Upper gastrointestinal bleeding (UGIB) of neoplastic origin is a rare but life-threatening cause of bleeding. Endoscopic treatment is challenging due to diffuse oozing blood and high rates of rebleeding, despite coagulation and hemoclip use. Hemostatic powders can be an option in those patients. We aimed to determine the initial hemostatic rate and the rebleeding rate at 7 and 30 days, using hemostatic powders in patients with malignant UGIB at a national referral center in Mexico.
Material and methodsA retrospective, observational study was conducted on patients with malignant UGIB treated with hemostatic powder between 2018-2023. Demographic and clinical variables, endoscopic findings, and treatment results were analyzed. A central tendency analysis and the chi-square test were employed.
ResultsThe study included 54 patients (54.7% were men), with a mean age of 54 years. A total of 40.7% were diagnosed with gastric cancer and presented with an episode of malignant UGIB. Of the endoscopic findings, 52% of patients had active malignant UGIB, most presenting with oozing bleeding (57.4%). EndoClot™ was the main monotherapy employed (81.5%), achieving initial homeostasis in 100% of cases. The rebleeding rate was 22.2% at 7 days and 44.4% at 30 days, with a 30-day accumulated mortality rate of 35.2%.
ConclusionsMalignant UGIB is a potentially life-threatening complication. Hemostatic powder use is highly recommendable due to its efficacy in the immediate control of bleeding. Nevertheless, its effect is temporary, suggesting its use as bridging therapy, facilitating bleeding stabilization and enabling the implementation of definitive hemostatic treatments.
La hemorragia digestiva alta de origen tumoral (HDT) es una causa rara, pero mortal de sangrado. El tratamiento endoscópico es desafiante debido al sangrado difuso, en capa y con altas tasas de resangrado con coagulación o hemoclips Los polvos hemostáticos pueden ser una opción en estos pacientes. Nuestro objetivo es determinar la tasa de hemostasia inicial y de resangrado a 7 y 30 días con el uso de estos polvos en pacientes con HDT en un centro de referencia nacional.
Material y métodosEstudio retrospectivo, observacional de pacientes con HDT tratados con polvo hemostático entre 2018-2023. Se analizaron variables demográficas y clínicas, así como hallazgos endoscópicos y resultados del tratamiento. Se utilizó análisis de tendencia central, prueba de Chi-cuadrado.
ResultadosSe incluyeron 54 pacientes, edad media 54 años, 57.4% masculinos, con diagnóstico de cáncer gástrico en 40.7% que presentaron un episodio de HDT. Entre los hallazgos endoscópicos: 52% mostró HDT activa, la mayor parte presentaban sangrado en capa (57.4%). Se empleó EndoClot™ principalmente como monoterapia (81.5%) logrando hemostasia inicial en 100% La tasa de resangrado a 7 días fue del 22.2% y a 30 días del 44.4%, con una mortalidad acumulada a 30 días del 35.2%.
ConclusionesLa HDT es una complicación potencialmente mortal. El uso de polvo hemostático es altamente recomendable debido a su eficacia en el control inmediato del sangrado. No obstante, su efecto es temporal, por lo que se sugiere su uso como terapia puente, facilitando la estabilización de la hemorragia y permitiendo implementar tratamientos hemostáticos definitivos.
Upper gastrointestinal bleeding (UGIB) of neoplastic origin, or malignant UGIB, is a rare cause of gastrointestinal bleeding proximal to the ligament of Treitz (5%)1,2 that has an elevated mortality rate (37.5%).2 The main etiology of malignant UGIB is gastric cancer, at 36-58%.3,4 Most cases present with melena or anemia, the first symptoms in 79%,2–5 and unfortunately are found at advanced stages (75%).4
Endoscopy is essential for diagnosing and treating this disease, and should be carried out within the first 24h of presentation, once the patient is hemodynamically stable.6 Endoscopic therapy (ET) reduces the occurrence of new bleeds (OR 0.38, 95% CI 0.32-0.45), the need for surgery (OR 0.36, 95% CI 0.28-0.45), and deaths (OR 0.55, 95% CI 0.40-0.76).6 Initial hemostasis rates with conventional ET vary between 67-100% but the rebleeding rate in malignant UGIB is high: 41-80% compared with 8-24% in cases of benign etiologies.6 Conventional endoscopic tools include therapy with injection, mechanical therapy with hemoclips, thermal therapy, and argon plasma ablation.1,6,7 Neither hemoclip placement nor thermal coagulation has been shown to be effective in malignant UGIB because bleeding tends to be diffuse, multifocal, or with indurated ulcers.1,3,7,8 Therefore, there is a need for using other ET methods, such as hemostatic powders.7,9 These powders are pulverized topical agents that can induce hemostasis without direct contact, preventing destruction of the surrounding tissue. They also have the advantage of covering multiple areas, as in cases of malignant UGIB.1
EndoClot™ PSH (EndoClot Plus Co., Santa Clara, CA, USA) was the first of these powders, and has been approved since 2012.3 It is a starch-based polymer that is applied through a plastic catheter and distributed through a constant airflow, enabling its dispersion over the mucosa, absorbing the water from the bleeding bed, and consequently forming a gelatinous matrix that favors clot formation.1–3 Different hemostatic powders, such as Hemospray® (TC-325, Cook Medical Inc, Winston-Salem, NC, USA), have recently been compared. It is a bentonite compound, an inert mineral, that is applied through pressurized CO2,3 absorbing water and forming an adhesive barrier, creating a clot. Used in all types of gastrointestinal bleeding, Hemospray® showed an initial hemostasis rate of 92.3% and a rebleeding rate of 20.6%, in a systematic review of prospective and retrospective studies.10
The use of hemostatic powders is a safe and innovative option for endoscopic hemostasis. However, evidence on its indication and efficacy for malignant UGIB is still limited. Thus, our aim was to analyze hemostatic powder efficacy in relation to the initial hemostasis rate and frequency of rebleeding at 7 and 30 days, in patients with malignant UGIB at a Mexican national referral center.
Material and methodsType of study: An original, retrospective, and observational study was conducted, utilizing the STROBE checklist. It included the case records of patients with a history of malignant UGIB, who underwent endoscopy and treatment with hemostatic powders, within the time frame of January 2018 and May 2023, at a national referral center in Mexico.
The primary aim was to determine efficacy, in relation to the initial hemostasis rate and the rebleeding rate, at 7 and 30 days, of the hemostatic powders utilized as ET, in patients with malignant UGIB at a national referral center. The following operational definitions were used:
- •
Malignant UGIB: the presence of clinical data of gastrointestinal bleeding (melena, hematemesis, hematochezia) associated with a decrease of 2g/dl of hemoglobin from the initial baseline or an initial hemoglobin value below 10g/dl, with signs of gastrointestinal bleeding.
- •
Active bleeding: signs of continuous diffuse or oozing bleeding during the endoscopy, that was not self-limited during the exploration.
- •
Initial hemostasis: cessation of bleeding for 5 minutes after the application of the hemostatic powder.
- •
Rebleeding: considered positive in patients with initial hemostasis, who presented with new signs of gastrointestinal bleeding or with a decrease of hemoglobin of 2g/dl, at a determined time range after the procedure.
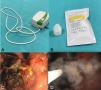
The decision to apply hemostatic powder was made based on each patient’s individual clinical scenario. The choice of the type of powder (EndoClot™ or Hemospray®) was made, based on the endoscopist’s preference and product availability. The procedure was standardized: the system was assembled, connecting the air compressor to the application catheter, whose tip was sealed with a piece of non-toxic commercial modeling clay to prevent the entrance of fluids and catheter occlusion. Once the source of bleeding was located, the compressor was activated in low airflow mode and the bottle containing the powder was gently and intermittently tapped to favor continuous release over the desired area, until completely covered. Initial hemostasis and the need for adjuvant treatments were then checked (Fig. 1A-D).
The population sample was obtained sequentially and indistinctly from the case records that met the following inclusion criteria: patients above 18 years of age, of either sex, and diagnosed with malignant UGIB, who underwent ET and hemostatic powder application between January 2018 and May 2023. The exclusion criteria were: patients with benign lower gastrointestinal bleeds or unpassable stricture or alteration impeding the complete endoscopic study. An effort was made to minimize bias by selecting the sample, in strict accordance with the inclusion and exclusion criteria, and precisely and consistently collecting the data. In addition, the authors declared there were no conflicts of interest that could be a risk of bias.
Statistical analysisThe demographic and clinical variables of age and sex, patient functional status through the validated ECOG and Karnofsky scales, primary tumor location, oncologic treatment prior to the malignant UGIB episode, initial clinical manifestation of bleeding, laboratory test results on admission, need for and number of blood product transfusions, and the Glasgow-Blatchford and AIM 65 predictive scale scores. The endoscopic findings were described as the presence of active bleeding, type of bleeding, and location. The following information was also collected: the type of hemostatic agent used, powder application as monotherapy or combined with other tools, whether initial hemostasis was reached, adverse events, days of hospitalization, the presence of rebleeding at 7 and 30 days, mortality at 30 days, whether a new endoscopic treatment was required, and if hemostatic therapy was definitive.
For the statistical analysis, the data were collected on an Excel® calculation sheet and then migrated to the SPSS® version 25.0 program. The results were described utilizing measures of central tendency for the normally distributed continuous variables. Means, medians, modes, and standard deviations were obtained using the Student’s t test for comparing means. Frequencies and percentages were reported for the qualitative variables and compared utilizing the chi-square test.
ResultsDemographics and clinical characteristicsSeventy case records were reviewed of patients with malignant UGIB at our institution seen within the time frame of 2018-2023. Fifty-four of those cases met the inclusion criteria, and of the other 16 patients, 3 were excluded due to benign disease, 3 were eliminated due to having an incomplete clinical case record, and 10 were eliminated because they were lost to follow-up. Table 1 specifies the demographic and clinical characteristics of the sample.
Demographic and clinical characteristics of the sample
| Characteristics (n=54) | Frequency or mean (%/SD) |
|---|---|
| Anthropomorphic characteristics | |
| Men | 31 (57.4) |
| Age (years) | 54 (14.9) |
| Karnofsky | 70 (13.9) |
| ECOG | 2 (0.8) |
| Relevant history | |
| Oncologic diagnosis | |
| Esophagus | 4 (7.4) |
| Adenocarcinoma of the stomach | 22 (40.7) |
| MALT lymphoma | 7 (13) |
| Duodenum | 5 (9.3) |
| Pancreas | 6 (11.1) |
| Other tumors with gastrointestinal tract infiltration | 10 (18.5) |
| Oncologic treatment before the episode | |
| No treatment | 12 (22.2) |
| Chemotherapy | 25 (46.3) |
| Chemotherapy-radiotherapy | 7 (13) |
| Surgery | 5 (9.3) |
| Combination of chemotherapy and surgery | 5 (9.3) |
| Malignant UGIB episode | |
| Initial clinical manifestation | |
| Melena | 32 (59.3) |
| Hematemesis | 18(33.3) |
| Hematochezia | 1 (1.9) |
| Chronic anemia | 3 (5.5) |
| Initial laboratory test results | |
| Hemoglobin at admission g/dl | 7.82 (2.55) |
| Platelets ×103 at admission/μl | 278 (127) |
| INR | 1.3 (1.22) |
| 24h peri-endoscopic blood product transfusions | |
| No. of packed red blood cell units | 1.56 (1.6) |
| No. of fresh frozen plasma units | 0.11 (0.5) |
| No. of apheresis platelet units | 0.09 (0.2) |
| At the time of endoscopy: | |
| Hemoglobin g/dl | 9.16 (1.7) |
| Glasgow-Blatchford scale | 9 (4) |
| AIM 65 scale | 2 (1.62) |
ECOG: Eastern Cooperative Oncology Group; MALT: mucosa-associated lymphoid tissue; SD: standard deviation; UGIB: upper gastrointestinal bleeding.
The main oncologic diagnosis was gastric adenocarcinoma, with 22 cases (40.7%), followed by esophageal adenocarcinoma in 4 patients (7.4%), gastric lymphoma in 7 (13%), duodenal adenocarcinoma in 5 (9.3%), pancreatic cancer in 6 (11.1%), and other tumors with upper gastrointestinal tract infiltration in 10 (18.5%), of which 5 cases were melanoma, 2 were adenocarcinoma of the breast, 2 were prostate cancer, and one was ovarian cancer. The most frequent clinical manifestations were melena, in 32 patients (59.3%), and hematemesis, in 18 cases (33.3%). The mean hemoglobin value at admission was 7.82 mg/dl (SD 2.55), with a normal platelet count (mean of 278×10³/μl, SD 127) and a mean INR of 1.3 (SD 1.22). The mean number of blood product transfusions before the endoscopy was 1.56 packed red blood cell units (SD 1.6). The mean predictive gastrointestinal bleeding scores before the endoscopy were 9 points (SD 4) on the Glasgow-Blatchford scale and 2 points (SD 1.62) on the AIM 65 scale.
During the endoscopy, 52% (28) of the patients presented with active UGIB (defined as signs of arterial bleeding or continuous bleeding that was not self-limited during the examination). The endoscopic findings included oozing blood from the tumor bed in 31 patients (57.4%), a visible vessel in the tumor in 5 patients (9.3%), fresh adhered clot in 8 (14.8%), and fibrin-covered lesion with abundant necrosis in 10 patients (18.5%). The most frequent tumor location was the proximal stomach (cardia, body and/or fundus), presenting in 16 cases (29.6%), followed by diffuse gastric lesions in 13 patients (24.1%), distal gastric lesions in 7 cases (13%), lower esophageal lesions in 7 cases (13%), and duodenal lesions in 11 cases (20.3%) (Table 2).
Endoscopic characteristics during malignant UGIB episode
| Findings during endoscopy (n=54) | Frequency or mean (%/SD) |
|---|---|
| Active malignant UGIB during procedure | 28 (52) |
| Endoscopic finding | |
| Oozing blood | 31 (57.4) |
| Vessel visible in the tumor bed | 5 (9.3) |
| Fresh clot adhered to the tumor bed | 8 (14.8) |
| Fibrin-covered lesion/necrosis | 10 (18.5) |
| Bleeding location | |
| Esophagus | 7 (13) |
| Proximal stomach | 16 (29.6) |
| Distal stomach | 7 (13) |
| Diffuse stomach | 13 (24.1) |
| Duodenum | 11 (20.3) |
SD: standard deviation; UGIB: upper gastrointestinal bleeding.
EndoClot™ was used in 96% (n=52) of the sample, and Hemospray® in 3.7% (n=2). Hemostatic powders were applied as monotherapy in 44 cases (81.5%), whereas in 18.5% they were rescue therapy, in combination with other techniques, such as argon plasma coagulation in 4 cases, adrenaline injection in 2 cases, application following hemoclip placement in 3 cases, and after over-the-scope clip placement in one case.
Regarding hemostatic efficacy, initial hemostasis at 5 min from application was achieved in 100% of the cases, regardless of the type of powder utilized. The mean hospital stay was 13 days (SD 16), with a mean hemoglobin value at 30 days of 9.03g/dl (SD 2.1), with no other adverse events (Table 3).
Analysis of hemostatic powder efficacy in malignant UGIB
| Hemostatic powder results | Total |
|---|---|
| n=54 | |
| Frequency or mean (%/SD) | |
| Hemostatic powder | |
| EndoClot™ | 52 (96.2) |
| Hemospray® | 2 (3.7) |
| Monotherapy | 44 (81.5) |
| Additional endoscopic therapy | 10 (18.5) |
| Adrenaline | 2 (3.7) |
| Hemoclips | 3(5.5) |
| APC | 4 (7.4) |
| Over-the-scope clip | 1 (1.85) |
| Initial hemostasis (5min) | 54 (100) |
| Adverse events | 0 (-) |
| Days of hospitalization | 13.1 (16) |
| Hemoglobin control at 30 days | 9.03 (2.1) |
| Rebleeding at 7 days | 12 (22.2) |
| Rebleeding at 30 days | 24 (44.4) |
| Deaths at 30 days | 19 (35.2) |
| Accumulated deaths at 90 days | 29 (53) |
| Endoscopy during rebleeding | |
| No treatment required | 44 (81.5) |
| EndoClot™ | 7 (13) |
| APC | 1(1.9) |
| Over-the-scope clip | 1 (1.9) |
| Self-expandable metallic stent | 1 (1.9) |
| Definitive hemostatic therapy | |
| No | 38 (70.4) |
| Hemostatic radiotherapy | 9 (16.7) |
| Angioembolization | 3 (5.6) |
| Surgery | 2 (3.7) |
| Radiotherapy/angioembolization | 2 (3.7) |
APC: argon plasma coagulation; SD: standard deviation; UGIB: upper gastrointestinal bleeding.
Twelve patients (22.2%) presented with rebleeding at 7 days, with accumulated rebleeding at 30 days in 24 patients (44.4%). There were no differences between patients that had hemostatic powder as monotherapy and those who had the combination with argon plasma coagulation (6 and 3 patients, respectively; p=0.146). Most of the patients with rebleeding (81.5%) required no new endoscopic hemostatic therapy. Only 10 patients required endoscopic re-intervention; hemostatic powder was re-applied to 7 of them (13%), one patient was treated with argon plasma coagulation, one patient needed OVESCO over-the-scope clip placement, and one patient required self-expanding metallic stent placement (Table 3).
It should be emphasized that definitive hemostatic therapy after endoscopic treatment with hemostatic powder was not necessary in 70.4% of the cases. Only 9 patients (16.7%) received hemostatic radiotherapy (8-10Gy/7 fractions), 3 patients (5.6%) required selective angioembolization (right gastroduodenal artery in 2 cases, left gastric artery in one case), 2 patients (3.7%) underwent total gastrectomy, and 2 patients (3.7%) required the combination of radiotherapy and chemotherapy. The 30-day mortality rate was 35.2% (n=19) (Table 3).
Discussion and conclusionsThe demographic and clinical characteristics of our study population were similar to those reported by other national2 and international3 authors. As in other studies, the main cause of malignant UGIB in our sample was gastric cancer, accounting for 40.7% of the cases. This concurs with the 36-58% range reported in 2015 by Kim et al.4 The patients with malignant UGIB in our analysis presented with functional status deterioration, with limitations for carrying out daily activities, that were reflected in the Karnofsky score of 70% and the ECOG score of 2. Some of the patients (22.2%) had not received oncologic treatment at the time of the bleeding episode. The main symptom reported was melena in 59.3% of the cases, and on admission, the patients presented with anemia (hemoglobin under 8g/dl), requiring a mean of 1.5 packed red blood cell units. In a Mexican study on an oncologic population, melena was the main symptom, presenting in 79% of patients.2
It is relevant to point out that during endoscopy, the findings of active bleeding were corroborated in 52% of the cases of malignant UGIB, primarily manifesting as oozing bleeding at the tumor bed (57.4%), visible vessel in the tumor (9.3%), fresh clot adhered to the tumor bed (14.8%), and fibrin-covered tumor with abundant necrosis (18.5%). In that context, conventional ET has not been reported as efficacious.1 Since the approval of hemostatic powder use in 2012, different authors have reported results on the application of powders, such as EndoClot™ (EndoClot Plus Co, Santa Clara, CA, USA) and Hemospray® (TC-325, Cook Medical Inc., Winston-Salem, NC, USA). Those powders are polymers that can be diffusely dispersed over the bleeding tumor bed, forming a gelatinous matrix that favors the concentration of erythrocytes, platelets, and coagulation factors.9,10
In our study, EndoClot™ was more frequently used (in 96.3% of the cases), explained in part by its greater availability at our hospital, whereas Hemospray® was used in only 2 patients (3.7%). EndoClot™ demonstrated important effectiveness, achieving initial hemostasis at 5 min in 100% of the cases. It was used as monotherapy in 81.5% of the cases and as rescue therapy in 18.5%, combined with other techniques, such as argon plasma coagulation, hemoclips, or adrenaline (10 cases). This effectiveness coincides with initial hemostasis figures of 73-100% in patients with gastric cancer.8 In a multicenter study on Mexican patients with gastrointestinal bleeding treated with Hemospray®, the initial hemostasis rate was 98.8%, with a failure rate of 1.2%, and a rebleeding rate of 20%,11 similar to our results.
The exact duration of the adhesion of EndoClot™ to the mucosa is unknown but ranges of 1-48h are managed, depending on patient and lesion factors.5 This could partially explain our population’s short-term rebleeding rate (7 days) of 22%. Other authors, such as Prei et al. (2016), have reported lower rebleeding rates (11%).3 In a retrospective study on 173 patients with non-variceal UGIB that required endoscopic treatment with hemostatic powder, only 6.3% of the cases had malignant UGIB. For that subgroup, the initial hemostasis rate was 100%, with a 4.8% rebleeding rate and a 19% mortality rate, compared with non-variceal UGIB due to benign ulcers, in which the rebleeding rate was 8.8% and the mortality rate was 13.9%.5
The main advantages of hemostatic powder use are its direct application and the ease of dispersing it at sites that are difficult to reach, after a short training period.12 In addition, hemostatic powders can serve as bridging therapy for stabilizing bleeding and enabling definitive treatment to be carried out, once the patient’s condition is better.12 In our population, only one-third of the cases received definitive therapy with radiotherapy, angioembolization, or surgery.
In 2019, a meta-analysis of 24 studies reported a rebleeding rate at 8 days of 17.9% (range: 10.3-25%), with a tendency toward greater rebleeding when the hemostatic powder was used as rescue therapy.12 Rebleeding at 30 days was reported at a rate of 16.9% (range: 9.8-24%), which was lower than that of our population (44.4%).
The 30-day mortality rate in our sample was 35.2%, coinciding with that of another study on a Mexican population that reported a 30-day mortality rate of 35.7%.2 Other authors have reported a 30-day mortality rate of 7.6%, albeit they recognized the great heterogeneity between the studies analyzed.13 In contrast, Paoluzi et al. reported that, in patients with malignant UGIB treated with combined endoscopy, there could be a short-term rebleeding rate of 40-80% and a 90-day rebleeding rate of 95%.14 In their study, Pittayanon et al. found that a deteriorated ECOG performance status>3 and an INR>1.3 were associated with poorer prognosis, whereas using a hemostatic powder was predictive of less blood loss.15
Among the limitations of the present study are its retrospective and single-center design and the marked tendency to apply EndoClot™ rather than other commercially available powders due to availability at our hospital center. Our study’s main strengths are that it explicitly addresses upper gastrointestinal bleeds of tumor origin, as well as their complementary treatments and results, regarding initial hemostasis, early rebleeding, and associated mortality. We suggest carrying out prospective and randomized studies to confirm such findings.
In conclusion, it is important to recognize that malignant UGIB is a potentially life-threatening complication. In the context of endoscopic treatments, hemostatic powders are highly recommendable due to their efficacy in immediate bleeding control and their capacity to act as rescue therapy. Nevertheless, their efficacy is limited in patients with a deteriorated functional status. Thus, their optimum use is as bridging therapy, facilitating bleeding stabilization and enabling the implementation of definitive hemostatic therapies, such as radiotherapy, angioembolization, and surgery.
Ethical considerationsThis study was conducted following the current bioethical research regulations and registered as No. 2023/095 with the research committee of the Instituto Nacional de Cancerología, after meeting the criteria of protection of persons and animals, data confidentiality following workplace protocol on their publication, preservation of anonymity, right to privacy, and informed consent. The authors declare there is no information that could identify patients.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
The authors declare that there is no conflict of interest.
The authors wish to give special thanks to Dr. Jaqueline Paola Bran Alvarado, gastroenterologist at the H. Universitario de Monterrey, for her support and guidance in database management and the statistical analysis.