Surgical or endoscopic treatments play an essential role in the management of achalasia. The probability of adverse events in the performance of said treatments is a relevant aspect, when establishing the risk-benefit balance. The present study aimed to establish the association between serious adverse events and the performance of those procedures, in adult patients with achalasia.
Materials and methodsA systemic search of randomized and nonrandomized clinical trials, retrospective cohorts, and cases series on adult patients with achalasia that underwent laparoscopic Heller myotomy (LHM), peroral endoscopic myotomy (POEM), or endoscopic balloon dilation, that reported serious adverse events, was carried out on the Medline, CENTRAL, and EBSCO databases. Serious adverse events were defined as: death at 30 days, Clavien-Dindo grade III or higher classification, esophageal or gastric perforation, pneumothorax, mucosal tear, leakage, emphysema, pneumonia, and chest pain. The methodology included the PRISMA guidelines for reporting systematic reviews.
ResultsThirty-five studies were found that reported information on 1,276 patients that underwent POEM, 5,492 that underwent LHM, and 10,346 that underwent endoscopic balloon dilation. The proportions of adverse events for the three techniques were 3.6, 4.9, and 3.1%, respectively.
Discussion and conclusionsThe 3 therapeutic interventions evaluated had similar proportions of adverse events. There were few reports of death at 30 days as an outcome and the lack of standardization in reporting adverse events in the studies analyzed was prominent.
Los tratamientos quirúrgicos o endoscópicos juegan un papel fundamental en el manejo de acalasia. La probabilidad de eventos adversos en la realización de estos tratamientos es un aspecto relevante a la hora de establecer el balance riesgo beneficio, este estudio pretende establecer la asociación entre eventos adversos serios y la realización de estos procedimientos en pacientes adultos con acalasia.
Materiales y métodosSe realizó una búsqueda sistemática de ensayos clínicos aleatorizados y no aleatorizados, cohortes retrospectivas y series de casos de pacientes adultos con acalasia llevados a miotomía laparoscópica de Heller (LHM), Peroral Endoscopic Myotomy (POEM) o dilatación endoscópica con balón que reportarán eventos adversos serios en Medline, CENTRAL, y EBSCO. Los eventos adversos serios se definieron como: muerte a 30 días, clasificación de Clavien y Dindo grado III en adelante, perforación esofágica o gástrica, neumotórax, desgarro mucoso, fuga, enfisema, neumonía y dolor torácico. La metodología incluyó los lineamientos PRISMA para reporte de revisiones sistemáticas.
ResultadosSe encontraron 35 estudios que reportaron información de 1,276 pacientes intervenidos con POEM, 5,492 llevados a LHM, y 10,346 a dilatación endoscópica con balón, las proporciones de eventos adversos para las tres técnicas fueron de 3.6, 4.9 y 3.1% respectivamente.
Discusión y conclusionesLas 3 intervenciones terapéuticas evaluadas presentan proporciones similares de eventos adversos, los reportes de mortalidad a 30 días como desenlace fueron pocos y es notoria la falta de estandarización del reporte de eventos adversos en los estudios analizados.
Achalasia is an esophageal motility disorder characterized by insufficient lower esophageal sphincter (LES) relaxation, added to failed peristalsis. Its chronic and incurable nature creates important limitations for ingestion, as well as weight loss, bronchoaspiration, and an increased risk for esophageal cancer, all causing a notable decrease in patient quality of life1.
There are currently several treatment modalities for achalasia. Pharmacologic treatment includes the use of oral medications and botulinum toxin injection, but is considered minimally effective, with a high probability of symptom recurrence. More efficacious treatments include the surgical performance of myotomy of the LES (Heller myotomy) that is usually accompanied by antireflux surgery. Another treatment modality is endoscopic balloon dilation (EBD) of the LES. A more recent procedure is peroral endoscopic myotomy (POEM), which follows the same anatomic basis as the Heller myotomy, but with an endoscopic technique that does not include an antireflux procedure, given the technical limitations.
Endoscopic or surgical interventions in achalasia have been shown to have a favorable impact on symptom control, but even though clinical trials to prove the effectiveness hypothesis have been registered, the large majority have not employed a strict protocol for identifying and classifying safety outcomes, such as the appearance of serious adverse events, which is a relevant aspect, when establishing the risk-benefit balance regarding a given intervention. Through a systematic review of the literature, the present study aimed to establish the proportion of serious adverse events in surgical or endoscopic interventions, in adult patients with achalasia.
MethodologyThe analysis included randomized and nonrandomized clinical trials, cohort studies, and case series with a control group conducted on adult patients with achalasia that underwent laparoscopic Heller myotomy (LHM), POEM, or EBD, and that reported serious adverse events related to said procedures. The studies were in English and Spanish, had a follow-up of up to 30 days, and were not restricted by the year of publication.
Studies that did not report serious adverse events in their results were excluded.
Serious adverse events were defined as: death, a Clavien-Dindo grade III or higher classification, esophageal or gastric perforation, pneumothorax, mucosal tear, leakage, emphysema, pneumonia, and chest pain.
The primary outcome was the appearance of any serious adverse event associated with LHM, POEM, or EBD. The secondary outcome was death at 30 days after the surgical or endoscopic intervention. The search strategy for identifying the studies utilized the following terms in the Embase, Medline, CENTRAL, and EBSCO databases: ((((achalasia[MeSH Terms]) OR (achalasia, esophageal[MeSH Terms])) OR (achalasias, esophageal[MeSH Terms])) AND ((((((clinical trial[MeSH Terms]) ) OR (analyses, cohort[MeSH Terms])) OR (analysis, cohort[MeSH Terms])) OR (retrospective studies[MeSH Terms])) OR (review of reported cases[MeSH Terms]))) AND (((POEM) OR (laparoscopic myotomy)) OR (endoscopic balloon dilation)). An additional search was conducted on the LILACS database, and “snowballing” was carried out to find studies not reported in the databases utilized.
The systematic search of the literature was independently performed by 4 researchers and the results were placed in a previously established register for the control and follow-up of the findings. Abstracts of the articles that met the inclusion and exclusion criteria were reviewed, after which the pertinence of reading the entire article to establish how the report of the adverse events was carried out, was determined.
To evaluate the quality of information and the risk for bias, each article was assessed through the RevMan 5.0 tool that evaluates the method of allocation sequence, allocation concealment, the comparability of study groups, blinding, and the reporting of adverse events, as well as the prior specification of the expected adverse events, according to the type of intervention.
Statistical analysisThe qualitative information was synthesized, using a table, and the statistical analyses were carried out using the RevMan 5.0 software. The occurrences of adverse events were managed as dichotomous data, summarized by odds ratios and reported with 95% confidence intervals. A risk subgroup was defined to establish the association with death at 30 days, derived from each procedure. The random-effects method was employed for the meta-analysis, given the expected elevated heterogeneity for the outcomes, together with measures to assess consistency, such as the I2. A forest plot was utilized to evaluate bias between studies.
Ethical considerationsGiven that the present study was a systematic analysis of the medical literature, at no time was patient personal information or data managed, and therefore, informed consent was not required in the study protocol.
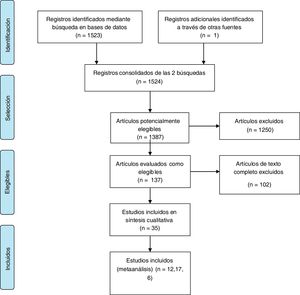
ResultsFig. 1 shows the study selection process. A total of 35 articles were found that compared all the possible combinations of the 3 treatments analyzed.
Table 1 summarizes the characteristics of the studies included in the analysis.
Qualitative synthesis of the evidence.
| Study, year, reference | Country | Type of study | Intervention, n, reported events (%) | Type of adverse event | |
|---|---|---|---|---|---|
| Harvey et al., 20181 | England | Retrospective cohort | LHM 2190, 173 (7.8) | EBD 4748, 195 (4.1) | Death, bleeding, perforation |
| Lynch et al., 20122 | The United States | Retrospective cohort | LHM 295, 6 (2) | EBD 272, 1 (0.1) | Perforation |
| Markar et al., 20183 | England | Retrospective cohort | LHM 1742, 21 (1.2) | EBD 4534, 108 (2.3) | Death, perforation |
| Zheng et al., 20185 | China | Retrospective cohort | POEM 26, 0 (0) | EBD 40, 0 (0) | Perforation, infection, bleeding |
| Bhayani et al., 20147 | The United States | Retrospective cohort | POEM 37, 4 (10.8) | LMH 64, 11 (17.1) | Perforation |
| Boeckxstaens et al., 20118 | The Netherlands | Randomized clinical trial | LHM 106, 13 (12.2) | EBD 95, 4 (4.2) | Perforation, tear |
| Borges et al., 20149 | Brazil | Randomized clinical trial | LHM 44, 0 (0) | EBD 48, 2 (4.1) | Perforation |
| Chan et al., 201610 | China | Retrospective cohort | POEM 33, 5 (15.1) | LHM 23, 3 (13) | Emphysema, pneumothorax, bleeding |
| de Pascale et al., 201711 | Italy | Retrospective cohort | POEM 32, 4 (12.5) | LHM 42, 7 (16.6) | Pneumothorax, pneumoperitoneum, tear |
| Hamdy et al., 201512 | Egypt | Randomized clinical trial | LHM 25, 4 (16) | EBD 25, 2 (8) | Perforation, mucosal tear |
| Hungness et al., 201213 | The United States | Retrospective cohort | POEM 18, 1 (5.5) | LHM 55, 1 (1.8) | Perforation, aspiration pneumonia |
| Khashab et al., 201714 | The United States | Retrospective cohort | POEM 52, 5 (9.6) | RLHM 52, 1 (1.9) | Emphysema, pneumothorax, wound infection |
| Kumagai et al., 201515 | Switzerland | Retrospective cohort | POEM 42, 1 (2.3) | LHM 41, 2 (4.8) | Pneumonia, leakage |
| Leeds et al., 201716 | The United States | Prospective cohort | POEM 12, 3 (25) | LHM 11, 3 (27.2) | Perforation, mucosal necrosis, leakage |
| Miller et al., 201717 | The United States | Retrospective cohort | POEM 98, 4 (4) | LHM 27, 2 (7.4) | Not specified |
| Moonen et al., 201618 | Belgium | Randomized clinical trial | LHM 96, 5 (5.2) | EBD 105, 5 (4.7) | Perforation |
| Novais et al., 201019 | Brazil | Randomized clinical trial | LHM 47, 0 (0) | EBD 47, 2 (4.2) | Perforation |
| Peng et al., 201720 | China | Retrospective cohort | POEM 13, 1 (7.6) | LHM 18, 1 (5.5) | Pneumothorax, wound infection, emphysema |
| Persson et al., 201421 | Switzerland | Randomized clinical trial | LHM 25, 0 (0) | EBD 28, 2 (7.1) | Perforation |
| Ponds et al., 201922 | The Netherlands | Randomized clinical trial | POEM 63, 0 (0) | EBD 63, 2 (3.1) | Perforation, chest pain |
| Ramirez et al., 201723 | Argentina | Retrospective cohort | POEM 35, 1 (2.8) | LHM 35,1 (2.8) | Perforation, leakage, pneumothorax |
| Schneider et al., 201624 | The United States | Retrospective cohort | POEM 25, 1 (4) | LHM 25, 3 (12) | Perforation, abdominal distension, leakage |
| Ujiki et al., 201325 | The United States | Retrospective cohort | POEM 18, 1 (5.5) | LHM 21, 1 (4.7) | Perforation, emphysema |
| Werner et al., 201926 | Germany | Randomized clinical trial | POEM 112, 3 (2.6) | LHM 109, 8 (7.3) | Perforation |
| Nickel et al., 201927 | Germany | Retrospective cohort | LHM 19, 2 (10.5) | EBD 17, 0 (0) | Perforation, leakage, pneumothorax |
| Wirsching et al., 201928 | The United States | Retrospective cohort | POEM 23, 2 (8.6) | LHM 28, 1 (3.5) | Perforation |
| Kim et al., 201829 | The United States | Retrospective cohort Retrospective cohort | LHM 35, 4 (11.4) | LMHR 37, 1 (2.7) | Perforation |
| Wang et al., 201630 | China | POEM 21, 1 (4.8) | EBD 10, 0 (0) | Perforation, emphysema | |
| Kim et al., 201931 | South Korea | Retrospective cohort | POEM 64, 4 (6.2) | EBD 177, 3 (1.6) | Perforation, bleeding |
| Podboy et al., 201932 | The United States | Retrospective cohort | POEM 55, 1 (1.8) | LHM 43, 3 (7) | Perforation |
| Costantini et al., 201933 | Italy | Retrospective cohort | POEM 318, 5 (1.5) | LHM 242, 3 (1.2) | Perforation |
| Kumbhari et al., 201434 | The United States | Retrospective cohort | POEM 49, 0 (0) | LMH 26, 0 (0) | Not specified |
| Chan et al., 2012 35 | China | Retrospective cohort | LHM 18, 1 (5) | EBD 50, 3 (6) | Perforation, emphysema |
| Meng et al., 201636 | China | Retrospective cohort | POEM 32, 0 (0) | EBD 40, 0 (0) | Not specified |
| Chystoja et al., 201637 | Canada | Randomized clinical trial | LHM 23, 0 (0) | EBD 22, 1 (4,5) | Heartburn |
EBD: endoscopic balloon dilation; POEM: peroral endoscopic myotomy; LHM: laparoscopic Heller myotomy.
The large majority of the 35 studies in the present analysis were retrospective cohorts (70%). The studies provided information on 1,276 patients that underwent POEM, 5,492 that underwent LHM, and 10,346 that underwent EBD. Only one-fourth of the studies included a report of adverse events related to the interventions, in their methods sections. The proportions of adverse events reported varied greatly (from 0 to 27%). The proportion of adverse events was 3.6% for POEM, 4.9% for LHM, and 3.1% for EBD. There were no significant differences between the adverse events from LHM and EBD (Fig. 2) and the association of serious adverse events was similar in the LHM and POEM groups (Fig. 3), as well as in the LHM and EBD groups (Fig. 4).
Only 3 publications included reports of mortality at 30 days1–3. The proportion of deaths was 0.09% for LHM and 1.4% for EBD (Fig. 5). No reliable information was found for establishing the association of that outcome with POEM.
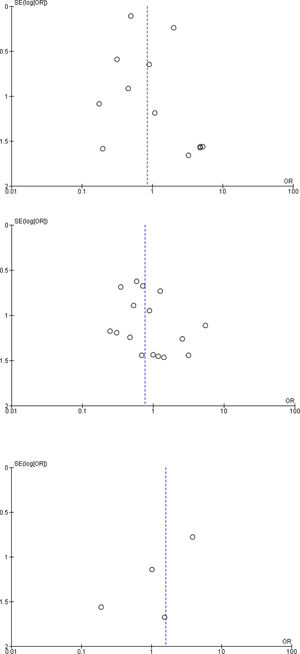
The majority of the studies analyzed had elevated risks for selection bias, performance bias, detection bias, and attrition bias. No publication biases were found (Fig. 6).
Discussion and conclusionOur findings showed a proportion of serious adverse events below 5%, for the 3 techniques, with no significant differences between them. A minority of studies included reports on mortality, but in those that did, there was a higher mortality rate with EBD.
In 2017, Haito-Chavez et al., and in 2019, Zheng et al. published two series of adverse events related to POEM4,5. The proportion of adverse events was 3.3% and 7.5%, respectively, similar to our findings. In 2017, Ngamruengphong et al.6 described the adverse events related to LHM, with a higher proportion (8%) than that found in our study.
Even though EBD had a lower association with serious adverse events in the one-point estimate, it also had a higher association with the mortality outcome. That finding was most likely influenced by biases and confounding variables.
A strength of the present study was the fact that it focused on relevant safety aspects for establishing a risk profile for the management of achalasia. However, the quality of evidence for the analysis was low, the risk for bias was high in the majority of the studies, and there was no standardization for reporting adverse events in the majority of the clinical trials.
In conclusion, the studies that are currently available have appropriately defined the effectiveness of the therapeutic interventions performed for achalasia, but the safety hypotheses have not received the same attention. The majority of the literature at hand has a high risk for biases and the majority of the studies are retrospective cohorts. Thus, the information that is available at present is inconclusive for establishing a safety profile for each intervention.
Standardization in the reporting of adverse events in the methodology of clinical trials is still lacking. The clear identification of adverse events, carried out with the same methodological rigor employed when evaluating efficacy, is essential for establishing guidelines that guarantee quality in the therapeutic procedures for achalasia.
Financial disclosureThis study was carried out utilizing the authors’ own resources.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Niño-Ramírez S, Ardila O, Rodríguez FH, Londoño J, Pérez S, Sánchez S, et al. Eventos adversos mayores relacionados con procedimientos endoscópicos o laparoscópicos en acalasia. Revisión sistemática y metaanálisis. Rev Gastroenterol Méx. 2023;88:36–43.