes detected through impedance measuring are parameters that can predict surgical treatment response in up to 79% of patients with refractory GERD, especially if regurgitation is the persistent symptom.80
Recommendation 19.In patients with persistent GERD symptoms, we recommend the following measures for optimizing treatment:
- a)
Confirm treatment adherence and the correct PPI dosage, in relation to meals
- b)
Utilize double-dose PPIs or divide the total dose
- c)
Switch to a different PPI
- d)
Opt for P-CAB use
- e)
Use adjuvant treatments
Medical treatment adherence is crucial for achieving effective symptom control and preventing complications in the long term. Studies have shown that optimal adherence to PPI therapy not only improves gastric acid suppression but also reduces relapse frequency and the need for additional interventions. In addition, correct adherence to the recommended dose, especially intake on an empty stomach, maximizes treatment efficacy by preventing premature degradation of the drug in the stomach.81 Importantly, there are several modified versions of PPIs, such as prolonged-release (dexlansoprazole) and second-generation (with longer half-lives, such as ilaprazole) formulations that may not strictly require administration under fasting conditions. However, the general recommendation for all PPIs is preferably taking them on an empty stomach.82
Different studies have shown that increasing the PPI dose significantly improves symptom control and gastric acid suppression. A Japanese study reported that doubling the dose of rabeprazole from 10 mg to 20 mg resulted in significant improvement in symptom control in 74% of patients, compared with 45% of patients that continued with the standard dose (p < 0.001).83 Likewise, a recent meta-analysis of 25 studies, with 592 subjects, found that the standard dose of pantoprazole (40 mg) taken twice a day maintained intragastric pH above 4 for an average time of 68% on day 3, whereas esomeprazole 40 mg twice a day maintained pH above 4 for 88% of the time.84 Such evidence underlines the importance of adjusting the PPI dose for optimizing refractory GERD management.
Dividing the total dose into 2 takes is another useful strategy. One study showed that 20 mg of omeprazole twice a day, instead of 40 mg once a day, significantly improved acid suppression from the first dose, reaching maximum benefit on the second day.85 Another study found that dividing the dose of pantoprazole (20 mg twice a day) maintained intragastric pH above 4 during an average time of 68% on day 3, whereas esomeprazole (40 mg twice a day) maintained it 88% of the time. Such results underline the effectiveness of divided doses in patients with refractory GERD, providing a valuable option for patients that do not respond adequately to the conventional dose.86
The strategy of switching PPIs is based on individual variability in the response to different PPIs due to factors like metabolism and pharmacokinetics. A study conducted by Fass et al. evaluated the efficacy of dexlansoprazole in patients with refractory GERD that did not respond to other PPIs.82 In that study, 142 patients were switched to dexlansoprazole 30 mg after a 6-week selection period. The results showed that heartburn was controlled in 88% of cases after the switch, compared with patients that continued with the placebo. That study underlines the importance of considering a PPI switch in patients who do not adequately respond to a starting treatment, offering an effective alternative for improving symptom control and quality of life.
P-CABs are a new therapeutic class of drugs because they act by reversibly blocking potassium channels in the parietal cells, offering faster and sustained acid secretion inhibition, compared with PPIs.87 Vonoprazan is one of the most widely studied P-CABs and has been shown to be effective in the control of refractory GERD. A study conducted on 124 Japanese patients showed that 20 mg of vonoprazan normalized esophageal acid exposure in 46% of patients, improving symptoms and promoting mucosal healing, compared with conventional PPIs.88 Tegoprazan, another P-CAB, has been approved and registered in the LATAM countries, showing significant improvement in nocturnal reflux symptoms and sleep quality, compared with esomeprazole.89 Fexuprazan, a new P-CAB, has also shown efficacy in GERD management. Those studies highlight P-CABs as a promising option for patients with refractory GERD, offering an efficacious alternative when traditional PPIs do not provide the necessary relief.
Sodium alginate is a viscous gel that acts as a physical barrier for the acid pocket, reducing esophageal acid exposure and alleviating residual or difficult-to-control symptoms in patients with GERD.79,90 One study demonstrated that adding a bioadhesive formulation of hyaluronic acid and chondroitin sulfate significantly improved symptoms and quality of life in patients with partial response to PPIs.66 Even though the evidence on their efficacy in refractory GERD is limited, alginates may be particularly useful in the management of postprandial heartburn and nocturnal episodes. Those findings suggest that antacids, alginates, and mucosal protectors may play an important role as adjuvant therapy in patients with refractory GERD, improving symptom control and quality of life.
Recommendation 20.We recommend the use of neuromodulators and psychologic therapies in patients with functional heartburn and reflux hypersensitivity.
In cases of acid hypersensitivity or the coexistence of GERD with functional disorders (functional heartburn), antisecretory treatment should be optimized, concomitantly utilizing neuromodulators and psychologic therapies. Neuromodulators, such as tricyclic antidepressants (e.g., amitriptyline, trazodone) and selective serotonin reuptake inhibitors (SSRIs), have been shown to significantly decrease symptoms by reducing esophageal pain perception and improving patient quality of life.91 A study conducted by Oudenhove et al. found that the SSRI, citalopram, reduced functional heartburn symptoms, compared with placebo.92 In addition, neuromodulator use has also been supported by evidence of its effectiveness in reducing esophageal hypersensitivity, by modifying the neurologic pathways that mediate pain perception. Those treatments not only encompass physical symptoms but also have a positive impact on the psychologic comorbidities of anxiety and depression, often associated with said disorders, thus improving the general wellbeing of the patient.
In addition, a recent panel of 15 experts, made up of 10 gastrointestinal psychologists and 5 esophageal specialists, issued recommendations regarding therapies centered on modulating the brain-esophagus axis. According to the panel, a psychosocial evaluation is essential, identifying hypervigilance, symptom-specific anxiety, and healthcare-related quality of life. Hypnotherapy and cognitive behavioral therapy are efficacious in the management of functional heartburn.93
Recommendation 21.The overlap of GERD with dyspepsia or irritable bowel syndrome is frequent. We recommend the thorough study of their diagnostic criteria and that of other disorders of gut-brain interaction (DGBI) in patients with GERD.
GERD and dyspepsia are highly prevalent and frequently coexist in the same individual, increasing symptomatology and worsening quality of life. The prevalence of that overlap varies, according to the criteria utilized, ranging from 12-35% with the Rome II criteria and increasing to 20-48% with the Rome III criteria.94 Their coexistence is not random, given that pathophysiologic mechanisms, such as altered gastric accommodation, delayed emptying, and visceral hypersensitivity, play a role in symptom production.95
Similarly, the overlap of GERD with irritable bowel syndrome (IBS) is common, affecting 5 to 30% of the population.96 Patients with nonerosive GERD are more prone to that combination due to visceral hypersensitivity, present in 74.3% of cases. Prevalence varies according to IBS subtype and is greater in patients with diarrhea (40.9%), compared with those with constipation (32.9%). Said overlap is associated with a decline in quality of life and poorer control of GERD symptoms, due to the high prevalence and pathophysiologic mechanisms the two conditions share.97
Treatment of extraesophageal manifestationsRecommendation 22.We recommend treatment with a double-dose PPI for 12 weeks in patients with extraesophageal manifestations and objective evidence of GERD. That therapeutic test is not recommended in patients with extraesophageal manifestations and undemonstrated GERD.
A PPI therapeutic test has traditionally been considered appropriate when there are typical symptoms, even though a positive response could be due to the placebo effect. However, evidence shows that assuming an association of GERD with cough, hoarseness, and other “isolated” extraesophageal symptoms (i.e., in the absence of typical symptoms of GERD) has overestimated atypical GERD diagnoses, leading to inappropriate antisecretory drug use, a high economic burden, and waste of the limited resources for tests. Therefore, at present and according to the Lyon Consensus 2.0, diagnostic tests should be carried out to have objective evidence of GERD when the patient presents with atypical symptoms.14
PPI use twice a day is clearly superior to PPI use once a day, regarding gastric acid suppression, and is probably more effective for extraesophageal symptoms. In a prospective cohort study, there was a 54% higher response rate in patients that did not respond to standard-dose PPIs after 8 weeks.98 In addition, even though P-CABs are expected to be used in the context of extraesophageal symptoms of GERD, at present there is no evidence on such use.
Endoscopic and surgical treatment of gastroesophageal reflux diseaseRecommendation 23.We recommend considering endoscopic treatment with transoral incisionless fundoplication (TIF) in strictly selected patients (hiatal hernia < 2 cm, Hill I or II, esophagitis A and B), performed by duly trained endoscopists.
TIF with EsophyX 2.0 may be considered a treatment option for GERD in strictly selected patients with hiatal hernia < 2 cm, Hill I or II, esophagitis A and B, and performed by trained endoscopists. The device is designed to create a full thickness serosa-to-serosa plication and reconstruct the valve approximately 3 cm in length, with a 270º to 300º circumference, based on the principles of laparoscopic fundoplication.99 Clinical trials, such as the RESPECT trial that compared the EsophyX® 2.0 versus placebo with a PPI, showed subjective symptom improvement for the treated group (67 vs. 45%, p = 0.023), a decrease in esophageal acid exposure from 9.3 to 6.3% (p < 0.001) and in the DeMeester score from 33.6 to 23.9 (p < 0.001), in the intention to treat analysis.100 Results were similar in the TEMPO trial, in which the efficacy of TIP was compared with that of PPIs. At 6 months, TIF was superior to PPI therapy in terms of improving regurgitation (97 vs. 50%) and extraesophageal manifestations (62 vs. 5%).101 Haseeb et al. evaluated TIF efficacy on atypical symptoms of GERD through a meta-analysis (10 studies, 564 patients).102 In the follow-up at 6 and 12 months, there was a reduction in the reflux symptom index, after TIF, of 15.72 points (95% CI 12.15-19.29) and 14.73 points (95% CI 11.74-17.72), respectively. The technical success rate was 99.5% and the adverse event rate was 1%. In a cohort study, Testoni et al. described stable and permanent regurgitation results with TIF and PPI use in 14 patients followed for 10 years.103 Thus, TIF has been positioned as the endoscopic procedure with greater evidence, compared with nonablative radiofrequency (Stretta®), ultrasonic endostapler (MUSE), endoscopic mucosectomy (ARMS), and mucosal ablation (ARMA). Future studies, correctly designed, that show the efficacy and durability of TIF are needed. In Latin America, experience with this technique is just beginning to be gained in some of the countries.
Recommendation 24.We recommend anti-reflux surgery in patients with a definitive diagnosis of GERD, who present with a large hiatal hernia and regurgitation as the predominant symptom, in the absence of a severe esophageal motility disorder.
Laparoscopic fundoplication is the procedure of choice for the surgical treatment of GERD. Numerous studies have shown that not only medium and long-term symptoms improve with efficacy equal to or greater than that of medical therapy, but also physiologic alterations related to the disease are corrected. Before considering surgery, GERD must be objectively demonstrated and peristaltic function evaluated through HRM, to rule out achalasia and other motor disorders. Patients with hypercontractile esophagus and distal esophageal spasm without obstructive symptoms can be referred for fundoplication. In contrast, surgery should be cautiously considered in patients with motor disorders associated with obstructive symptoms, such as dysphagia or chest pain.19 A recent randomized trial compared medical treatment with surgery in 78 patients with GERD and refractory heartburn. Surgical treatment was significantly superior to active medical treatment and control medical treatment in symptom relief (67, 28, and 12%, respectively; p = 0.007).104 A systematic review and meta-analysis that evaluated fundoplication, compared with medical treatment with PPIs, found overall results in favor of fundoplication in short-term and long-term symptom resolution (SMD 0.18; 95% CI 0.01-0.35 vs. SMD 0.33; 95% CI 0.13-0.54).105 Likewise, a Cochrane review reported that in the long term, quality of life and symptom control favored fundoplication, compared with medical therapy, but adverse events were greater in the surgery group (18.1 vs. 12.4%).106
Symptom control duration for fundoplication is variable. A long-term follow-up study reported that, after 10 years, 62% of patients were again taking antireflux medication.107 Studies on fundoplication versus medical therapy conducted at referral centers have reported GERD recurrence rates of 10-27% at 3 to 5-year follow-up periods.108,109 Factors related to good surgical outcome are the presence of hiatal hernia, favorable response to PPIs, and adequate peristaltic reserve in esophageal manometry.110 Partial fundoplications (Toupet and Dor) appear to have similar efficacy to complete fundoplication (Nissen). Two meta-analyses of comparative studies have shown equivalent results between the 2 types of fundoplication, but partial fundoplications produce less dysphagia and less inability to belch and vomit.111,112
A population cohort study analyzed the results at 5 years of fundoplication performed on 2,655 patients and found that 17.7% had reflux recurrence, 4.1% had nonsevere complications, and 0.8% had dysphagia. With those data it can be concluded that fundoplication has a low morbidity rate and provides lasting relief of symptoms of GERD for the majority of patients.113
In summary, medical treatment and laparoscopic fundoplication have comparable therapeutic efficacy for the resolution of symptoms and esophagitis. The majority of patients that have been well-selected and operated on by expert surgeons obtain long-term benefits and a higher level of satisfaction than those obtained with chronic medical treatment.
Gastroesophageal reflux disease in special conditionsEosinophilic esophagitisRecommendation 25.Patients who present with persistent symptoms of GERD after optimal treatment with antisecretory drugs, with dysphagia, a history of atopy, and esophageal food impaction, should undergo endoscopy with biopsies from the proximal and distal esophagus for ruling out eosinophilic esophagitis.
The prevalence of EoE in the LATAM countries is unknown but it is lower than in Anglo-Saxon countries. Nevertheless, it should be suspected in atopic patients with persistent symptoms of GERD, despite optimal treatment with PPIs or P-CABs and a history of food impaction. Those patients should undergo endoscopy, carefully looking for endoscopic signs of EoE, such as rings or trachealization of the esophagus, eosinophilic abscesses, grooves, or furrows. Biopsies should also be taken from the distal and proximal esophagus. The diagnosis of EoE is made with the presence of > 15 eosinophils per high power field.114,115
PregnancyRecommendation 26.Typical symptoms of GERD can appear or increase during pregnancy. We recommend lifestyle modifications, using antacids or sucralfate in patients with mild symptoms and PPIs in those with severe symptoms. All PPIs are considered category B throughout pregnancy, except omeprazole (category C). No category has been defined for P-CABs.
Approximately two-thirds of pregnant women experience heartburn in some trimester of pregnancy. GERD has the same presentation as in the adult population, but symptoms worsen at advanced stages of pregnancy. Regurgitation and heartburn are present with the same frequency. In the first trimester of pregnancy, GERD is related to an altered physiologic response.116,117 The possibility of heartburn during pregnancy increases when the patient previously presented with symptoms, in addition to cases of greater parity and longer pregnancy duration. Maternal age is inversely correlated with heartburn.118 Diagnosis is made basically by patient-described symptoms. Endoscopy and pH monitoring are rarely necessary. Symptoms usually resolve after giving birth and endoscopy tends to be deferred to the postpartum period due to the greater risk to the fetus from sedation. On the other hand, obesity after giving birth may lead to symptom persistence.117 Treatment should start with lifestyle modifications, and antacids or sucralfate can be safely used in mild cases. When symptoms are not controlled with those agents, H2RAs or PPIs are indicated, both of which are category B medications, except omeprazole, which is category C in the Food and Drug Administration (FDA) classification in pregnancy.
Older adults and gastroesophageal reflux diseaseRecommendation 27.We recommend performing endoscopy in older adults with suspected GERD. In this group of patients, the disease is more frequent and severe and manifests with atypical symptoms. When carrying out endoscopy, comorbidities and risks must be taken into account.
GERD in older adults tends to be more severe, even though they present with fewer symptoms, compared with younger populations. Protective factors of the esophagus, such as salivary bicarbonate, have been described to be reduced in older adults due to xerostomia, and motility disorders and hiatal hernia are also frequently found in this age group.119 Symptoms can be mild or atypical (dyspepsia, epigastric pain, anorexia, dysphagia, odynophagia, weight loss, anemia, and belching), which may result in a delayed diagnosis.119 Therefore, esophagogastroduodenoscopy is indicated in older adults to opportunely detect and treat severe cases of esophagitis. PPI or P-CAB use is the most efficacious medical treatment, regardless of age, and requires no dose adjustment. Treatment in older adults should follow the same principles as in young patients.120
ObesityRecommendation 28.In obese patients that are indicated for bariatric surgery and have objective evidence of GERD, we recommend Roux-en-Y gastric bypass as the procedure of choice.
Overweight and obesity are risk factors for GERD. The increase in intra-abdominal fat and intra-abdominal pressure predisposes to the development of hiatal hernia and GERD.121–123 The prevalence of GERD in obese patients is greater than that in nonobese patients (OR 1.73; 95% CI 1.46-2.06).124 In persons with a BMI > 35, the prevalence of GERD is at least 6-times higher.125
Roux-en-Y gastric bypass (RYGB) may control GERD in obese patients because the residual stomach produces less acid, and the surgical bypass prevents biliary reflux. This technique should be considered as the first-choice surgical option for obese patients with GERD who are indicated for bariatric surgery, and as an alternative for correcting a failed fundoplication in obese patients.4 Even though RYGB may bring benefits, it must be remembered that it is a surgical technique that produces great anatomic alterations and can lead to early or late complications. Thus, in patients with morbid obesity and GERD or obese patients with refractory GERD, who are candidates for the surgical option for GERD, the risk-benefits of RYGB must be considered.4 RYGB may also be an option in nonobese patients with worsening of GERD symptoms after sleeve gastrectomy.4
Helicobacter pylori infectionRecommendation 29.Helicobacter pylori (H. pylori) has no causal effect in GERD. Its eradication does not increase the risk of esophageal adenocarcinoma, so if H. pylori infection is diagnosed during the study of GERD, its treatment is not contraindicated.
H. pylori infection is very prevalent worldwide and there is more morbidity and mortality due to its causal relation to peptic ulcer and gastric cancer than to its association with GERD.126 Therefore, H. pylori eradication is increasingly more frequent, including the indication for eradication in the context of populations at intermediate-to-high risk for gastric cancer, even when they are asymptomatic.127
Distinguishing between symptoms attributable to GERD, peptic ulcer, and functional symptoms is difficult, so the relative indications for H. pylori eradication have increased and expanded in clinical guidelines.128 Even though epidemiologic studies show a negative association between the prevalence of H. pylori infection and the prevalence and severity of GERD, H. pylori should be treated according to the recommendations of the guidelines on the subject.129
Studies with esophageal pH monitoring have determined that there is no correlation between abnormal acid exposure and the presence of H. pylori. In the majority of patients, H. pylori infection has no effect on the symptom severity, recurrence, or treatment efficacy of GERD. Eradicating H. pylori does not exacerbate GERD, but it may recover the secretory capacity of patients, post-eradication, producing a non-significant trend in the post-H. pylori eradication group in relation to erosive GERD (OR 1.11) and GERD symptoms (1.22).130
Additionally, given that the association of PPI use with H. pylori infection increases the prevalence of gastric atrophy and intestinal metaplasia, which are known risk factors for gastric adenocarcinoma in the context of GERD with prolonged PPI therapy, looking for and treating H. pylori is recommended, especially in young patients.131,132 A recent Nordic study showed that H. pylori eradication did not increase the risk for esophageal adenocarcinoma, so there are no compelling reasons against H. pylori eradication in the context of GERD.133
GLP-1 analogues and gastroesophageal reflux diseaseRecommendation 30.The use of GLP-1 analogues is not contraindicated in patients with GERD, but they may increase the frequency and intensity of the symptoms of the disease.
Glucagon-like peptide-1 (GLP-1) agonists are medications mainly used in treating type 2 diabetes and obesity. Examples of these drugs are liraglutide, semaglutide, exenatide, and dulaglutide, and they imitate the action of GLP-1, a natural hormone that regulates insulin release, glucagon secretion, and appetite. However, their use also has implications for the digestive system, which may affect GERD.134
Possible negative effects of GLP-1 agonists in patients with GERD are: 1) delayed gastric emptying may increase intra-abdominal pressure, favoring acid reflux and worsening symptoms of GERD and 2) secondary effects, such as nausea and vomiting, may injure the esophagus and aggravate symptoms of GERD.134
Positive effects of these drugs in GERD are: 1) weight loss associated with GLP-1 agonists may improve symptoms of GERD in persons with overweight or obesity, and 2) reduced meal sizes, thanks to increased satiety, may aid in controlling reflux episodes.134
It is important for patients with GERD and type 2 diabetes or obesity to speak with their physician, evaluating the risks and benefits of their specific case, before starting a treatment with GLP-1 agonists.
Recommendation 31.We recommend individualizing the patient with GERD treated with GLP-1 agonists, who will undergo endoscopy, evaluating the daily or weekly medication dose and indication (diabetes, overweight, obesity), as well as the presence of symptoms suggestive of gastroparesis prior to the endoscopic procedure, to reduce the risk of bronchoaspiration.
The use of GLP-1 agonists for managing diabetes, overweight, and obesity has significantly increased. Their effects on gastric motor function, delaying gastric emptying and increasing food retention in the stomach, are a cause of concern among gastroenterologists because of the possible higher risk of bronchoaspiration during endoscopic studies.134 Case series have shown that the retention of solid foods in the stomach is greater in diabetic patients or patients with symptoms suggestive of gastroparesis, such as nausea, vomiting, fullness, or early satiety. Said symptoms are more common with the use of long-acting GLP-1 agonists than with those of a short half-life.134,135
Unfortunately, there is presently not enough evidence for making recommendations on sedation and endoscopic procedures in patients treated with GLP-1 agonists. Nevertheless, a recent review of the American Gastroenterological Association (AGA)4 suggests the following:
Patients treated with GLP-1 agonists with no symptoms of nausea, vomiting, dyspepsia, or bloating should follow the standard protocol for an endoscopic examination (8 h fasting). In patients presenting with the symptoms suggestive of gastric content retention, suspending the GLP-1 agonist one week before the procedure and liquid diet the day before the endoscopy is suggested. The use of transabdominal ultrasound before the endoscopy for identifying food retained in the stomach can be a resource in this group of patients, but there is no evidence that determines the true usefulness of such conduct.136
Fig. 1 presents a diagnostic and therapeutic GERD algorithm that establishes that a patient with typical symptoms of GERD with no alarm symptoms can receive a PPI test. If the patient responds with improvement > 50%, he/she can continue with the minimum antisecretory drug dose necessary for symptom control. When there are alarm symptoms, the patient should be studied by endoscopy. The diagnosis of GERD is made with the presence of Los Angeles grade B, C, or D esophagitis, Barrett’s esophagus, or peptic stricture. The patient with normal endoscopy or esophagitis A should undergo gastroesophageal reflux evaluation through wireless capsule pH monitoring, pH catheter monitoring, or pH-impedance monitoring without PPIs. An AET > 6% is diagnostic of GERD. An AET < 4% rules out GERD and supports the presence of esophageal functional disorders. An AET between 4 and 6% requires additional parameters, such as nocturnal baseline impedance (NBI) < 1,500 Ohms, more than 80 reflux episodes in 24 h, or a HRM with an elevated Milan score, metrics that support the diagnosis of GERD. A patient with demonstrated evidence of GERD through endoscopy or reflux measuring that does not respond to optimal treatment with PPIs or P-CABs should be studied with endoscopy and pH-impedance with antisecretory treatment. The presence of esophagitis or an AET > 4% or > 80 reflux episodes or NBI < 1,500 Ohms are diagnostic of refractory GERD and the patient should be evaluated for anti-reflux surgery, especially if he/she has a hiatal hernia > 3 cm, or for LINX or endoscopic treatments in the absence of hiatal hernia or a small-sized hernia. The morbidly obese patient with demonstrated GERD should be treated with gastric bypass. Patients with esophageal functional disorders should be treated with neuromodulators and psychologic therapies.
Patients with extraesophageal manifestations of GERD should undergo wireless capsule reflux measuring with pH monitoring or pH-impedance without PPIs. Patients with an AET < 4% should be evaluated by specialists (otorhinolaryngologist, pneumologist, dentist, allergologist) to investigate an origin of symptoms other than GERD. Patients with an AET between 4% and 6% or an AET > 6% should follow the algorithm exactly as suggested for patients with typical symptoms.
Ethical considerationsProtection of persons and animals. The authors declare that no experiments were carried out on humans or animals for this research.
Data confidentiality. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Financial disclosureThis work was carried out with no funding and no participant received fees for developing these guidelines.